Gut Microbiota Signature in Atopics Dermatitis: Experience gained at the “L. Sacco” University Hospital, in Milan, Italy
Rimoldi SG*, 1, Caron L2, Alvaro A2, Comandatore F3, Curreli D1, Salari F1, Pagani C1, Lombardi A1, Micheli V1, Tamoni A1, Iemoli E3, Gismondo MG1
1Laboratory of Clinical Microbiology, Virology and Bioemergency, ASST Fatebenefratelli Sacco, L. Sacco” University Hospital, Italy
2Allergy and Clinical Immunology Unit ASST Fatebenefratelli Sacco, “L. Sacco” University Hospital Milan, Italy
3Romeo ed Enrica Invernizzi Pediatric Research Center, Department of Biomedical and Clinical Sciences L. Sacco Hospital, University of Milan, Italy
*Corresponding author: Rimoldi SG, Laboratory of Clinical Microbiology, Virology and Bioemergency, ASST Fatebenefratelli Sacco, “L. Sacco” University Hospital, Milan, Italy.
Received: 04 July 2023; Accepted: 11 July 2023; Published: 24 July 2023
Article Information
Citation: Rimoldi SG, Caron L, Alvaro A, Comandatore F, Curreli D, Salari F, Pagani C, Lombardi A, Micheli V, Tamoni A, Iemoli E, Gismondo MG. Gut Microbiota Signature in Atopics Dermatitis: Experience gained at the “L. Sacco” University Hospital, in Milan, Italy. Archives of Microbiology and Immunology. 7 (2023): 111- 120.
View / Download Pdf Share at FacebookAbstract
Literature have suggested that gut microbiota is a key factor in the development and have a role in the clinical progress of atopic dermatitis (AD) affected patients. We aimed in the current study to better investigate the gut microbiota in AD before and after probiotics subministration. Faecal samples collected from 63 subjects (48 AD patients that completed probiotic assumption and 15 (HC)), were microbiologically investigated with a 16S rRNA amplicon metagenomics analysis. The microbiota results indicated that microbial diversity is lower in AD patients than healthy controls, according with Chao Index. The relative abundance showed the presence at the class level, of Bacilli, less abundant in atopic dermatitis with gastroenteritis (AD_GE) in comparison to the other categories (pvalue 0.052), while Deltaproteobacteria and Erysipelotrichi were more abundant in AD_worsened than in the other groups. At family level, Porphyromonadaceae were more abundant in AD_GE than AD or AD_worsened (pvalue 0.0044), Ruminococcaceae were less abundant in AD_GE than the other categories and Gracilibacteraceae were significantly less abundant in AD than Control patients and more abundant in AD_worsened than AD. In future a larger cohort is needed to support our data.
Keywords
<p>atopic dermatitis, species composition, probiotics</p>
Article Details
1. Introduction
Atopic dermatitis (AD) is an inflammatory skin disease characterized by recurrence, dry skin, erythema and itchiness. The incidence of AD gradually increases with the development of industrialization and urbanization, affecting 15-30% of children and 10% of adults all over the world [1, 2]. The causes of AD are complex and include genetic and environmental factors. Individuals with AD are commonly stimulated by allergens including pollen, dust mite and animal dander around them [3, 4]. Skin flora, especially, Staphylococcus aureus and Malassezia infect the lesions resulting in more severe AD clinical symptoms [5, 6]. The gut microbiota is a potential target for regulating immune responses in the host and a correlation between gut microbiota and atopic dermatitis is revealed in many studies [7].
The gut microbial community plays an essential role in the maturation of the human immune system, beginning at the first month of life and affecting individual immune responses. The acquisition of the gut microbiota begins at birth, after which it diversifies around six months. Several related studies have shown that microorganisms in the intestine are related to chronic diseases ranging from gastrointestinal inflammatory and metabolic conditions to neurological, cardiovascular, and respiratory illnesses [8]. The onset and the development of AD are closely associated the gut microbial alterations. Probiotics consumption may be an effective alternative to alleviate AD clinical symptoms (A) but with controversial beneficial role in AD outcomes.
2. Materials and Methods
2.1 Patients and study design
56 patients (24 male, 32 female) aged from 19 to 62 years (mean age 37 ys) with mild-moderate AD of different severity were recruited during routine visits to the Allergy and Clinical Immunology Unit of the L. Sacco University Hospital of Milan. The study was performed from January to June 2021. Clinical history was collected and evaluation of dermatitis with questionnaires was conducted. Fifteen (HC) not affected from AD were also enrolled (control group). All the patients unrolled were COVID-2019 free. Due to different conditions, regarding compliance’s patient and COVID, the faecal sample was collected in 48 out of 56 patients. Every patient received probiotics and a violet extract for 30 days. Patients were allowed to use the needed therapy for their dermatitis (emollients, antihistamines, topical steroids, others if needed); they weren’t asked to change diet habits; they were asked to avoid commercial probiotics. This study evaluates the gut microbiota’s patients at different times: enrolment (T0), at the probiotic administration (T1), on the thirtieth day from the probiotic administration (T2), on the thirtieth day from the wash out (T3). Differences in dermatitis extension and symptoms after the use of probiotics were investigated.
