Neutrophils and Pregnancy-Associated Malaria
Moussa Djimde*,1,2,3,4, Kassoum Kayentao1, Charles Arama1, Alassane Dicko1, Petra F. Mens2,3 and Henk H.D.F. Schallig2,3
1Malaria Research and Training Center (MRTC), University of Sciences of Techniques and Technologies of Bamako (USTTB), Mali
2Amsterdam University Medical Centres, Academic Medical Centre at the University of Amsterdam (AMC), Amsterdam, the Netherlands
3Amsterdam Institute for Infection and Immunity, Infectious Diseases Programme, Amsterdam, the Netherlands
4Biomedical Research and Training Center (BioMed-RTC), Mali
*Corresponding author: Moussa Djimde, Malaria Research and Training Center (MRTC), University of Sciences of Techniques and Technologies of Bamako (USTTB), Mali.
Received: 31 July 2023 Accepted: 08 August 2023 Published: 28 September 2023
Article Information
Citation: Moussa Djimde, Kassoum Kayentao, Charles Arama, Alassane Dicko, Petra F. Mens and Henk H.D.F. Schallig. Neutrophils and Pregnancy- Associated Malaria. Archives of Microbiology and Immunology. 7 (2023): 222-229
View / Download Pdf Share at FacebookAbstract
Purpose: Pregnant women living in areas with transmission of Plasmodium falciparum are exposed to malaria and its harmful consequences on pregnancy outcomes. Neutrophils are the most abundant white blood cells (WBC) in the bloodstream and are innate immune key effectors against infections. Substantial work has been done to study the role of neutrophils in malaria, but little on pregnancy-associated malaria (PAM). This review focuses on neutrophil responses to malaria during pregnancy that may help us to understand their dynamics and effects on pregnancy outcomes.
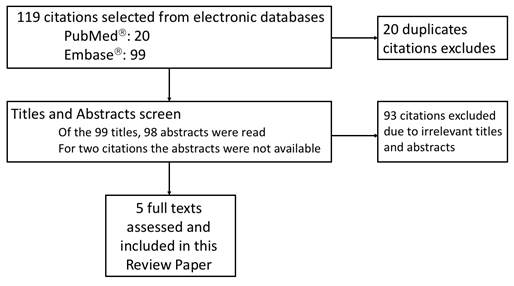
Source: A literature review covering the topic of PAM and neutrophils were accessed via PubMedâ and Embaseâ databases. In total, 20 unique publications were found in PubMed while 99 in Embaseâ. After excluding 114 irrelevant titles and abstracts, 5 original articles full texts were assessed and included in this review.
Results: Due to oestrogen stimulation, the number of neutrophils is higher in pregnant women compared to non-pregnant women. This increase in neutrophil numbers reaches a plateau in the second and third trimesters of pregnancy. However, the number of circulating neutrophils in peripheral blood is lower in pregnant women with Plasmodium falciparum malaria than in pregnant women without malaria. The decrease in circulating neutrophils in the context of PAM may reflect the accumulation of neutrophils in the infected placenta. Data showed that the prevalence of children with low birth weight (LBW) was higher in pregnant women with high number of pigmented peripheral neutrophils compared to malaria-infected pregnant women with low number of pigmented peripheral neutrophils.
Conclusions: This review aids our understanding of the dynamics of neutrophils during a malaria infection in in pregnant women by providing scientific evidence that suggests that neutrophil levels decrease in pregnant women with malaria infection. A negative association between the number of pigmented neutrophils in women with malaria and the birth weight of children points towards prioritizing future research in pregnant women with malaria on these cells involved in the first line of innate immunity.
Keywords
<p>neutrophil, pregnancy, malaria, immunity</p>
Article Details
1. Introduction
Despite ongoing efforts, malaria continues to be a major public health problem. The number of malaria deaths per 100,000 population at risk has been estimated at 14.8 in 2021 [1]. Malaria in pregnancy can occur in any country in which transmission of Plasmodium falciparum occurs. It is a refractory disease that can affect the blood, placenta, brain and other internal organs. These are distinctive features of P. falciparum malaria [2]. The World Health Organization's (WHO) reported in 2021 [3] that among the estimated 33.8 million pregnancies that occur worldwide annually, 34% (11.6 million) were exposed to malaria infection in sSA alone. Although pregnant women are more vulnerable to malaria than non-pregnant women, this vulnerability is greater in women who are in their first or second pregnancy and who have not yet developed adequate pregnancy-specific immune responses against the parasite strains that sequester into the placenta microvasculature [4]. Pregnancy-associated malaria (PAM) is responsible for maternal anaemia and low birth weight (LBW) [5], both increasing the risk of maternal and infant mortality.
