Interventions with Potential to Mitigate Injection Site Reactions Following Subcutaneous Elamipretide Administration: A Phase 1, Crossover Study
Alana Sullivan1, Sandrin C. Bergheanu1, Laura E. Kropp1, Li Zhang2, Lisa A. Beck3, Benjamin McNeil2,*, Anthony Abbruscato1,*
1Stealth BioTherapeutics Inc., Needham, MA, USA
2Division of Allergy and Immunology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
3Carol & Lowell Goldsmith Professor of Dermatology, Medicine & Pathology, University of Rochester Medical Center, Rochester, NY, USA
*Corresponding author: Anthony Abbruscato, Stealth BioTherapeutics Inc., Needham, MA, USA.
Benjamin McNeil, Division of Allergy and Immunology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Received: 28 September 2023 Accepted: 10 October 2023 Published: 15 November 2023
Article Information
Citation: Alana Sullivan, Sandrin C. Bergheanu, Laura E. Kropp, Li Zhang, Lisa A. Beck, Benjamin McNeil, Anthony Abbruscato. Interventions with Potential to Mitigate Injection Site Reactions Following Subcutaneous Elamipretide Administration: A Phase 1, Crossover Study. Archives of Microbiology and Immunology. 7 (2023): 306-317.
View / Download Pdf Share at FacebookAbstract
Aim: Elamipretide is a mitochondria-targeting agent in development for treating mitochondrial dysfunction-associated diseases. While prior studies showed that subcutaneous elamipretide is generally safe/well tolerated, most subjects reported injection site reactions (ISRs). We evaluated the efficacy of interventions to mitigate ISRs, identify underlying ISR mechanisms, and evaluate the pharmacokinetic and safety profile of subcutaneous elamipretide.
Methods: Subcutaneous elamipretide 60mg was administered to healthy subjects (N=10) on six separate occasions with/without potential ISR interventions (mometasone furoate, ice application, tacrolimus ointment, doxepin cream, and oral diphenhydramine). ISR clinical/self-assessments, blood samples, and safety data were collected at predetermined intervals. Preclinical studies investigated mast cell-specific receptor MRGPRX2 mediation of ISRs.
Results: Mometasone significantly reduced the incidence of induration/swelling and pruritus. Diphenhydramine significantly decreased the incidence of induration; 50% reported somnolence. Ice application significantly reduced the incidence of pain, although it reduced elamipretide’s maximum plasma concentration and area-under-the-curve from time 0-6hrs versus elamipretide alone. Preclinical data suggest that subcutaneous-elamipretide induced ISRs by activating MRGPRX2 in humans and its ortholog, Mrgprb2, in mice.
Conclusion: Elamipretide activated MRGPRX2 and Mrgprb2 receptors, resulting in activation of mast cells and inflammation in mouse models, suggesting that targeting mast-cell activation may ameliorate elamipretide ISRs. Topical mometasone prior to subcutaneous elamipretide demonstrated significant reductions in ISR signs and symptoms and did not cause significant changes in elamipretide plasma exposure or additional adverse events. Therefore, mometasone prior to subcutaneous injection of elamipretide warrants further investigation in clinical studies for alleviating ISRs.
Keywords
<p>Elamipretide, injection site reaction, mitigation, mometasone, safety, tolerability, MRGPRX2</p>
Article Details
1. Introduction
Elamipretide is an aromatic-cationic tetrapeptide that readily penetrates the cell membrane and transiently localizes to the inner mitochondrial membrane (IMM) where energy (adenosine triphosphate [ATP]) production occurs, thereby improving mitochondrial function by restoring the physical and biochemical properties of the IMM by irreversibly associating with cardiolipin (CL), a mitochondrial-membrane-unique phospholipid [1-4]. This association improves membrane stability; enhances ATP synthesis in several organs including the heart, kidney, neurons, and skeletal muscle; and reduces reactive oxygen species (ROS) production [5-16]. Elamipretide has been extensively examined in multiple preclinical and clinical studies for diseases involving mitochondrial dysfunction, consistently demonstrating amelioration of pathologic symptoms, including improvement in skeletal muscle strength, cardiac stroke volume, and kidney function [2, 6, 8, 9, 12, 17-23].
Elamipretide is being developed for the treatment of patients with a variety of diseases, such as Barth syndrome (BTHS) and Primary Mitochondrial Myopathy (PMM), among others, in which genetic abnormalities affecting the mitochondria lead to life-long symptoms requiring long-term elamipretide administration [20, 21, 23, 24]. Clinical development of elamipretide has focused on subcutaneous (SC) administration. Prior studies showed that SC elamipretide up to 80mg once daily is generally safe and well tolerated, although most longer-term studies have used SC elamipretide 40mg or 60mg once daily [data on file, Stealth BioTherapeutics Inc.]. However, injection site reactions (ISRs) were reported in the majority of subjects receiving treatment. In multiple-dose clinical trials lasting >8 days, ISRs were relatively common in subjects: injection site erythema (47%), pruritus (45%), pain (22%), induration (19%), swelling (14%), urticaria (13%), bruising (12%), hemorrhage (6%), and mass (6%). Although typically mild in nature and resolving within 4 hours of elamipretide administration, they can lead to subject withdrawal or discontinuation during chronic daily administration [data on file, Stealth BioTherapeutics Inc].