2.2 Ingredients characteristics
Every patient received probiotics and a violet extract for 30 days, dosed in 2 different capsules to be taken once a day.
The probiotics were administered once a day in capsule containing at least 1 billion of each of the following Lactobacilli, Lactiplantibacillus plantarum DSM 24937 (LP), Limosilactobacillus reuteri DSM 25175 (LR), Lacticaseibacillus rhamnosus DSM 25568 (LRH) (ROELMI HPC srl, Origgio, Italy) and 2 billion of Bacillus clausii SIN spores (BC) (Sanofi, Origgio, Italy), sprayed on a microcrystalline cellulose solid matrix with fluid bed dryer technology.
The strains were selected based on in vitro studies that demonstrated the ability to produce antimicrobial and anti-inflammatory compounds, showing inhibitory activity against skin pathogens and positive modulation of pro-inflammatory cytokines. The combination of Lactobacilli and spores allowed to reach a product with high stability, without need of further excipients in the final blend, necessary for technological reason or to compensate the strains assay to reach the targeted daily dose. Stability studies have been launched at 25°C, 60% relative humidity up to 9 months and results met the product specifications and the total count for the different strains. The violet extract (Viola Tricolor L., provided by BICT srl) was selected based on literature research [9], for its claim linked to the body purifying functions and therefore to the well-being of the skin. An extract with a drug-extract ratio 5:1 was selected and studied for its cytotoxicity (MTT test) and its anti-inflammatory properties in vitro (as modulation of TNF-α and IL-8). The stability of the extract was also tested at 25°C for 1 year.
2.3 Ethic statement
The study protocol was approved by the Sacco Hospital Ethics Committee (Area 1-2019/ST/049) and all patients gave informed consent when assessed for eligibility. All procedures were conducted according to the guidelines stipulated and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki, as revised in 2013. The patients and healthy volunteers provided their written informed consent to participate in this study.
2.4 Symptom scores and questionnaire
Sign and symptoms of AD were investigated using the Eczema Area and Severity Index (EASI) and the SCORing Atopic Dermatitis score (SCORAD) (10,11). Dermatology Life Quality Index (DLQI) was self-administered to patients to evaluate impact of AD on different aspects of life (12). EASI grades extension and severity of dermatitis in 4 body areas (head and neck, trunk, arms, lower limbs); 0 point mean patient clear, 0,1-5,9 mild dermatitis, 6,0–22,9 moderate dermatitis, 23,0–72 severe dermatitis [13]. SCORAD score evaluates extension and intensity of dermatitis (0-83 points) and subjective symptoms like itching and sleep loss (each rated from 0 to 10); from 0 to 27 total points dermatitis was considered mild, from 28 to 56 moderate and >57 severe [14]. Objective SCORAD was obtained excluding itching and loss of sleep evaluation from final score. DLQI is a ten-question questionnaire that measures the impact of AD on the quality of life, investigating subjective symptoms, embarrassment and impact on social, working, sexual life; we considered mild impairment in quality of life from 0 to 5 points, moderate impairment from 6 to 10, severe impairment from 11 to 30 [15]. We considered the Minimal Clinically Important Difference (MCID) of 4 points to look for differences before and after probiotic assumption. Scores were performed at T0-T1 and T2.
2.5 Faecal sample collection and storage
127 faecal samples were collected in all the patients unrolled at T0, T1 and if possible at T2; samples were stored at 4° C temperature ad transferred to the microbiological laboratory. Due to the COVID-19 disease in was difficult to guarantee all the follow up required by the study.
2.6 Samples and DNA isolation
Total genomic DNA was extracted using Stool DNA isolation kit (Norgen, Thorold CA) according to manufacturer’s instructions with minor modifications. To purify, the samples were introduced into a silice filter tube and washed the eluate obtained was about 35 μl DNA concentration was quantified using Qubit dsDNA HS Assay kit
2.7 NGS analyses
2.7.1 Amplicon library preparation
For amplicon library preparation the Ion 16S™ Metagenomics Kit (Thermo Fisher, USA). was performed; V2-4-8 and V3-6, V7-9 hypervariable regions of the 16S rRNA gene were amplified from total bacterial DNA. The prepared templates were sequenced on the Ion Torrent PGM instrument (LifeTechnologies) in the Microbiome Core Facility using the Ion PGM 400 sequencing reagents. All kits were used according to the manufacturer’s instructions. Initial data analysis, base pair calling and trimming of each sequence was performed on Ion Torrent browser to yield high quality reads.