Neutrophils (also known as polymorphonuclear cells) are the most abundant white blood cells in the human bloodstream [6]. They are involved in innate immune defence and play a crucial role against bacterial and fungal pathogens, and also participate in the development of inflammatory reactions [7]. They are key effectors of innate immunity by eliminating pathogens through phagocytosis, by generating reactive oxygen species (ROS) and antimicrobial peptides, or by forming neutrophil extracellular traps (NET) [8]. In addition, they also play a role in the activation and regulation of the immune response, through the secretion of cytokines and chemokines [9].
During PAM, P. falciparum-infected erythrocytes sequester in the placenta, often resulting in monocyte infiltration [10] with increased production of pro- and anti-inflammatory mediators [11,12]. Given the abundance of neutrophils in the white blood cell count and their role in innate immunity, it is essential to understand their role in PAM. This will contribute to the management of this disease during pregnancy. However, little is known about the dynamics of neutrophils during PAM and their contribution to control malaria infection. Therefore, this review synthesized the available data on how neutrophils respond to malaria infection in pregnant women. It aims to get an insight into the behaviour of neutrophils during PAM and how they can affect the pregnancy outcome. This review also examined the role of PAM in immune system activation.
2. Methods
2.1 Search Strategy
Original peer-reviewed publications in English, French, or Spanish covering the topic of "pregnancy-associated malaria and neutrophil" were accessed via PubMedâ and Embaseâ databases. From 8th June 2022 to 13th June 2022, bibliographic research was conducted for this review. The database search has been updated on 5th April 2023. The literature search involved the articles indexed in PubMedâ (from 1928 to April 2023) or in Embaseâ (from 1947 to April 2023). In PubMedâ, the database search was done using the topic of malaria, pregnancy and neutrophils. Searching for publications on malaria, the search term used was: "malar*"[All Fields] OR "palud*"[All Fields]. About publications on pregnancy, the search term used was: "gravid*" OR "grossess*"[All Fields] OR "embaraz*"[All Fields] OR "pregnan*"[All Fields]. About neutrophils, the search term used was "neutro*"[All Fields]. Then, looking for publications on malaria and pregnancy, the search term used was: ("malar*"[All Fields] OR "palud*"[All Fields]) AND ("gravid*" OR "grossess*"[All Fields] OR "embaraz*"[All Fields] OR "pregnan*"[All Fields]).
Malaria and neutrophil publications search required the use of search term: ("malar*"[All Fields] OR "palud*"[All Fields]) AND ("neutro*"[All Fields]).
Neutrophil and pregnancy publications search was done using the term: ("gravid*" OR "grossess*"[All Fields] OR "embaraz*"[All Fields] OR "pregnan*"[All Fields]) AND "neutro*"[All Fields]. Finally, for the publications investigating malaria in pregnancy and neutrophils the following search term was used: "malar*"[All Fields] OR "palud*"[All Fields]) AND ("gravid*" OR "grossess*"[All Fields] OR "embaraz*"[All Fields] OR "pregnan*"[All Fields]) AND ("neutro*"[All Fields]).
This search identified 7,109 publications addressing PAM, but only 20 publications addressing PAM in which there was an investigation of the neutrophils.
A database search was also done in Embaseâ (a biomedical research database) for all publications using the topic of malaria: (search term: (malar* OR palud*)); pregnancy (search term: (pregnan* OR gravid* OR grossess* OR embaraz*)); neutrophil (search term: (neutro*)), malaria and pregnancy (search term: (malar* OR palud*) AND (pregnan* OR gravid* OR grossess* OR embaraz*)), malaria and neutrophil (search term: (malar* OR palud*) AND (neutro*)), neutrophil and pregnancy (search term: (pregnan* OR gravid* OR grossess* OR embaraz*) AND (neutro*)) and PAM and neutrophils (search term: (malar* OR palud*) AND (pregnan* OR gravid* OR grossess* OR embaraz*) AND (neutro*)). It identified 9,546 publications addressing PAM, but only 99 publications addressing PAM in which there was an investigation of the neutrophils.