The Mas-related G-protein coupled receptor member X2 (MRGPRX2), expressed almost exclusively by mast cells that populate connective tissues, including the skin [25, 26], has relatively recently been linked to ISRs [27]. Skin mast-cell-activation causes immediate inflammation, and many therapeutic drugs associated with high frequencies of ISRs have been shown to activate MRGPRX2 directly, thereby triggering inflammation via mast cells. MRGPRX2 is preferentially activated by drugs with cationic and aromatic properties [28], such as elamipretide, making it an MRGPRX2 agonist. While the current management of ISRs consists of alternating daily injection sites around abdominal quadrants, interventions that target mast cell activation or the effects of mast-cell-derived mediators warrant investigation. The aim of the present study was to evaluate the efficacy of potential interventions used to mitigate elamipretide-induced ISRs, identify any role of the MRGPRX2 receptor in elamipretide ISRs, and further understand the pharmacokinetics (PK) and safety of 60mg SC elamipretide administration. The study agents (mometasone, tacrolimus, doxepin, diphenhydramine) have dosing regimens supported by the product label and their ability to reduce inflammatory responses by targeting mast cell activation.
2. Materials and Methods
2.1 Study Design
This was a Phase 1, open-label, 4-week, 6-part, crossover study. After a screening period (up to 28 days), ten eligible subjects received SC elamipretide 60mg (0.75mL) to alternating abdominal quadrants on each of six occasions with or without potential ISR interventions. The first treatment arm (Arm 1) started on Day 1 with SC administration of elamipretide only, followed by the remaining treatment arms (Arms 2-6) with the following interventions sequentially. On Day 3, Arm 2 received mometasone furoate 0.1% ointment applied once to an area ~8cm in diameter and covered with a hydrocolloid occlusive dressing (DuoDERM® Extra Thin) prior to SC elamipretide administration 1 week later on Day 10. Arm 3 received ice application to an area ~10cm in diameter around the injection site 5 minutes before and 5 minutes after elamipretide administration on Day 12. Arm 4 received tacrolimus 0.1% ointment applied to an area ~10cm in diameter around the injection site 15 minutes before elamipretide administration on Day 14. Arm 5 received doxepin 5% cream applied to an area ~10cm in diameter around the injection site 15 minutes before elamipretide administration on Day 16. Arm 6 received diphenhydramine 50mg orally taken 2 hours prior to elamipretide administration on Day 18. All treatments were administered by and/or under investigative staff supervision, ensuring compliance. Drug classes and/or mechanisms of action of ISR interventions are summarized in Table 1. All patients participated in all arms of the study; therefore, randomization and blinding were not applicable.
For each treatment arm, blood samples for determining elamipretide plasma concentrations and metabolites were collected pre-dose and at 0.25, 0.5, 1, 2, 4, and 6 hours after elamipretide administration. Vital signs were recorded pre-dose and at 0.5 and 6 hours after elamipretide administration. ISR clinical and self-assessments were performed before and at 0.5, 1, 2, 4, 6, 12, 24, and 48 hours after elamipretide administration. Injection sites were photographed at 0.5, 1, 2, 4, and 12 hours after elamipretide administration.
This study was conducted in accordance with international ethics guidelines, including the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, International Conference on Harmonisation Good Clinical Practice guidelines, and all applicable laws and regulations. The protocol was approved by the institutional review boards and all participants provided written informed consent prior to entering the trial.
Table 1: Drug class/mechanism of actions of the potential ISR interventions
|
Arm |
Intervention |
Drug class/mechanism of action |
|
1 |
None |
Control (elamipretide only) |
|
2 |
Mometasone |
Topical corticosteroid + occlusive dressing; anti-inflammatory and mast cell inhibition |
|
3 |
Ice |
Vasoconstriction |
|
4 |
Tacrolimus |
Calcineurin inhibitor |
|
5 |
Doxepin |
Tricyclic antidepressant; used topically to alleviate itching via histaminic blockade |
|
6 |
Diphenhydramine |
H1 antihistamine antagonist |
2.2 Participants
Healthy subjects ≥18 and ≤65 years-of-age were enrolled with body mass index ≥18.5 and ≤32.0kg/m2 and body weight ≤120kg. Participants had to be willing/able to provide consent and adhere to trial requirements. All participants of childbearing potential were on acceptable birth control. Main exclusion criteria were estimated creatinine clearance <90mL/min, history of clinical hypersensitivity or allergy to any pharmaceutical agent, chronically administered antihistamines or corticosteroids within the last 28 days, and significant mental illness. Tattoos, scarring, or other abdominal skin characteristics which could confound assessment of ISRs were also considered exclusion criteria.