2.7.2 Bioinformatic and statistical analyses
The bioinformatic analyses of sequencing data to determine and taxonomically annotate the Operational Taxonomic Units (OTU) were conducted using the Thermo Fisher Ion Reporter Software. The obtained results were then used to study alpha and beta diversity. For each taxonomic level (phylum, class, order, family, genus, and species) the bacterial frequencies between Control, T0, T1, T2 and T3 groups were compared using the non-parametric Anova Kruskal−Wallis and pairwise Wilcoxon-Mann-Whitney tests. The same analyses were used to compare Controls, AD patients who developed gastroenteritis, AD patients that have worsened during the treatment, and all the other remaining AD patients, regardless of time. SCORAD and EASI scores were compared between T1 and T2 utilizing the Wilcoxon-Mann-Whitney test with continuity correlation, and standard deviation was calculated for each score at every time point. All the statistical analyses were run in the R environment.
2.7.3 Objectives
Aim of this study was to evaluate the gut microbiota underling the onset and chronicity of atopic dermatitis before and after administration of a probiotic. The gut composition was investigated by NGS sequencing on samples collected before and after the use.
3. Results
3.1 Clinical data and adherence to the study
In this 6-month study (January - June 2021) 56 patients unrolled at Allergy and Clinical Immunology Unit of the L. Sacco University Hospital of Milan started probiotic treatment, dosed in 2 different capsules to be taken once a day, containing at least 1 billion of each of the following Lactobacilli and Bacillus clausii, and violet extract. 48 (27 female, 21 male) out of 56 patients (87,5%) completed 30 days of assumption. Five patient out of 56 (7,1%) were lost at follow up, 2 patients (3,6%) stopped assumption and one patient chose to leave the study. Forty-four had mild AD (SCORAD <25), 4 moderate AD (scorad from 28 to 56). Nonsevere AD were identified at the time of the enlistment. Mean age was 37 years. Allergic asthma/rhinitis or food allergy were the most frequent comorbidities in our population, found in 75% of patients.
Table 1: Probiotic and botanical capsule composition
|
billion/capsule |
mg/capsule |
|
|
LP |
1 |
10 |
|
LR |
1 |
25 |
|
LRh |
1 |
15 |
|
BC |
2 |
200 |
|
Total |
5 |
250 |
|
|
mg/capsule |
|
Violet extract |
200 |
|
Maltodextrin |
50 |
|
Total |
250 |
Demographic characteristics of AD patients are reported in table 2. The different score used were reported as followed: before treatment mean Scorad score was 17,4 (mean objective scorad 11,1), mean EASI was 2,7. At T2 after 30 days of probiotic assumption mean scorad score was 11,4 (objective scorad 7,8) and mean EASI was 1,9 (see table 3). A statistically significant difference was observed in SCORAD and EASI scores between T1 and T2 (paired Wilcoxon-Mann-Whitney test, pvalue 1.233e-06 and 1.79e-06, respectively, Table 3). Considering DLQI and Minimal Clinically Important Difference (MCID) of 4 points, 23 patients (50%) patient had improvement of DLQI score, 20 patients (40%) had no change in DLQI score and 5 patients (10%) had worsening of DLQI score after probiotic assumption (table 4). The food supplement was well tolerated by most of the patients examined. Clinical condition of the 10 patients who developed side effects was better investigated.