2.2 Eligibility Criteria
This review included originals articles reporting on malaria in pregnancy that assessed the dynamics and / or activity of neutrophils, including in vitro studies. Excluded from this review were review papers, editorials, case reports, conference abstracts and commentaries. Animal studies were also not included in this systematic review.
3. Results
Of the 119 articles that met the search criteria, 114 were excluded on the basis of title and abstract (Fig. 1). An additional 47 papers, identified from the references of the 5 original papers, contributed to this review. In total 52 manuscripts were used for this review and are listed as references 4, 5 and from 11 to 61 in the reference list.
3.1 Leukocytes and neutrophils variations during pregnancy
The total white blood cell (WBC) count was found to be higher in pregnant women than in non-pregnant women due to an increase in neutrophils[13]. The number of neutrophils increases at the time of the oestrogen peak of a normal menstrual cycle and, if fertilization has occurred, neutrophils continue to increase[14]. Subsequently, there is an increase in neutrophils from day 45 of pregnancy, reaching a plateau during the second and third trimesters[12]. Breastfeeding may prolong the rise in neutrophil counts (reviewed by Cruickshank et al.[14]). Increases in neutrophil count and metabolic activity are apparently the result of oestrogen stimulation [15-17].
3.2 Neutrophils and cytokine variations in PAM
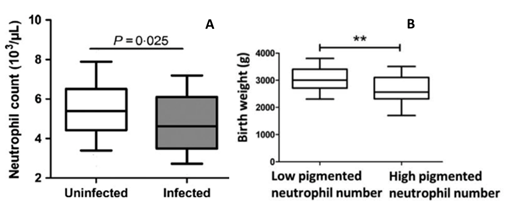
In response to stress, such as inflammation or infection, neutrophils are released into the blood circulation. This is a fast-early response to stimuli, together with early release. Normally, neutrophils are released in large numbers, more than any other leukocytes. In case of PAM, recently, Okezie Caleb Okamgba et al showed that the number and type of WBC lineages that may be preferentially increased against the pathogenic malaria parasite in peripheral and placental blood [18]. Comparison of the mean of the neutrophil counts between malaria-infected peripheral and placental blood, showed that the number of neutrophils is higher in placental blood [18]. Bostrom S et al. showed that the number of neutrophils in the peripheral blood stream was reduced in pregnant women with P. falciparum infection compared to pregnant women without malaria infection (Fig. 2 A) [19]. In contrast, other studies reported an increase in neutrophil counts in African children with uncomplicated malaria [20,21]. In contrast to African children, a significant reduction of neutrophil counts in Thailand adults with P. falciparum malaria was described [22]. This difference in immune response may reflect different inflammatory responses and differences in neutrophils functions. Age difference and hormonal status could also play a role in immune response.
Figure 2: Neutrophils count according malaria infection and Birth weight according pigmented neutrophil number. In pregnant women, the neutrophil count drops when infected with malaria parasites (Fig. 2 A). The birth weight of babies is lower when the number of pigmented neutrophils in the peripheral blood is high during pregnancy (Fig. 2 B). Adapted from Boström et al., 2017 [19] (Fig. 2 A, Reprinted with permission of Wiley Parasite Immunology) and Chua et al., 2015 [23] (Fig. 2 B; Reprinted with permission of International Journal for Parasitology).
Although undetectable in the peripheral blood, the malaria parasite may be present in the placenta and induce an immune response detectable in the peripheral blood. It has been investigated that the number of maternal peripheral granulocytes decreases with placental malaria (PM) in the presence and absence of pre-existing human immunodeficiency virus (HIV) infection [23]. Even though the study did not perform a differential analysis targeting the number of neutrophils among granulocytes, these results are consistent with previous publications that have reported reduced numbers of neutrophils in malaria-infected pregnant women compared with uninfected women [19,24]. This reduction in the number of neutrophils in the peripheral blood may be explained by their accumulation in the placenta infected with malaria parasites. Other publications confirmed an important accumulation of neutrophils in the placenta during PM [18,25].