2.3 Study Assessments
Primary endpoints consisted of the following efficacy measures: clinical assessments and subject self-assessments. Primary endpoints compared grading of each ISR parameter following administration of elamipretide with each separate intervention versus grading of each ISR parameter following administration of elamipretide alone. For clinical assessments, a standard procedure adapted from the Division of Aids Table for Grading the Severity of Adult and Pediatric Adverse Events to score pain, erythema, induration/swelling, and pruritus using a 4-point scale based on severity (1=mild, 2=moderate, 3=severe, and 4=potentially life threatening) was used. Self-assessments were based on a questionnaire to determine how bothered the patient was following each injection of elamipretide and included parameters of pain, burning sensation, cold sensation, itching, redness, swelling, and bruising (Not at all, A little, Moderately, Very, Extremely).
Secondary endpoints consisted of PK and general safety assessments. Plasma samples were analyzed for elamipretide and its M1/M2 metabolites using a validated liquid chromatography/tandem mass spectrometry assay. The lower limits of assay quantitation were 3.0, 1.5, and 1.0ng/mL for elamipretide, M1, and M2, respectively. Maximum plasma concentration (Cmax) and area under the plasma concentration-time curve from time zero to 6 hours (AUC0-6h) were calculated using Phoenix™ WinNonlin® software.
Safety assessments compared treatment-emergent adverse events (TEAEs) reported following administration of elamipretide with each separate intervention versus TEAEs reported following administration of elamipretide alone. Safety measurements for determination of TEAEs included routine clinical laboratory tests, 12-lead ECG, physical examination, and vital signs. TEAEs were graded based on severity (mild/moderate/severe) and relationship to study drug (unrelated or unlikely/possible/probably related). Injection sites were photographed at 0.5, 1, 2, 4, and 12 hours post-elamipretide-dose for qualitative purposes.
2.4 Data and Statistical Analysis
All statistical analyses were performed using SAS GRID Linux/SAS Studio (Version 9.4, or higher). PK parameters for elamipretide and its metabolites, M1 and M2, were summarized using descriptive statistics. Comparisons between treatments (elamipretide with each separate intervention versus elamipretide alone) were evaluated by an analysis of the log-transformed PK parameters (Cmax and AUC0-6h) by performing an analysis of variance with treatment effects. From these analyses, least square means (LSMs), least square treatment differences, and 90% confidence intervals (CIs) for the treatment differences on log-scale were obtained. The results were transformed back to the original scale by exponentiation to provide treatment geometric LSM, point estimates of the geometric test (elamipretide alone)/reference (elamipretide with each separate intervention) LSM ratios, 90% CI for these ratios, and p-values. Chi-square test or Fisher exact test were used to examine the differences in ISR responses between treatment arms at each timepoint. Data and statistical analyses complied with the recommendations on experimental design and analysis in pharmacology. Thresholds for statistical significance and trends toward significance were defined as p<0.05 and p<0.20, respectively.
2.5 Preclinical MRGPRX2, Mrgprb2, Mast Cell Activation, and Evans Blue Assays
Calcium mobilization in heterologous cells - Clonal HEK293 cells expressing human MRGPRX2 and Galpha15 were plated in wells of a 96-well plate, loaded with Fluo-4 acetoxymethyl ester (Fluo-4 AM) for 45minutes at 37°C, and allowed to rest for 30 minutes before use [29]. 2X elamipretide was added by manual pipetting at designated time points, and fluorescence intensity before, during, and after elamipretide incubation was measured with a confocal microscope using the FITC filter. A similar protocol was used for MRGPRX2-transfected Chem-1 cells and their parental cell line, Chem-1. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) high glucose medium (4.5g/L) with 10% fetal bovine serum, non-essential amino acids, and HEPES. Geneticin (G418) was used to maintain receptor expression in the MRGPRX2 cell line. These cells were seeded to 96-well plates, loaded with Fluo-4 AM for 30 minutes at 37°C, washed, and allowed to rest for 30 minutes before use. Cells were resuspended in 50mL of PBS with calcium and magnesium and 50mL of 2X elamipretide or ionomycin wasadded to stimulate the cells. A no-drug vehicle control and ionomycin positive control were also included. Plates were read every six seconds for two minutes using a Biotek plate reader.
EC50 calculation - Clonal HEK293 cells expressing human MRGPRX2 and Galpha15 were plated in a 96-well plate, loaded with Fluo-4 AM for 45 minutes at 37°C, and allowed to rest for 30 minutes before use. The assay was performed using a fluorescent plate reader and baseline fluorescence was calculated as the average of a 30-second read. 2X elamipretide was added manually after baseline recordings, and response was defined as the maximum signal within 90 seconds after addition of elamipretide minus the baseline fluorescence signal. Concentrations were tested in duplicate, the assay was performed six times, and the curve was calculated as a four-parameter non-linear fit with variable slope.
Peritoneal mast cell activation assay - Primary peritoneal mast cells were isolated from wild type and Mrgprb2 knockout mice [29], selected because Mrgprb2 is the mouse ortholog of MRGPRX2. Mast cells were incubated in DMEM and supplemented with 10% fetal bovine serum and 100ng/mL human stem cell factor for 2 hours in a 96-well plate. Cells were then loaded with Fluo-4 AM for 30 minutes at room temperature, washed, and allowed to rest for 30 minutes before use. Free intracellular calcium levels in each mast cell were measured using a fluorescence microscope. If the Fluo-4 signal rose by at least 50% for ³10 seconds, cells were identified as responding. Evans Blue assay - Anesthetized wild type and Mrgprb2 knockout mice up to 8 months of age were injected intravenously with 50mL of 12.5mg/mL Evans Blue in saline, followed by injection of 5mL of 0.5mg/mL elamipretide in one paw and saline in the other paw. Mice were sacrificed 15 minutes after elamipretide injection and paw tissue was collected, dried for 24 hours at 50°C, and weighed. Evans Blue was extracted by a 24-hour incubation in formamide at 50°C and the optical density was read at 600nm and 740nm using a spectrophotometer. The value at 740nm was subtracted from the value at 600nm to attain the final readout.