Table 2: Median score SCORAD test before and after treatment
|
T1 |
T2 |
|
|
Mean SCORAD |
17,4 |
11,4 |
|
Mean objective SCORAD |
11,1 |
7,8 |
|
Mean EASI |
2,7 |
1,9 |
Table 3: Test Wilcon signed rank with continuity correlation used to compare SCORAD and EASI scoring atopic dermatitis before and after treatment in the table Median value, Standard deviation and (p-value)
|
Mean + Standard deviation – T1 |
Mean + Standard deviation – T2 |
Wilcoxon signed rank test p value |
|
|
SCORAD |
17,4 +- 10.53 |
11,4 +-10.8 |
1.23E-06 |
|
EASI |
2,7 +-3.9 |
1,9 +-3.12 |
1.79E-06 |
Table 4: Representation of the quality of life based on score DLQI; in this evaluation patient with “stable” score included AD patients with gastrointestinal problems
|
N . patients |
|
|
Improved DLQI score |
23 (49%) |
|
Stable DLQI score |
20 (41%) |
|
Worsened DLQI score |
5 (10%) |
Two of the five worsened patients needed to add oral corticosteroids, ciclosporine or pimecrolimus during or soon after the probiotic treatment; one of them was the only Japanese patient in our group, the other one was subsequently diagnosed with severe AD and months later started biological treatment. Therefore, we can suppose that their dermatitis would have worsened anyway, regardless of the cycle with the treatment. All 5 patients had higher post treatment SCORAD score including subjective symptoms (pruritus and sleep loss), but only two had worsened in extension and intensity of AD using objective SCORAD or EASI. No significant differences existed in comorbidity profiles between these 5 patients and other partecipants. Five patients experienced gastrointestinal symptoms during the treatment reported a stable DLQI score. 3 out of 5 had mild adverse gastrointestinal events, not requiring interruption of treatment; al T2 their DLQI score was stable, so these patients didn’t report worsening of quality of life, but dermatitis was unchanged. On the contrary the other two patients reported exacerbation of pre-existing gastrointestinal problem (in one case gastrointestinal symptoms for years without a specific diagnosis, and in the other known hemorrhoidal symptoms) that led a difficult to conclude probiotic assumption. No significant differences existed in comorbidity profiles between these 5 patients and other partecipants. Two patients, one with benefits and one with worsening after the treatment, were later diagnosed with severe AD and started a biological therapy.
3.2 Alpha and beta diversity compared with clinical severity of AD
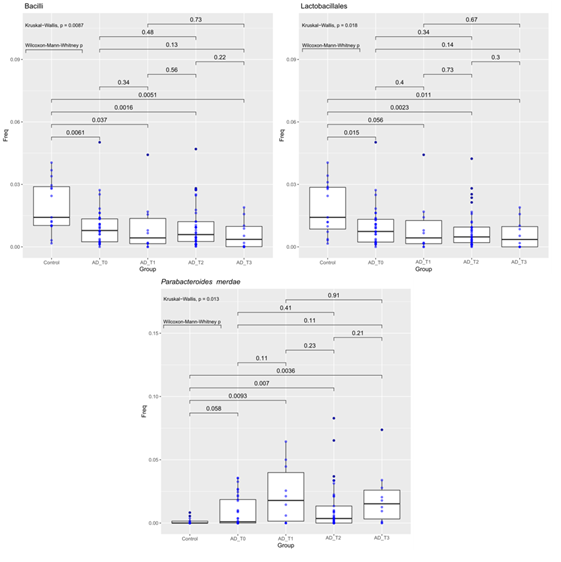
One Hundred twenty-seven fecal samples, collected from 63 subjects (48 AD and 15 HC), were analyzed using 16S rRNA sequencing, including 48 patients with AD and 15 healthy control subjects. The samples collection were investigated at different times as follow: at the probiotic administration (N=56), on the thirtieth day from the probiotic administration (N=48). Due to COVID-19 disease in many cases (N=48) the patient’s enrollment coincided with the food supplement administration; for the same reasons a low patient’s compliance on the thirtieth day from the wash out (N=10) was reported. For what concerns the alpha diversity, Chao1 index was significantly higher at family level in Control vs AD_T1 and AD_T2 vs AD_T1 (pairwise Wilcoxon-Mann-Whitney, pvalue 0.0071 and 0.012, respectively, Figure 1). The Shannon index (Wilcoxon-Mann-Whitney, pvalue 0.028) showed a higher diversity in control than AD_T0 at the species level.
Figure 1: Abundance of bacteria Bacilli (Class, Fig A), Lactobacillales (Order, Fig B) and Parabacteroides merdae (species, Fig C) compared between Controls and AD patients at different time points. The median is compared across groups using the Kruskal-Wallis test and Wilcoxon-Mann-Whitney test for pairwise comparisons. Boxes represent the interquartile range, lines indicate medians.
The Kruskall-Wallis and the Wilcoxon-Mann-Whitney tests have been used to compare the operational taxonomic unit frequencies between HC and AD at different time points (T=0, 1, 2, 3) (see Table 1). AD patients at all the four time points of the probiotic’ s administration (T0-T3), were less abundant in the class Bacilli and the order Lactobacillales than the HC. On the contrary, AD patients before and after probiotic treatment (at all four time points), were more abundant in Parabacteroides merdae species (Fig 2).
