Clinical Analysis of 14 Pediatric Cases of Listeria Monocytogenes Meningitis in Southwest China
Cai Xiaotang1,2, Zhou Wei1,3, Yu Dan1,2, Xie Yongmei1,2, Wang Zhiing1,2, Zhou Hui1,2*
1Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, 610041, Sichuan, China
2Key Laboratory of Obstetric & Gynaecologic and Pediatric Diseases and Birth Defects of Ministry of Education, Sichuan University, Chengdu, 610041, Sichuan, China
3Department of Medical Laboratory Science, West China Second University Hospital, Sichuan University, Chengdu, 610041, Sichuan, China
*Corresponding Authors: Zhou Hui, Department of Pediatrics, West China Second University Hospital, Sichuan University, No. 20 Ren Min Nan Road, Chengdu 610041, China
Received: 30 April 2019; Accepted: 14 May 2019; Published: 17 May 2019
Article Information
Citation:
Cai Xiaotang, Zhou Wei, Yu Dan, Xie Yongmei, Wang Zhiing, Zhou Hui. Clinical Analysis of 14 Pediatric Cases of Listeria Monocytogenes Meningitis in Southwest China. Archives of Microbiology & Immunology 3 (2019): 039-049.
View / Download Pdf Share at FacebookAbstract
Background: Pediatric meningitis caused by Listeria monocytogenes is rare and is associated with high mortality and morbidity. Because L. monocytogenes meningitis in children from Southwest China has rarely been reported, we aimed to summarize the clinical data of pediatric L. monocytogenes meningitis cases encountered at our hospital to improve disease diagnosis and treatment.
Methods: Predisposing factors, clinical manifestations, laboratory tests, and cranial images of 14 pediatric patients were retrospectively analyzed.
Results: Among the patients, 57% were neonates (87.5%, preterm infants; 50%, maternofetal infection cases). In non-neonatal cases, 50% had predisposing factors, including cancer-associated chemotherapy and congenital heart disease with rickets. All neonatal cases had positive blood cultures, with poor response, frequent apnea or tachypnea, birth asphyxia, and seizure being the predominant manifestations. All non-neonates had positive cerebrospinal fluid (CSF) cultures, with fever, vomiting, headache, and neck stiffness being the predominant symptoms. Pediatric patient mortality was 21.4% (two neonates with maternofetal infection; one non-neonate). Excluding three deaths and one self-discharge, three patients had hydrocephalus (two neonates; one non-neonate); four patients had developmental retardation (three neonates; one non-neonate).
Conclusions: L. monocytogenes meningitis had numerous predisposing factors, commonly including mother-tochild transmission in neonates (particularly preterm infants) and hypoimmunity in non-neonates. Differently aged patients showed different clinical manifestations. Neonatal and non-neonatal cases, mostly occurred secondary to bloodstream infections and CSF infections, r
Keywords
<p>Listeria monocytogenes; Meningitis; Pediatric patients</p>
Article Details
1. Introduction
Listeriosis is a rare and invasive disease associated with high mortality [1,2]. Globally, the incidence of listeriosis varies between 0.1 and 11.3/1,000,000 [1]. Listeriosis is a foodborne disease caused by ingesting foods contaminated with L. monocytogenes. Listeria monocytogenes infection may cause focal infection or systemic listeriosis depending on an organism’s immune state. Invasive listeriosis might be life-threatening, with high case fatality rates, and warrants hospitalization. Immunocompromised populations, including neonates, pregnant women, elderly, and immunocompromised adults are more vulnerable to invasive listeriosis infection [3]. Sepsis and meningitis are the common manifestations of invasive listeriosis [3,4]. Even with effective antibiotic treatment, invasive listeriosis has an average case fatality rate of 20–30% [5, 6].