The decreased neutrophil counts observed in peripheral blood might partially reflect the accumulation of neutrophils in the infected placenta as part of immune response. Neutrophils, eosinophils, basophils and mast cells have in fact often been observed in histological studies of placental biopsies from pregnant women with malaria infection [26,27]. Moreover, cytokine levels such as interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-4 (IL-4), IL-6, and IL-10 were elevated in pregnant women with malaria compared to those uninfected by malaria parasites [14]. Other studies reported that IFN-γ and IL-6 were significantly higher in malaria-infected pregnant women than in non-pregnant uninfected women [15,16] . Additional finding (Torre et al., 2002) [30], showed that a significantly higher levels of IFN-γ, TNF-α, and IL-10 were found in malaria-infected pregnant women than in their control counterparts. Data from East Africa, published by Bayoumi et al [31] and Boström et al [32], show a significant increase in IL-10 in malaria infected pregnant women compared to uninfected pregnant women. However, data from Nigeria [18] described that IFN-γ, IL-4 and IL-10 were higher in peripheral blood not infected with malaria parasites than in infected peripheral blood. The authors believe that this may be due to regional differences, as Nigeria is a region where malaria is very prevalent. From these findings, it can be speculated that the intensity of malaria prevalence can modulate cytokine levels.
In addition to in vivo studies, in vitro models have also been used to observe the activation of immunological parameters including neutrophils. In vitro studies showed that stimulation of human placental cells line that originates from a choriocarcinoma (BeWo cells) [33] with malaria infected red blood cells (iRBCs) resulted in a pronounced release of interleukin 8 (IL-8) and strongly induced neutrophil migration in a transwell assay. This is in the same line with other co-culture studies where iRBCs incubated with whole blood [34] or brain endothelial [35] cells also led to IL-8 production. In addition to this, IL-8 as well as macrophage inflammatory protein-1 alpha (MIP-1a) were elevated in placenta plasma of pregnant women with malaria [11]. This suggests that the PM associated with choriocarcinoma promote neutrophil migration and activities.
3.3 Neutrophil levels in the peripheral bloodstream and poor birth outcome during PAM
It has been reported based on data collected in Papua New Guinea that women in the second or third trimester of pregnancy with increased numbers in pigmented neutrophils gave birth to babies with lower weights (Fig. 2 B) [36]. Furthermore, the prevalence of children with low birth weight (LBW) was higher in pregnant women with high number of pigmented peripheral neutrophils than in PAM women with low numbers [36]. When the women were grouped into primigravidae, secundigravidae and multigravidae, the study found no significant difference in either birth weight or pigmented neutrophil count among the different groups [36]. The role of neutrophils in the pathogenesis of PAM needs to be further explored, but the available data are consistent with the role of neutrophil activation in the pathogenesis of idiopathic intrauterine growth restriction and preeclampsia [37,38]. Evidence suggests that abnormally pigmented neutrophils clearance in malaria-infected women may be delayed in the peripheral circulation, leading to chronic inflammation and thus impacting fetal growth [39]. Furthermore, pigmented neutrophil counts were positively correlated with levels of TNF-a [40], a pro-inflammatory cytokine steady associated with LBW [4,41]. Similarly, sequestration of maternal monocytes in placental intervillous blood has been linked to LBW in placental malaria [5,42]. It could be that their excessive cytokine production impairs placental nutrient transport [43] and placental trophoblast invasion [44], thereby contributing to poor fetal growth. Circulating pigmented neutrophils counts can be used to predict malaria fatality with more accuracy compared to peripheral parasitemia [45] but remains speculative regarding poor delivery outcome.
3.4 Neutrophil activation markers and malaria or malaria and HIV coinfection in pregnant women
Neutrophil activation results in massive production of its markers such as myeloperoxidase (MPO), matrix metalloproteinase (MMP9), neutrophil elastase (NE) and proteinase 3 (PRTN3). MPO is involved in the oxidative burst of neutrophils and macrophages. Interestingly, the placental blood MPO and PRTN3 levels are increased with PM in both HIV negative and HIV positive pauci-gravidae women relative to women negative to both PM and HIV [23]. In addition, MPO was found in placental tissue sections, suggesting that this factor may be produced by both neutrophils and intervillous macrophages. However, most MPO-expressing cells observed by immunofluorescence also stain for NE, identifying them as neutrophils. Furthermore, it has been found that placental levels of MPO, MMP9, and PRTN3 correlated positively with placental malaria parasite density [23].