3. Results
3.1 Participants
Of the 13 subjects screened, ten underwent treatment and completed the study (50% male). Mean (standard deviation [SD]) age was 40.7 (5.89) years, weight 75.31 (11.35) kg, height 167.98 (9.25) cm, and body mass index 26.58 (2.92) kg/m2. All participants were White with 90% classified as Fitzpatrick skin type III (darker white [tans after initial burn]) and 10% as Fitzpatrick skin type IV (light brown [burns minimally, tans easily]).
3.2 Efficacy outcomes
Mometasone significantly (p=0.0031) reduced the incidence of induration/swelling at 0.5 hours post-elamipretide (Table 2). There were trends to significance in reduction of induration/swelling at 1 hour post-dose (p=0.0736) and pruritus at 0.5 hours post-dose (p=0.0573) with mometasone (Tables 2 and 3). Diphenhydramine significantly (p=0.0198) decreased the incidence of induration/swelling at 1 hour post-elamipretide dose, trending to significance (p=0.0698) in reduction of induration/swelling at 0.5 hours post-dose (Table 2). For subject-reported assessment of ISR signs/symptoms, ice application significantly (p=0.0325) reduced the incidence of pain at 0.5 hours post-elamipretide dose (Table 4) and trended to significance (p=0.0573) for reduction of itching at 0.5 hours post-elamipretide dosing (Table 5). Mometasone trended to significant reductions in swelling incidence at 1 hour post-elamipretide dose (p=0.3698), bothersome itching at 1 hour post-dose (p=0.1409), and increased redness at 12 hours post-dose (p=0.0867): the latter attributed to an occlusive dressing reaction. Tacrolimus and doxepin demonstrated no significant differences in ISR signs/symptoms compared to elamipretide administered alone in all clinical and self-assessments. Injection site photographs aligned with the signs/symptoms of ISRs commonly described following SC administration of elamipretide. Photographs supported the clinical assessments of ISRs conducted at the same timepoints. Overall, photograph-captured ISRs were resolving at 4 hours post-elamipretide dose (exception of bruising which appeared to form after the 12-hour post-dose timepoint in those affected). In the mometasone arm, photographs aided in deciphering erythema grading in subjects where the pattern of redness appeared to be related to the use of the occlusive dressing and not the injection.
Table 2: Incidence of induration/swelling as reported through the clinical assessments at early timepointsa (N = 10)
aUp to 4 hours post dose.
Table 3: Incidence of pruritus as reported through the clinical assessments at early timepointsa (N = 10)
aUp to 4 hours post dose.
Table 4: Incidence of pain as reported through the subject-reported assessments at early timepointsa (N = 10).
aUp to 4 hours post dose.
Table 5: Incidence of itching as reported through the subject-reported assessments at early timepointsa (N = 10)
aUp to 4 hours post dose.
3.3 Pharmacokinetics
In all study arms where elamipretide was administered with a topical or systemic drug, elamipretide AUC0-6h was not significantly different compared to elamipretide alone. Ice application arm statistically significantly impacted elamipretide Cmax and AUC0-6h compared to elamipretide alone. Mean ± SD Cmax of elamipretide was reduced by ~23% (1693.0±369.23 vs 2213.0±634.54ng/mL, p=0.0003) and the AUC0-6h of elamipretide was reduced by ~12% (4747.8±818.99 vs 5401.3±1096.06ng/mL•h, p<0.0001). Ice application arm showed a statistically significant reduction of Cmax (407.9±81.72 vs 454.1±70.24ng/mL, p=0.0028) and AUC0-6h (1849.1±352.12 vs 2079.1±278.81ng/mL•h, p<0.0001) for the M1 metabolite and AUC0-6h (210.7±32.65 vs 239.4±27.09ng/mL•h, p=0.0135) for M2 metabolite.
3.4 Safety
Apart from data relating to ISRs, which were not captured as AEs (designated study endpoints), few TEAEs were reported on daily SC elamipretide 60mg. The only TEAE observed in more than one subject was mild somnolence in the diphenhydramine treatment arm (five subjects [50%]). It is reasonable to suspect that night-time diphenhydramine could reduce the occurrence of somnolence, but was not a part of the study protocol; therefore, a conclusion cannot be drawn. There were no apparent differences between treatment arms in vital signs, ECG parameters, or laboratory values.