Children and elderly adults are two groups of highly susceptible to L. monocytogenes meningitis. Yet, there are differences between children and elderly groups in aspects of clinical manifestations, etiology and risk of severe factors [7]. Therefore, it is necessary to summarize the clinical features, etiology and risk of severe and mortality factors of Listeria monocytogenes in children. To improve diagnosis and treatment efficacy, we conducted a retrospective analysis of the clinical data of 14 pediatric patients with L. monocytogenes meningitis treated in the signal center hospital in Southwest China, from January 2012 to December 2016.
2. Patients and Methods
Patients: Fourteen pediatric patients with L. monocytogenes meningitis admitted to the West China Second Hospital, Sichuan University, from January 2012 to December 2016 were included in the present study. Inclusion criteria were as follows: (1) patients who showed clinical manifestation of purulent meningitis; (2) patients who showed cerebrospinal fluid (CSF) characteristics with pleocytosis (≥10 cells/μl) and/or hypoglycorrhachia; (3) Patients with positive L. monocytogenes blood and/or CSF cultures.
Patients with the following manifestations were defined as critically ill: septic shock; dyspnea requiring mechanical ventilation; recurrent seizure; coma; and cerebral herniation.
2.1 Research Methods
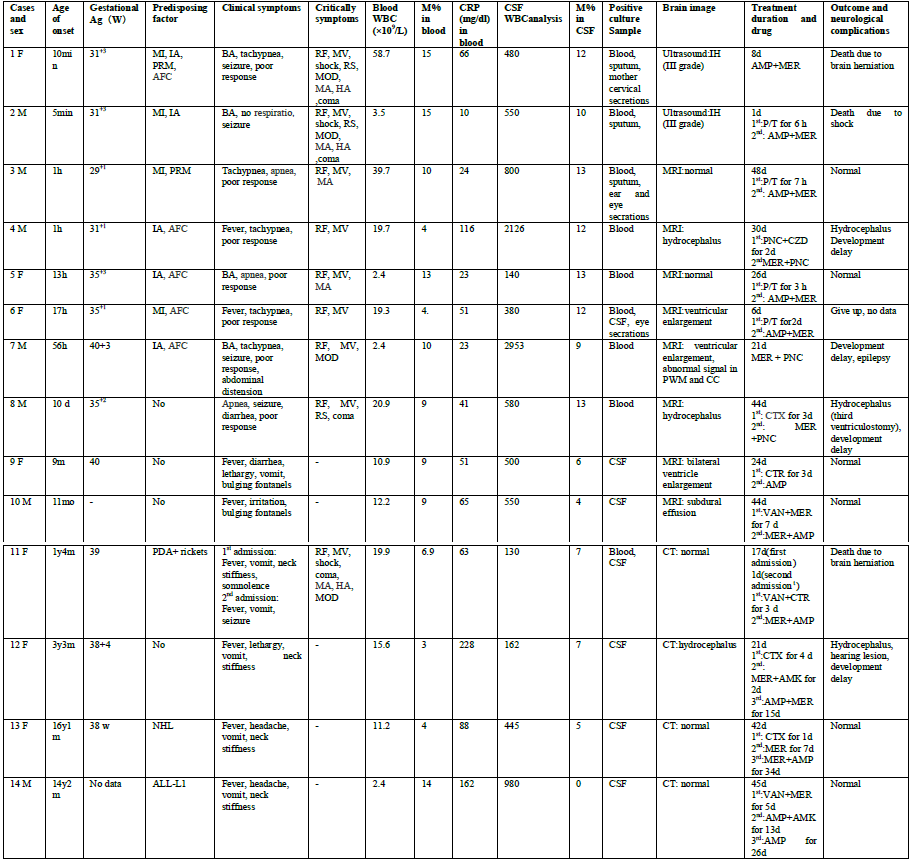
Retrospective analysis of clinical data, including manifestations, laboratory tests, bacterial culture, drug sensitivity tests, and cranial images, of 14 pediatric in-patients with L. monocytogenes meningitis was conducted. Differences between neonatal and non-neonatal cases were compared. (Table 1)
3. Results
3.1 Patient Information
Seven male and seven female pediatric patients were included, ranging from newborns to adolescents who were 16 years old. Eight cases (57.1%) were neonatal, including 7 preterm births, whereas six cases (42.9%) were non-neonatal (two patients were aged <1 year, 2 patients were aged between 1 and 4 years, and 2 patients were aged >12 years).