This further confirms that MPO is derived from neutrophils whose activation is proportional to the parasite density. MPO levels in placental blood were significantly increased in chronic, inflammatory PM in primigravida. MPO is associated with vascular dysfunction [46], which underlies the pathophysiology of many vascular inflammatory diseases, including arteriosclerosis and coronary artery disease [47,48]. High neutrophil activation, proved by higher plasma concentrations of not only MPO but also PRTN3 and NE, is correlated with severe pediatric malaria [49]. Relatedly, MMP9, an endopeptidase discharged by neutrophils and monocytes, is incriminated in the pathogenesis of severe malaria [50-52]. A polymorphism in MMP9 protects against PM, implying an important role for this enzyme in P. falciparum infection [53]. High concentrations of neutrophil-derived antimicrobial compounds can render these cells harmful to the host [54-56]. Neutrophils can rapidly be recruited to sites of infection and tissue injury [8], generate ROS which can exacerbate preeclampsia [57-60].These findings suggest that malaria during pregnancy associated with HIV infection perturb granulocyte activation. Further exploration is needed to better understand neutrophil function in pregnant women co-infected with malaria and HIV.
4. Conclusions
This review highlighted that in case of malaria infection, in contrast to the increase observed in children, neutrophil levels decrease in pregnant women. Data gathered in this review suggest a negative association between the number of pigmented neutrophils in women with malaria and the birth weight of the child, pigmented neutrophils being predictive of disease severity. On the other hand, gravidity does not have an effect on birth weight or the number of pigmented neutrophils. In addition, PM and PM/HIV co-infection disrupt granulocyte levels, and soluble signatures of neutrophil activation are associated with indicators of PM infection and associated symptoms. This paper showed that PAM has the ability to induce immune activation with the release of cytokines but also that this activation could be exacerbated when choriocarcinoma is associated.
Future Investigations
Neutrophils and their potential role(s) in PAM remain understudied. We have listed below future research priorities that will greatly help us to understand the role of neutrophils during malaria in pregnancy and reduce the burden of this disease in pregnant women:
- Determine whether pigmented neutrophils became pigmented in the peripheral circulation after schizogony or whether they became pigmented in the intervillous space of the placenta and were recirculated.
Approach: PM is a special forthcoming of PAM. Pigmented neutrophil levels are known to be negatively correlated with birth weight [19]. If pigmented in the placenta, pigmented neutrophils may be absent in the peripheral blood while colonising the placenta intervillous space. This can be an additional hidden threat because during pregnancy, microscopy will not detect these pigmented neutrophils.
- Assessment of the effect of neutrophil levels variation in artemisinin-based combination therapies (ACTs) effectiveness in pregnant women with malaria.
Approach: Decreased neutrophil counts in children have been shown to decrease the effectiveness of ACTs (Moussa Djimde et al., in press at the Journal of Infection in Developing Countries (JIDC)). The same study showed that the rate of reappearance of malaria parasites after treatment in neutropenic patients was higher among those treated with artemether lumefantrine. Many phenomena occur during pregnancy including hormonal and immunological changes and could affect ACTs effectiveness, with a general shift from cell-mediated immunity toward humoral immunity [62]. If pregnant women based on their neutrophil count respond as children to ACTs (Moussa Djimde et al., in press at JIDC), a drug with longer prophylactic coverage may be advised.
Abbreviations
HIV Human immunodeficiency virus
IFN-γ Interferon gamma
IL- Interleukin
iRBCs infected red blood cells
LBW Low birth weight
MIP-1α Macrophage inflammatory protein-1α
MMP9 Matrix metalloproteinase
MPO Myeloperoxidase
NE Neutrophil elastase
NET Neutrophil extracellular traps
PAM Pregnancy-associated malaria
PM Placental malaria
PRTN3 Proteinase 3
- falciparum Plasmodium falciparum
- vivax Plasmodium vivax
ROS Reactive oxygen species
SSA Sub-Sahara Africa
TNF-α Tumor necrosis factor alpha
WBC White blood cell
Ethics approval and consent to participate
The various original studies have been approved by the ethics committees in the respective countries.
Consent for publication
Not applicable
Availability of data and Material
Not applicable
Competing interests
None of the authors declare a conflict of interests.
Author’s Contributions
MD did the database search and wrote the first draft of the paper. KK, CA, AD, PFM and HDFHS have made a substantial intellectual contribution to the work. All authors approved this paper for publication.