3.5 Preclinical MRGPRX2, Mrgprb2, and Mast Cell Data
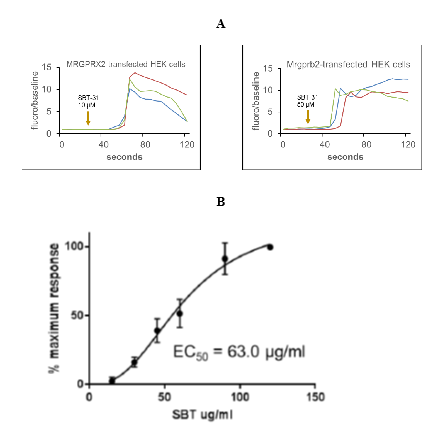
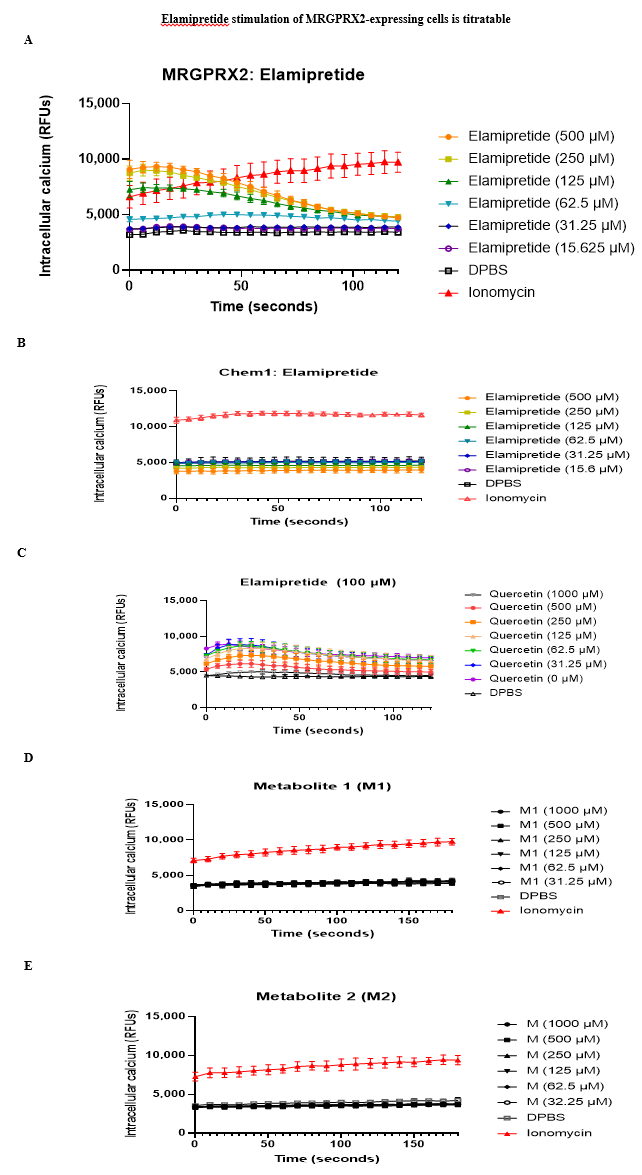
We examined whether ISRs may be caused by activation of mast cells through MRGPRX2. HEK293 cells stably transfected with MRGPRX2 showed massive calcium mobilization - a key component of MRGPRX2-mediated intracellular signaling - after incubation with elamipretide, as assessed by an increase in fluorescence intensity of the calcium sensitive dye Fluo-4 (Figure 1A). Untransfected cells did not demonstrate calcium mobilization. This was also observed in Chem-1 cells transfected with MRGPRX2 (Figure 2A), but not untransfected cells (Figure 2B), confirming that the response was due to MRGPRX2 and that elamipretide is an MRGPRX2 agonist. Quercetin, proposed to antagonize MRGPRX2 signaling [30], inhibited elamipretide activation of MRGPRX2 (Figure 2C). Neither M1 (Figure 2D) or M2 metabolites (Figure 2E) elicited calcium mobilization in MRGPRX2 cells, suggesting that only the parent compound is responsible for mast cell activation.
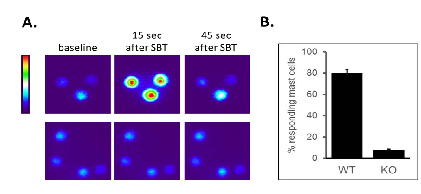
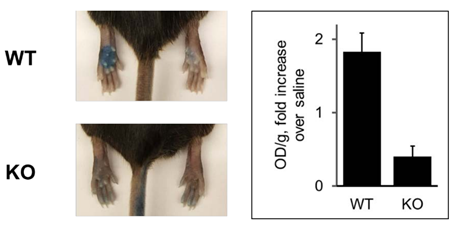
Using the HEK293 cell line showed that the half maximal effective concentration (EC50) was 63 ± 13µg/mL (Figure 1B), well under the 80mg/mL injection concentration used for human administration, suggesting that this elamipretide concentration readily activates skin mast cells. Elamipretide also activated Mrgprb2, the mouse ortholog of MRGPRX2 (Figure 3), and triggered calcium mobilization in wild type but not Mrgprb2 knockout primary mouse peritoneal mast cells, demonstrating that mast cell activation was Mrgprb2-specific (Figure 3B). Evans Blue assay was used to examine skin inflammation after in vivo administration of elamipretide because it binds to albumin in blood. Intravenous Evans Blue labels albumin blue, and when albumin-containing fluid escapes from the bloodstream and into tissues after mast cell degranulation, tissue becomes dye-filled. SC administration of elamipretide into wild-type mouse hind paws triggered immediate tissue swelling and fluid extravasation, reminiscent of ISRs, and was reduced by ~80% in Mrgprb2 knockout mice, demonstrating a reaction due to Mrgprb2 activation of mast cells (Figure 4).