3.2 Analysis of Predisposing Factors
Among the eight neonatal patients, seven cases (87.5%) were preterm births, and the gestational age ranged from 29+1 to 35+3 weeks. Five patients (62.5%) had intrauterine asphyxia; two (25%), premature membrane rupture; and five (62.5%), amniotic fluid contamination. In four cases (50%), the mother had prenatal infection with fever or elevated total white blood cell (WBC) count (9.4–26.4×109/L) and C-reactive protein (CRP, 37–82 mg/dL) level. The mother of one case had cervical secretion culture positive for L. monocytogenes.
Among six non-neonates, one patient (aged 16 month; 12.5%) had patent ductus arteriosus (PDA) and rickets, two patients had neoplastic diseases (aged >12 years; 33.3%; one patient had non-Hodgkin’s lymphoma and the other patient had acute lymphocytic leukemia), and three patients (50%) had no detectable predisposing factor.
3.3 Clinical Manifestations
Among eight neonates, six patients (75%) showed symptoms within 2 days after birth, and major manifestations were poor response (100%), frequent apnea or tachypnea (100%), birth asphyxia (50%), seizure (50%), and fever (25%). The neonates displayed critical symptoms including respiratory failure (100%), mechanical ventilation (100%), metabolic acidosis (50%), refractory seizure (37.5%), multiple organ dysfunction (37.5%), coma (37.5%), hyperlactic acidemia (25%), and shock (25%). None of them had bregmatic eminence.
The predominant manifestations in the six non-neonates were fever (100%), vomiting (83.3%), headache (33.3%), neck stiffness (66.7%), and bregmatic eminence (33.3%). Only one non-neonate had critical symptoms such as respiratory failure, mechanical ventilation, shock, coma, multiple organ dysfunction, metabolic acidosis, and hyperlactic academia.
3.4 Laboratory Tests
Among the 14 study patients, 9 (64.3%) had positive blood culture and 7 (50%) had positive CSF culture. Two patients (14.3%) had both positive blood and CSF cultures. All neonates had positive blood culture and one patient had both positive CSF and blood culture. Among the four neonatal cases with maternofetal infection, there were three cases with positive sputum culture, of which one was a multiple type, showing positive ear and eye secretion cultures. All non-neonatal cases had positive CSF culture, of which one case had both positive blood and CSF cultures.
Although all the 14 patients had elevated CRP levels, 10 (71.4%) had elevated WBC count and 9 (64.3%) had elevated monocyte levels. Four patients (25.8%) had reduced WBC counts and one had neutropenia. Ten patients (71.4%) had anemia and five (35.7%) underwent blood transfusion. One patient had thrombocytopenia.
All patients received lumbar puncture and had pleocytosis, but five (35.7%) had neutrocytosis. The monocyte ratio in CSF ranged from 0 to 13%, and only one non-neonate had no monocytes in CSF. Biochemical changes in CSF included obvious reductions in glucose level (<0.2–2.5 mmol/L), normal or elevated protein levels (423–3540 mg/L), and normal chloride levels.
3.5 Imaging Examinations
Chest X-ray scans of seven neonatal cases demonstrated patchy shadows, and those of three cases revealed pulmonary hyaline membrane lesion. Two of six non-neonatal cases showed patchy shadows on both sides of the lungs.
Two neonatal patients with critical illnesses underwent only cranial ultrasonography, and were found to have II-III grade intracranial hemorrhage. The remaining 12 underwent cranial computed tomography (CT) or magnetic resonance imaging (MRI), of whom five patients were normal results, three had hydrocephalus, three had widened lateral ventricle of which one experienced abnormal signals in the periventricular white matter and corpus callosum, and one had subdural effusion.