Authors' Information
Moussa Djimde: mdjimde@icermali.org
Kassoum Kayentao: kayentao@icermali.org
Charles Arama: charama@icermali.org
Alassane Dicko: adicko@icermali.org
Petra F. Mens: p.f.mens@amsterdamumc.nl
Henk H.D.F. Schallig: h.d.schallig@amsterdamumc.nl
Funding
Contributions of MD, KK, PM and HS were partly supported through the Efficacy and Safety of pyronaridine-artesunate for the treatment of uncomplicated malaria in African pregnant women project (PYRAPREG), which is part of the European and Developing Countries Clinicals Trials Partership (EDCTP2) programme supported by the European Union (grant number RIA2017MC-2025-PYRAPREG).
References
- World Health Organisation (WHO). World malaria report (2022)
- Arora DR AB. Malaria parasite In: Medical Parasitology. 3rd ed. New Delhi, India: CBS Publishers distribution Ltd (2010).
- World Health Organisation (WHO). World malaria report (2021).
- Fried M, Muga RO, Misore AO, Duffy PE. Friend et al. Malaria elicits type 1 cytokines in the human placenta: INF-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol 160 (1998): 2523-2.
- Stephen SJ, Pollina E, Getachew A, Tadesse E, Lema VM and Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg 68 (2003): 115-119.
- Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: Age, sex, smoking status, and ethnic differences. Ann Intern Med 146 (2007): 486-92.
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6 (2006): 173-82.
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol 13 (2013): 159-75.
- Tecchio C, Cassatella MA. Neutrophil-derived chemokines on the road to immunity. Semin Immunol 28 (2016): 119-28.
- Muehlenbachs A, Fried M, Mcgready R, Harrington WE, Mutabingwa TK, Ois Nosten F, et al. A Novel Histological Grading Scheme for Placental Malaria Applied in Areas of High and Low Malaria Transmission. J Infect Dis 202 (2010): 1608-16.
- Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg 68 (2003): 115-9.
- Abrams ET, Brown H, Chensue SW, Turner GDH, Tadesse E, Lema VM, et al. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J Immunol 170 (2003): 2759-64.
- Efrati P, Presentey B, Margalith M, Rozenszaln L. Leukocytes of normal pregnant women. Obstet Gynecol 23 (1964): 429-32.
- Cruickshank JM, Morris R, Butt WR, Crooke AC. The relationship of total and differential leukocyte counts with urinary oestrogen and plasma cortisol levels. J Obs Gynaecol Br Commonw 77 (1970): 634-9.
- Elder MG, BFEJ. Neutrophil alkaline phosphatase in pregnancy and its relationship to urinary estrogen excretion and serum heat-stable alkaline phosphatase levels. Am J Obstet Gynecol 111 (1971): 319-23.
- Cruickshank JM, Morris R, Butt WR, Corker CS. Inter-relationships between levels of plasma oestradiol, urinary total oestrogens and blood haemoglobin and neutrophil counts. J Obs Gynaecol Br Commonw 79 (1972): 450-4.
- Jacobs AA, Selvardj RJ, Strauss RR, Paul BB, Mitchell GW Jr, and Sbarra AJ. The role of the phagocyte in host-parasite interactions: XXXIX. Stimulation of bactericidal activity of myeloperoxidase-containing leukocytic fractions by estrogens. Am J Obstet Gynecol 117 (1973): 671-8.
- Okamgba OC, Ifeanyichukwu MO, Ilesanmi AO, Chigbu LN. Variations in the leukocyte and cytokine profiles between placental and maternal circulation in pregnancy-associated malaria. Res Rep Trop Med 9 (2018): 1-8.
- Boström S, Schmiegelow C, Abed UA, Minja DTR, Lusingu J, Brinkmann V, et al. Neutrophil alterations in pregnancy-associated malaria and induction of neutrophil chemotaxis by Plasmodium falciparum. Parasite Immunol (2017).
- Maina RN, Walsh D, Gaddy C, Hongo G, Waitumbi J, Otieno L, et al. Impact of Plasmodium falciparum infection on haematological parameters in children living in Western Kenya. Malar J 9 (2010): S4.