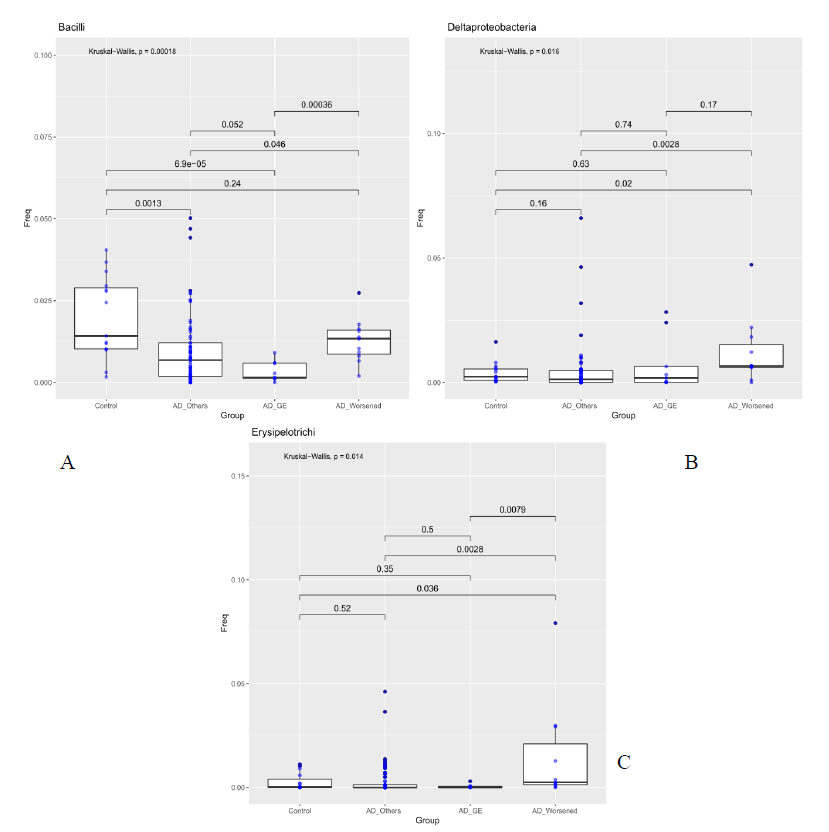
Figure 2: Abundance of bacteria Bacilli Bacilli (Class, Fig A), Deltaproteobacteria (Order, Fig B) and Erysipelotrichi (Order, Fig C) compared between Controls, patients who developed gastroenteritis (AD_GE), patients which worsened during treatment (AD_Worsened) and all the other AD patients (AD_Others). The median is compared across groups using the Kruskal-Wallis test and Wilcoxon-Mann-Whitney test for pairwise comparisons. Boxes represent the interquartile range, lines indicate medians.
3.3 Comparison of gut population in subgroups of AD
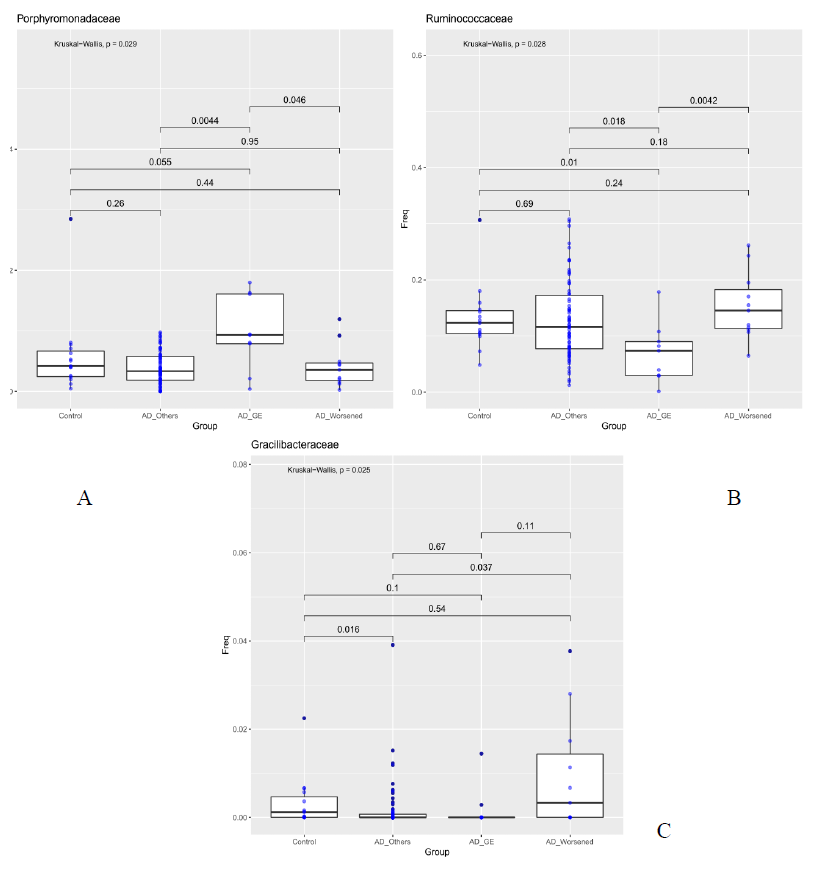
For each sample available, in the dataset at each taxonomic level, significance associated with taxa, was investigated considering the atopic dermatitis scores and the patient’s response to food supplement treatment. 5 out 10 patients had gastroenteritis (AD_GE), 5 out of 10 did not benefit (AD_W). The 48 patients were grouped in three categories: AD_GE (N=5), which includes those who developed gastroenteritis; AD worsened (N=5), which includes who got worse after the treatment; AD (N=38), which includes the remaining patients. The 15 Controls were also included in the analysis. At the class level, Bacilli were to be less abundant in AD_GE in comparison to the other categories (AD, AD_worsened and Control, pairwise Wilcoxon-Mann-Whitney, pvalue 0.052, 0.00036, 6.9e-05 respectively, Figure 3a), while Deltaproteobacteria and Erysipelotrichi were more abundant in AD_worsened than in the other groups (Figure 3 b and c). At family level, Porphyromonadaceae were more abundant in AD_GE than AD or AD_worsened (pairwise Wilcoxon-Mann-Whitney, pvalue 0.0044 and 0.046 respectively, Figure 3a), Ruminococcaceae were less abundant in AD_GE than the other categories (Figure 3b) and Gracilibacteraceae were significantly less abundant in AD than Control patients and more abundant in AD_worsened than AD (Figure 3c). All the data are summarized in table 5.