4. Discussion
The aim of this preliminary phase 1 study was to evaluate the potential efficacy of interventions that might be used to mitigate ISRs following the SC administration of 60mg elamipretide in healthy subjects, and any potential impact on PK and safety. We focused on treatments that target mast cells or factors upstream or downstream to their activation, due to our preclinical data that strongly suggest that elamipretide-associated ISRs are caused by direct activation of mast cells through MRGPRX2. Overall, mometasone appeared to mitigate pseudo-allergic reactions observed following SC elamipretide, reducing pruritus and induration/swelling with no effect on PK. Ice application ameliorated early injection site pain and itching, but reduced the absorption of elamipretide. Diphenhydramine demonstrated potential in reducing induration/swelling, but caused sedation in some cases. Tacrolimus and doxepin demonstrated little impact on reported ISRs compared to elamipretide alone; therefore, they are not recommended for ISR mitigation. There were no significant changes in elamipretide or M1/M2 metabolite plasma exposures with any of the treatments except for ice application, which reduced exposure to elamipretide and its metabolites.
Although ice application and diphenhydramine showed some reduction in ISR signs and symptoms, mometasone revealed the most promise. Reductions in pruritus and induration/swelling demonstrated by mometasone enabled patients to be more comfortable with elamipretide treatment. In addition, mometasone may further improve elamipretide tolerability by reducing scratching and subsequent scratching-related skin damage. In some subjects, the hydrocolloid occlusive dressing applied over the mometasone ointment resulted in redness of the covered area (injection site area), confounding the ability to identify erythema due to ISRs. ISR photographs proved useful in deciphering erythema grading in subjects where the pattern of redness appeared to be related to the use of the occlusive dressing, although the ISR erythema may have been overreported in the mometasone treatment arm.
Despite ice application and diphenhydramine reducing some ISR signs/symptoms, both presented undesirable effects. Ice application reduced early injection site pain and itching, but reduced the Cmax and AUC0-6h of elamipretide and its metabolites (M1 and M2), possibly due to vasoconstriction. Neither M1 nor M2 are biologically active and the potential reduction in plasma concentrations of these metabolites is not therefore anticipated to have a clinical impact [data on file, Stealth BioTherapeutics Inc.]. Although the potential ramification on efficacy of the disruption in absorption of the active parent drug is unclear, lowering plasma exposure of elamipretide is not desirable, making ice application less appropriate. Similarly, diphenhydramine showed some potential in reducing induration/swelling, but a significant incidence (50%) of mild somnolence was reported in this treatment arm. While second generation antihistamines were not included in this study, the observations with diphenhydramine suggest that other antihistamines that are less sedating could provide utility in mitigating ISRs.
Based on mometasone and antihistamine outcomes, further investigation is warranted to include separate/combined interventions with oral second-generation antihistamines (such as fexofenadine) and topical mometasone without an occlusive dressing. Fexofenadine, a selective peripheral H1 receptor antagonist, does not readily cross the blood-brain barrier, thereby causing less drowsiness in comparison to first-generation antihistamines, such as diphenhydramine. Dermatology and allergy organizations issued a common guideline on chronic urticaria management, recommending the regular use of second-generation antihistamines as first-line treatment. Fexofenadine appears safe and well tolerated; daily doses can be titrated upwards in case of no improvement [31]. Chronic urticaria could be considered the closest model to chronic ISRs in terms of available data on therapeutic management. Because elamipretide is injected daily at alternating sites, applications with mometasone twice daily (one prior and one post-injection) appear to be a more practical alternative to the use of mometasone with occlusive dressing.
Our focus on mast cells arose from preclinical experiments which established that elamipretide, in addition to its intended function, also acts as an agonist of the human G protein-coupled receptor MRGPRX2. MRGPRX2 is primarily expressed by mast cells, which are constitutive residents of the skin and other tissues, trigger rapid tissue inflammation, and mediate many of the symptoms of allergic diseases. We reasoned that MRGPRX2 might be involved because it can be activated by many cationic peptides and small molecules with properties similar to elamipretide and which also cause ISRs and other pseudo-allergic reactions [27]. We have several pieces of evidence to support the hypothesis that elamipretide-associated ISRs are due to MRGPRX2 activation. First, elamipretide triggers intracellular signaling pathways, as measured by calcium mobilization, in cell lines forced to express MRGPRX2 or its mouse ortholog Mrgprb2, but not in unmodified cell lines that do not natively express the receptors (Figures 2 A, B). Notably, this could be blocked by a molecule reported to inhibit MRGPRX2 signaling (Figure 2C). MRGRPX2 activation by elamipretide is physiologically relevant, as the calculated EC50 is over 1,000-fold lower than the injection concentration in our clinical trial. Second, elamipretide causes intracellular signaling, again as measured by calcium flux, in primary mouse mast cells from wild type but not Mrgprb2 knockout mice (Figure 3). Third, SC elamipretide injection into mouse hind paws triggers rapid swelling via fluid extravasation from the bloodstream, similar to human ISRs, in wild type mice, while extravasation was nearly absent in mice lacking Mrgprb2 (Figure 4). These effects almost certainly are due to the parent drug, as elamipretide’s metabolites have no effect on MRGPRX2 signaling (Figures 2 D, E).