- Olliaro P, Djimdé A, Dorsey G, Karema C, Mårtensson A, Ndiaye JL, et al. Hematologic parameters in pediatric uncomplicated Plasmodium falciparum malaria in sub-Saharan Africa. Am J Trop Med Hyg 85 (2011): 619-25.
- Kotepui M, Phunphuech B, Phiwklam N, Chupeerach C, Duangmano S. Effect of malarial infection on haematological parameters in population near Thailand-Myanmar border. 2 Malar J 13 (2014): 218.
- Chua CLL, Robinson LJ, Baiwog F, Stanisic DI, Hamilton JA, Brown GV, et al. High numbers of circulating pigmented polymorphonuclear neutrophils as a prognostic marker for decreased birth weight during malaria in pregnancy. Int J Parasitol 45 (2015): 107-11.
- Sarr D, Oliveira LJ, Russ BN, Owino SO, Middii JD, Mwalimu S, et al. Myeloperoxidase and Other Markers of Neutrophil Activation Associate with Malaria and Malaria/HIV Coinfection in the Human Placenta. Front Immunol 12 (2021): 682668.
- Sarr D, Marrama L, Gaye A, Dangou JM, Niang M, Mercereau-Puijalon O, et al. High prevalence of placental malaria and low birth weight in Sahelian periurban area. Am J Trop Med Hyg 75 (2006): 171-7.
- Ordi J, Menendez C, Ismail MR, Ventura PJ, Palacín A, Kahigwa E, et al. Placental Malaria Is Associated with Cell-Mediated Inflammatory Responses with Selective Absence of Natural Killer Cells. J Infect Dis 183 (2001): 1100-7.
- Garnham PCC. The placenta in malaria with special reference to reticulo-endothelial immunity. Trans R Soc Trop Med Hyg. 32 (1938): 13-34.
- Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, et al. Placental pathology in malaria: A histological, immunohistochemical, and quantitative study. Hum Pathol 31 (2000): 85-93.
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol 21 (2003): 713-58.
- Nmorsi OPG, Isaac C, Ohaneme BA, Obiozi H. Pro-inflammatory cytokine pro les in Nigerian pregnant women infected with Plasmodium falciparum malaria. Asian Pac J Trop Med 3 (2010): 731-733.
- Torre D, Speranza F, Giola M, Matteelli A, Tambini R et al. Role of TH1 and TH2 cytokines in immune response to uncomplicated malaria. Clin Diagn Lab Immunol 9 (2002): 348-3.
- Bayoumi NK, Bakhet KH, Mohmmed AA, Eltom AM, Elbashir MI, Mavoungou E, et al. Cytokine Profiles in Peripheral, Placental and Cord Blood in an Area of Unstable Malaria Transmission in Eastern Sudan. J Trop Pediatr 55 (2009): 233-7.
- Boström S, Ibitokou S, Oesterholt M, Schmiegelow C, Persson JO, Minja D, et al. Biomarkers of Plasmodium falciparum Infection during Pregnancy in Women Living in Northeastern Tanzania. PLoS One 7 (2012): e48763.
- Pattillo RA, Gey GO. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res 28 (1968): 1231-6.
- Walther M, Woodruff J, Edele F, Jeffries D, Tongren JE, King E, et al. Outcomes Correlate with Parasitological and Clinical Plasmodium falciparum to Blood-Stage Malaria: Heterogeneous Cytokine Responses Innate Immune Responses to Human. J Immunol 177 (2006): 5736-45.
- Tripathi AK, Sha W, Shulaev V, Stins MF, Sullivan DJ Jr. Plasmodium falciparum-infected erythrocytes induce NF-_B regulatedinflammatory pathways in human cerebral endothelium. Blood 114 (2009): 4243-4252.
- Sabatier F, Bretelle F, D'ercole C, Boubli L, Sampol J, Dignat-George F. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obs Gynecol 183 (2000): 1558-63.
- Belo L, Santos-Silva A, Caslake M, Cooney J, Pereira-Leite L, Quintanilha A, et al. Neutrophil Activation and C-Reactive Protein Concentration in Preeclampsia. Hypertens Pregnancy 22 (2003): 129-41.
- Von Dadelszen P, Watson RWG, Noorwali F, Marshall JC, Parodo J, Farine D, et al. Maternal neutrophil apoptosis in normal pregnancy, preeclampsia, and normotensive intrauterine growth restriction. Am J Obstet Gynecol 181 (1999): 408-14.