Table 5: Table 5.a Comparison of the taxonomic composition between HC and AD at different time points (T=0, 1, 2, 3)
|
AD-T0 |
AD-T1 |
AD-T2 |
AD-T3 |
|
|
AD |
||||
|
Family level |
Enterobacterales |
Enterobacterales |
none |
none |
|
Species level |
Parabacteroides merdae |
Parabacteroides merdae |
||
|
HC |
||||
|
Family level |
Bacilli |
|||
|
Species level |
Bacteroides dorei |
|||
Table 5.b Gut microbioma of the AD_GE patients compared to the other categories (AD_Others, AD_Worsened, and Control-HC)
abundancy
LA : less abundant
MA : more abundant
AD_W : AD Worsened
AD_GE : GASTROENTERITIS
4. Discussion
As afore mentioned, the gut microbiota plays an important role in disease development, which is characterized by cutaneous symptoms but also increasing intestinal permeability and inflammation, through induction of immunosuppression and immune tolerance [16, 17]. Purpose of our study was to characterize the differences in the composition of the gut microbiota between healthy control subjects and AD patients. Furthermore, following the administration of a food supplement based on probiotics and a botanical extract, microbiota has been investigated. Viola Tricolor L. is a traditional medical herb that demonstrated to reduce asthma symptoms in a mice model by inhibiting T-helper type 2 (Th2) cytokine production and to be effective in a trial involving chronic obstructive pulmonary disease (COPD) patients [18, 19]. It contains bioactive cyclotides which prevent the proliferation of activated lymphocytes through IL-2 [20]. Few studies to date have been conducted to clarify the role of probiotic administration in AD patients. Bifidobacterium animalis subsp lactis LKM512 alleviated itch in adult AD subjects [21]. A probiotic mix containing Bifidobacterium animalis subsp. lactis DGCC 420, Lactobacillus paracasei Lpc-37, and Lactobacillus acidophilus 74-2 was evaluated in the same category of patients, revealing a significant increase of Lactobacilli in stool samples, but not of Bifidobacteria or Bacteroidetes [22]. All the patients unrolled in the present study declare to have a balanced diet. Only one dietary difference can be found in the only patient of Japanese descent enrolled in the study. Recent evidence suggests that gut microbiota have been involved in AD [23] and it is a key factor in the development of AD. In addition, these results demonstrate the changes in microbiota composition in AD compared with a healthy control group, opening the way to future diagnosis or intervention studies [23].
Purpose of our study was to characterize the differences in the composition of the gut microbiota between healthy control subjects and patients with AD. Furthermore, following probiotic administration, microbiota has been investigated. To support the opinion reported in literature from different authors about AD [24], our data showed that patients unrolled had a lower alpha diversity, the bacterial diversity, than healthy control subjects. In particular, the total number of families inferred by the Chao 1 index resulted lower in the AD patients (AD-T1) than the controls and AD_T2. The effect of probiotic intake (Lactobacilli and Bacillus clausii) on the modulation of gut microbiota and the probiotic persistence were also evaluated. No statistical differences in gut microbioma was reported in our analysis after food supplement administration; these results are in contrast with previous internal data showing gut probiotic colonization after 10 days of probiotic intake. Our result could be justify by the small number of patients enrolled and their important initial gut dysbiosis. 38 out of 48 patients completed treatment without side effects. 10 out of 48 revealed adverse events during the treatment. A further evaluation was performed comparing microbiota of DA_GI and DA_worse with that of patients who positively ended the treatment. As reported in table 2, gut microbioma of the DA_GI patients was compared to the other categories (AD, DA_worse and HC, p value 0.052, 0.00036, 6.9e-05 respectively, Figure 3a), revealing a less abundance of Bacilli in their microbiome. Deltaproteobacteria and Erysipelotrichi were more abundant in AD_worsened than in the other groups (Figure 3 b and c). At family level, Porphyromonadaceae were more abundant in AD_GE than AD or AD_worsened (pairwise Wilcoxon-Mann-Whitney, pvalue 0.0044 and 0.046 respectively, Figure XXX2), Ruminococcaceae were less abundant in AD_GE than the other categories (Figure 4b) and Gracilibacteraceae were significantly less abundant in AD than Control patients and more abundant in AD_Worsened than AD (Figure 4c).