While itch produced in humans by injection of MRGPRX2 agonists can be blocked by antihistamines [32], development of inhibitors is still in its infancy with no candidates in clinical trial yet [27]. Indeed, a combination of H1 and H2 histamine receptor antagonists have demonstrated efficacy in blocking MRGPRX2-driven systemic and local reactions [33] and could be considered in future elamipretide-driven ISR studies. Although the topical steroid mometasone reduced pruritus and induration/swelling following SC elamipretide in this study, its anti-inflammatory mechanism is unclear but is thought to act by inhibition of the arachidonic acid pathway [34,35]. In addition to anti-inflammatory effects, topical steroids, such as mometasone, possess anti-mitotic, immunosuppressive, and vasoconstrictive effects [36], which may have played a role in mitigating ISR signs in this study.
Overall, the data collected in this study support prior findings that SC elamipretide is generally safe and well-tolerated [23, 24, 37]. With the exception of data relating to ISRs, very few TEAEs were identified in this study. The only TEAE seen in more than one subject was that of somnolence in the diphenhydramine treatment arm. Given that somnolence is a well-known side effect of diphenhydramine [38], this adverse event was considered likely related to diphenhydramine and not to elamipretide treatment. A limitation of the study was the relatively low number of subjects evaluated (N=10), which impacted the potential to demonstrate statistically significant results with respect to efficacy of mitigation. This study was not powered to show a statistically significant difference in the ISR profile between treatment arms but was meant to identify signals that could warrant further investigation in other clinical settings.
5. Conclusion
Application of topical mometasone prior to SC elamipretide administration demonstrated a significant reduction in ISR signs induced by elamipretide and did not cause significant changes in elamipretide plasma exposure or additional adverse events. Further investigation of mometasone is warranted for mitigation of elamipretide-induced ISRs and improving compliance. Treatment options that target mast cell activation may ameliorate elamipretide ISRs and should also be explored in future studies.
Acknowledgements
Writing and editing assistance, including preparation of a draft manuscript under the direction and guidance of the authors, incorporating author feedback, and manuscript submission, was provided by Jamie L. Dermatis, DPM and James A. Shiffer, RPh (Write on Time Medical Communications, LLC, NJ). The trial was funded by Stealth BioTherapeutics (Newton, MA).
Conflicts of interest
SCB, AS, LEK, and AA are employees of Stealth BioTherapeutics (Newton, MA). LZ, LAB, and BM have no conflicts to disclose.
References
- Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxidants & redox signaling 10 (2008): 601-619.
- Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. Journal of the American Society of Nephrology: JASN 24 (2013): 1250-1261.
- Grazioli S & Pugin J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Frontiers in immunology 9 (2018): 832.
- Mitchell W, Ng EA, Tamucci JD, Boyd KJ, Sathappa M, Coscia A, et al. The mitochondria-targeted peptide SS-31 binds lipid bilayers and modulates surface electrostatics as a key component of its mechanism of action. The Journal of biological chemistry 295 (2020): 7452-7469.
- Zhao K, Luo G, Giannelli S, & Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochemical pharmacology 70 (2005): 1796-1806.
- Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, et al. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. Journal of Alzheimer's disease : JAD 20 (2010): S609-S631.
- Szeto HH, & Schiller PW. Novel therapies targeting inner mitochondrial membrane--from discovery to clinical development. Pharmaceutical research 28 (2011): 2669-2679.
- Dai DF, Hsieh EJ, Chen T, Menendez LG, Basisty NB, Tsai L, et al. Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted peptides. Circulation. Heart failure 6 (2013): 1067-1076.
- Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, et al. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging cell 12 (2013): 763-771.
- Birk AV, Chao WM, Bracken C, Warren JD, & Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. British journal of pharmacology 171 (2014): 2017-2028.
- Brown DA, Hale SL, Baines CP, del Rio CL, Hamlin RL, Yueyama Y, et al. Reduction of early reperfusion injury with the mitochondria-targeting peptide bendavia. Journal of cardiovascular pharmacology and therapeutics 19 (2014): 121-132.
- Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, et al. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovascular research 103 (2014): 461-472.
- Nickel A, Kohlhaas M, & Maack C. Mitochondrial reactive oxygen species production and elimination. Journal of molecular and cellular cardiology 73 (2014): 26-33.
- Szeto HH, & Birk AV. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clinical pharmacology and therapeutics 96 (2014): 672-683.
- Alam NM, Mills WC, 4th Wong AA, Douglas RM, Szeto HH, & Prusky GT. A mitochondrial therapeutic reverses visual decline in mouse models of diabetes. Disease models & mechanisms 8 (2015): 701-710.