- Luty AJF, Perkins DJ, Lell ‡ Bertrand, Schmidt-Ott R, Lehman LG, Luckner D, et al. Low Interleukin-12 Activity in Severe Plasmodium falciparum Malaria †. Infect Immun 68 (2000): 3909-15.
- Stephen J. Rogerson, Heidi C. Brown, Elena Pollina, Elizabeth T. Abrams, Eyob Tadesse VML and MEM. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect Immun 71 (2003): 267-270.
- Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The Impact of Placental Malaria on Gestational Age and Birth Weight. J Infect Dis 181 (2000): 1740-5.
- Boeuf P, Aitken EH, Chandrasiri U, Lin C, Chua L, Mcinerney B, et al. Plasmodium falciparum Malaria Elicits Inflammatory Responses that Dysregulate Placental Amino Acid Transport. PLoS Pathog 9 (2013): e1003153.
- Umbers AJ, Stanisic DI, Ome M, Wangnapi R, Hanieh S, Unger HW, et al. Does Malaria Affect Placental Development? Evidence from In Vitro Models. PLoS One 8 (2013): e55269.
- Lyke KE, Diallo DA, Dicko A, Kone A, Coulibaly D, Guindo A, et al. Association of intraleukocytic plasmodium falciparum malaria pigment with disease severity, clinical manifestations, and prognosis in severe malaria. Am J Trop Med Hyg 69 (2003): 253-9.
- Vita JA, Brennan M-L, Gokce N, Mann SA, Goormastic M, Shishehbor MH, et al. Serum Myeloperoxidase Levels Independently Predict Endothelial Dysfunction in Humans (2004).
- Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol 158 (2001): 879-91.
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol 77 (2005).
- Catherine Manix Feintuch, Alex Saidi, Karl Seydel, Grace Chen, Adam Goldman-Yassen, Neida K. Mita-Mendoza, Ryung S. Kim, Paul S. Frenette, Terrie Taylor JPD. Activated Neutrophils Are Associated with Pediatric Cerebral Malaria Vasculopathy in Malawian Children. MBio 167 (2016): e0.
- Prato M, Giribaldi G, Polimeni M, Gallo V AP. Phagocytosis of Hemozoin Enhances Matrix Metalloproteinase-9 Activity and TNF-Alpha Production in Human Monocytes: Role of Matrix Metalloproteinases in the Pathogenesis of Falciparum Malaria. J Immunol 175 (2005): 64.
- Prato M, Gallo V, Giribaldi G, Arese P. Phagocytosis of haemozoin (malarial pigment) enhances metalloproteinase-9 activity in human adherent monocytes: Role of IL-1beta and 15-HETE. Malar J 7 (2008): 1-9.
- Prato M, Giribaldi G. Matrix Metalloproteinase-9 and Haemozoin: Wedding Rings for Human Host and Plasmodium falciparum Parasite in Complicated Malaria. J Trop Med (2011): 11.
- Apoorv TS, Babu PP, Meese S, Gai PP, Bedu-Addo G MF. Matrix Metalloproteinase-9 Polymorphism 1562 C > T (Rs3918242) Associated with Protection Against Placental Malaria. Am J Trop Med Hyg 93 (2015): 186-8.
- Bn P, Stein RT. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front Immunol 7 (2016): 311.
- Rada B. Neutrophil Extracellular Traps. Methods Mol Biol (2019).
- Rada B. pathogens Interactions between Neutrophils and Pseudomonas aeruginosa in Cystic Fibrosis (2017).
- Tsukimori K, Nakano H, Wake N. Difference in Neutrophil Superoxide Generation During Pregnancy Between Preeclampsia and Essential Hypertension (2007).
- Aly AS, Khandelwal M, Zhao J, Mehmet AH, Sammel MD, Parry S. Neutrophils Are Stimulated by Syncytiotrophoblast Microvillous Membranes to Generate Superoxide Radicals in Women with Preeclampsia. Am J Obstet Gynecol 190 (2004): 252.
- Gupta AK, Hasler P, Holzgreve W, Hahn S. Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia? Semin Immunopathol 29 (2007): 163-7.
- Marder W, Knight JS, Kaplan MJ, Somers EC, Zhang X, O’dell AA, et al. Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci Med 3 (2016): e000134.
- Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg. Infect. Dis 12 (2006): 1638-43.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