Five patients out of 48 had gastroenteritis. Three out of 5 had mild adverse gastrointestinal events, not requiring interruption of treatment and dermatitis was unchanged. On the contrary the other 2 patients reported exacerbation of pre-existing gastrointestinal problems that led to a difficult prosecution in food supplement assumption. However, in all cases it was not possible to correlate their symptoms with the probiotic administration. Two of the worsened patients needed to add oral corticosteroids, ciclosporine or pimecrolimus during or soon after the food supplement treatment; one of them was the only Asian ethnicity patient in the group, the other one was subsequently diagnosed with severe AD and few months later started a biological treatment. This study has several limitations. Mainly, the small cohort of patients. Despite the patients initially enrolled in the study were 56, only 48 concluded the study, mainly due to the COVID-19 pandemic situation that do not allowed a complete follow-up. A larger number of patients will be needed in future studies to confirm the significance of the results. Anyway, this paper is meaningful because it is the first Italian study looking at AD symptoms and gut microbiome in AD patients comparing them with healthy subjects before and after food supplement treatment using a deep sequencing analysis. In our opinion, the side effects arisen in some patients are not due to the use of the food supplement. Some authors, in fact, reported that different gut microbiome profiles of AD patients correlated to the presence of gastrointestinal symptoms [26]; moreover it is known that the symptoms worsening could have a subjective component. In 36 out of 48 patients in our study, an improvement was demonstrated even if a change in gut microbial diversity and composition was demonstrated with the disappearing after treatment of Enterobacteriales and Parabacteroides merdae bacteria [27]. Compared with healthy individuals gut microbial diversity decreased and the relative abundance of the beneficial microbes such as Lactobacillus reduced in AD patients as reported in our data. Melli et al demonstrated a high level of relative abundance of Bacteroides, Parabacteroides and Porphyromonadaceae in AD patients [19]. However further verification are required to clarify the role of these bacteria in AD [16].
Further investigation are needed in order to clarify the role of food supplement as possible adjuvant therapy for the treatment of AD in adult patients and for other immune-related disorders. The characterization of the gut microbiota by NGS is a powerful tool that will hopefully be implemented soon to be applied not only in research but also in clinical practice.
Funding
This study was performed in the frame of the Project “Studio e messa punto di nuovi prodotti pro- e prebiotici per la prevenzione ed il trattamento di patologie infiammatorie quali la sindrome del colon irritabile e la dermatite atopica (SCIDA)” ID Project 226149, financed by Lombardy Region POR FESR 2014-2020 Asse I Action I.1.b.1.3 as part of Support for collaborative R&D activities for development of new sustainable technologies, products and services.
References
- Hidaka T, Ogawa E, Kobayashi EH, Suzuki T, Funayama R, Nagashima T, et al. The Aryl Hydrocarbon Receptor Ahr Links Atopic Dermatitis and Air Pollution Via Induction of the Neurotrophic Factor Artemin. Nat Immunol 18 (2017): 64–73.
- Langan SM, Irvine AD, Weidinger S. Atopic Dermatitis. Lancet 396 (2020): 345–60.
- Jedrychowski W, Perera F, Maugeri U, Mrozek-Budzyn D, Miller RL, Flak E, et al. Effects of Prenatal and Perinatal Exposure to Fine Air Pollutants and Maternal Fish Consumption on the Occurrence of Infantile Eczema. Int Arch Allergy Immunol 155 (2011): 275–81.
- Goon A, Leow YH, Chan YH, Ng SK, Goh CL. Atopy Patch Testing with Aeroallergens in Patients with Atopic Dermatitis and Controls in Singapore. Clin Exp Dermatol 30 (2005):627–31.
- Reginald K, Westritschnig K, Linhart B, Focke-Tejkl M, Jahn-Schmid B, Eckl-Dorna J, et al. Staphylococcus Aureus Fibronectin-Binding Protein Specifically Binds Ige from Patients with Atopic Dermatitis and Requires Antigen Presentation for Cellular Immune Responses. J Allergy Clin Immunol 128 (2011): 82–91.