- Roshanravan B, Liu SZ, Ali AS, Shankland EG, Goss C, Amory JK, et al. In vivo mitochondrial ATP production is improved in older adult skeletal muscle after a single dose of elamipretide in a randomized trial. PloS one 16 (2021): e0253849.
- Stauffer B, Sparagna G, Chau S, RodegheriBrito J, Ambardekar A, Korst A, et al. MTP131, a cardiolipin targeting peptide, improves mitochondrial activity in the failing human heart. European Journal of Heart Failure 18 (2016): 289.
- Daubert MA, Yow E, Dunn G, Marchev S, Barnhart H, Douglas PS, et al. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circulation. Heart failure 10 (2017): e004389.
- Saad A, Herrmann SMS, Eirin A, Ferguson CM, Glockner JF, Bjarnason H. Phase 2a Clinical Trial of Mitochondrial Protection (Elamipretide) During Stent Revascularization in Patients with Atherosclerotic Renal Artery Stenosis. Circulation. Cardiovascular interventions 10 (2017): e005487.
- Karaa A, Haas R, Goldstein A, Vockley J, Weaver WD, & Cohen BH. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology 90 (2018): e1212-e1221.
- Sabbah HN, Gupta RC, Singh-Gupta V, & Zhang K. Effects of elamipretide on skeletal muscle in dogs with experimentally induced heart failure. ESC heart failure 6 (2019): 328-335.
- Allen ME, Pennington ER, Perry JB, Dadoo S, Makrecka-Kuka M, Dambrova M, et al. The cardiolipin-binding peptide elamipretide mitigates fragmentation of cristae networks following cardiac ischemia reperfusion in rats. Communications biology 3 (2020): 389.
- Thompson WR, Hornby B, Manuel R, Bradley E, Laux J, Carr J, et al. A phase 2/3 randomized clinical trial followed by an open-label extension to evaluate the effectiveness of elamipretide in Barth syndrome, a genetic disorder of mitochondrial cardiolipin metabolism. Genetics in medicine: official journal of the American College of Medical Genetics 23 (2021): 471-478.
- Karaa A, Haas R, Goldstein A, Vockley J, et al. A randomized crossover trial of elamipretide in adults with primary mitochondrial myopathy. Journal of cachexia, sarcopenia and muscle 11 (2020): 909-918.
- Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochemical and biophysical research communications 349 (2006): 1322-1328.
- Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 123 (2014): e58-e67.
- McNeil BD. Minireview: Mas-related G protein-coupled receptor X2 activation by therapeutic drugs. Neuroscience letters 751 (2021): 135746.
- Grimes J, Desai S, Charter NW, Lodge J, Moita Santos R, Isidro-Llobet A, et al. MrgX2 is a promiscuous receptor for basic peptides causing mast cell pseudo-allergic and anaphylactoid reactions. Pharmacology research & perspectives 7 (2019): e00547.
- McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519 (2015): 237-241.
- Ding Y, Che D, Li C, Cao J, Wang J, Ma P, et al. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCγ-IP3R related Ca2+fluctuations. International immunopharmacology 66 (2019): 185-197.
- Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. Endorsed by the following societies: AAAAI, AAD, AAIITO, ACAAI, AEDV, APAAACI, ASBAI, ASCIA, BAD, BSACI, CDA, CMICA, CSACI, DDG, DDS, DGAKI, DSA, DST, EAACI, EIAS, EDF, EMBRN, ESCD, GA²LEN, IAACI, IADVL, JDA, NVvA, MSAI, ÖGDV, PSA, RAACI, SBD, SFD, SGAI, SGDV, SIAAIC, SIDeMaST, SPDV, TSD, UNBB, UNEV and WAO. The EAACI/GA²LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 73 (2018): 1393-1414.
- Hasbak P, Eskesen K, Lind H, Holst J, & Edvinsson L. The vasorelaxant effect of adrenomedullin, proadrenomedullin N-terminal 20 peptide and amylin in human skin. Basic & clinical pharmacology & toxicology 99 (2006): 162-167.
- McNeil BD. MRGPRX2 and Adverse Drug Reactions. Frontiers in immunology 12 (2021): 676354.
- Spada F, Barnes TM, & Greive KA. Comparative safety and efficacy of topical mometasone furoate with other topical corticosteroids. The Australasian journal of dermatology 59 (2018): e168-e174.
- ELOCON® (mometasone furoate) Cream, 0.1% for topical use. Prescribing Information. Merck & Co., Inc., May (2018).
- Gabros S, Nessel TA & Zito PM. Topical Corticosteroids. In StatPearls. Treasure Island, Florida: StatPearls Publishing (2023).
- Mettu PS, Allingham MJ, & Cousins SW. Phase 1 Clinical Trial of Elamipretide in Dry Age-Related Macular Degeneration and Noncentral Geographic Atrophy: ReCLAIM NCGA Study. Ophthalmology science 2 (2021): 100086.
- Sicari V & Zabbo CP. Diphenhydramine. In StatPearls. Treasure Island, Florida (2022).


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