- Reginald K, Westritschnig K, Werfel T, Heratizadeh A, Novak N, Focke-Tejkl M, et al. Immunoglobulin E Antibody Reactivity to Bacterial Antigens in Atopic Dermatitis Patients. Clin Exp Allergy 41 (2011): 357–69.
- Stefanovic N, Flohr C, Irvine AD. The Exposome in Atopic Dermatitis. Allergy 75 (2020): 63–74.
- Durack J, Lynch SV. The Gut Microbiome: Relationships with Disease and Opportunities for Therapy. J. Exp. Med 216 (2019): 20–40.
- Abraham Fikru Mechesso, Seung-Jin Lee, Na-Hye Park, Jin-Yoon Kim, Zi-Eum Im, Joo-Won Suh & Seung-Chun Park; BMC Complementary and Alternative Medicine (23 February 2016). Preventive effects of a novel herbal mixture on atopic dermatitis-like skin lesions in BALB/C mice (2016).
- Hanifin JM, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 10 (2001): 11-8.
- Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 186 (1993): 23-31.
- Finlay AY and Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol 19 (1994): 210-216.
- Chopra R, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol 177 (2017): 1316-1321.
- Silverberg JI, et al. Severity strata for POEM, PO-SCORAD, and DLQI in US adults with atopic dermatitis. Ann Allergy Asthma Immunol 121 (2018): 464-468.
- 11^Basra MK, Salek MS, Camilleri I, Sturkey R, Finlay AY. "Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data".Dermatology230 (2015): 27-33.
- Majamaa H, Isoulari E. Evaluation of the gut mucosal barrier: evidence for increased antigen transfer in children with atopic eczema. J Allergy Clin Immunol 97 (1996): 985–990
- Rosenfeldt V, Benfeldt E, Valerius NH, et al. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J Pediatrics 145 (2004): 612–616
- Harati E, Bahrami M, Razavi A, et al. Effects of Viola tricolor Flower Hydroethanolic Extract on Lung Inflammation in a Mouse Model of Chronic Asthma. Iran J Allergy Asthma Immunol 17 (2018): 409-417
- Kirichenko TV, Sobenin IA, Markina YV, et al. Clinical Effectiveness of a Combination of Black Elder Berries, Violet Herb, and Calendula Flowers in Chronic Obstructive Pulmonary Disease: The Results of a Double-Blinded Placebo-Controlled Study. Biology (Basel) 9 (2020): 83.
- Hellinger R, Koehbach J, Fedchuk H, et al. Immunosuppressive activity of an aqueous Viola tricolor herbal extract. J Ethnopharmacol 151 (2014): 299-306.
- Matsumoto M, Ebata T, Hirooka J, Hosoya R, Inoue N, Itami S, Tsuji K, Yaginuma T, Muramatsu K, Nakamura A, Fujita A, Nagakura T. Antipruritic effects of the probiotic strain LKM512 in adults with atopic dermatitis. Ann Allergy Asthma Immunol 113 (2014): 209-216.
- Roessler A, Forssten SD, Glei M, Ouwehand AC, Jahreis G. The effect of probiotics on faecal microbiota and genotoxic activity of faecal water in patients with atopic dermatitis: a randomized, placebo-controlled study. Clin Nutr 31 (2012): 22-9.
- Siqi Ye, Fenggen Yan, Haiyan Wang, Xiumei Mo, Junfeng Liu,Yu Zhang, et al. Diversity analysis of gut microbiota between healthy controls and those with atopic dermatitis in a Chinese population. Journal of Dermatology 48 (2021): 158-167.
- Zhifeng F, Lingzhi L, Hao Z, Jianxin Z, Wenwei L, Wei C. Gut microbiota, probiotics and their interactions in prevention and treatment of atopic dermatitis: a revive. Frontiers in immunology (2021).
- Mezzasalma V, Manfrini E, Ferri E, Sandionigi A, La Ferla B, Schiano I, et al. A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviaaing Symptoms of Irritable Bowel Syndrome Associated with Constipation. Biomed Res Int (2016): 4740907.
- Chang-Yi Han, Soon-Kyeong Kwon, Mijung Yeom, Dae-Hyun Hahm, Jae-Woo Park, Hi-Joon Park, et al. Exploring the differences in the gut microbiome in AD according to the presence of gastrointestinal symptoms. J Clin Med 11 (2022): 3690.
- Melli LC, do Carmo-Rodrigues MS, Araújo-Filho HB, Solé D, de Morais MB. Intestinal microbiota and allergic diseases: A systematic review. Allergol Immunopathol (Madr) 44 (2016): 177-88.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