3.6 Treatment and Prognosis
Among the 14 patients, 2 patients were initially treated with meropenem plus penicillin/ ampicillin; 2 patients with meropenem plus vancomycin; and 1 patient with penicillin plus ceftazidime; however, the remaining 9 patients were treated with piperacillin/tazobactam (4 cases), vancomycin (1 case), and third-generation cephalosporin (5 cases, of which 1 case was already receiving vancomycin at that time). After confirming the presence of L. monocytogenes in blood or CSF, three patients received combination therapy with meropenem and penicillin; seven, combination therapy with meropenem and ampicillin; one, combination therapy with meropenem and amikacin; one, therapy with ampicillin alone; one, therapy with meropenem alone; and one, combination therapy with ampicillin and amikacin. Excluding three deaths and one self-discharge case, the remaining 10 patients received treatment for an average of 34.5 (range, 21–48) days.
Three patients died (mortality, 21%), including two neonates and one 16-month-old child. The two neonates who died were preterm infants with maternofetal infection, of whom one neonate died 1 day after treatment and the other died 8 days after treatment. One non-neonatal patient received antibiotic treatment for the first 17 days and showed a slight improvement, but discontinued the treatment owing to financial difficulties. This patient developed hernia and died 7 days after treatment cessation. Apart from the three cases of death, one patient discontinued the treatment after 6 days, thus giving rise to incomplete data.
Among 10 patients who survived, 4 experienced developmental retardation (3 neonates, 1 non-neonate) and 6 had normal development (2 neonates, 4 non-neonates). Three patients had hydrocephalus (two neonates, 1 non-neonate); one of these patients underwent endoscopic third ventriculostomy. One non-neonate experienced subdural effusion; one, epilepsy; and one, hearing loss.
4. Discussion
The globally incidence of L. monocytogenes meningitis has not been clarified. Koopmans et al. [7] reported 375 cases of L. monocytogenes meningitis, based on a national survey conducted in the Netherlands from 1985 to 2014. The two groups that had the highest incidences of L. monocytogenes meningitis were the neonates group, with an incidence of 0.61 per 100,000 live births, and the elderly adults group, peaking at the age of 87 years, with an incidence of 0.53 cases per 100,000 population of the same age. The precise incidence of L. monocytogenes meningitis in China remains unclear; Feng et al. [8] reviewed 147 cases of infection caused by Listeria from Chinese literature between 1964 and 2010. They found 68 cases (46%) of sepsis, and 45 cases (31%) of CNS infection, which included 11 neonatal patients. The overall mortality of listeriosis was 26%, and neonatal mortality was 46%. Hsieh et al. [9] reported 14 cases of neonatal listeriosis in Taiwan from 1990 to 2007; in 50% of the cases, central nervous system infection was present, and the mortality rate of neonate listeriosis was 29%. Until now, pediatric L. monocytogenes meningitis has rarely been reported in China. The present study was a clinical analysis involving the largest sample of patients with pediatric L. monocytogenes meningitis in China.
4.1 Differences in predisposing factors among patients of different age groups
Among the eight neonates, 87.5% were preterm infants and 50% had maternofetal infection. Positive blood culture results were found in all newborns, and only one newborn had a positive CSF culture. All evidences suggest that neonatal CNS infections were secondary to sepsis. Maternofetal infection is also an important predisposing factor for neonatal listeriosis. Therefore, not only the blood culture but also other secretions and aseptic humoral cultures can present with positive results of L. monocytogenes. Data analysis from the United States of Foodborne Disease Active Surveillance Network showed the incidence of L. monocytogenes infection in pregnant women to be 13 times higher than that in the overall population [10]. Lv et al. [11] reported that mother-to-child transmission was an important route for neonatal L. monocytogenes infection. Pregnancy-associated listeriosis is an invasive disease affecting both pregnant women and newborns that can lead to fetal losses and neonatal deaths as well as miscarriages in pregnant women and maternal death. Fetus acquires infection owing to transplacental migration of L. monocytogenes via the mother’s bloodstream. Previous studies have reported the sporadic incidence of L. monocytogenes in Chinese adults, resulting from mother-to-child transmission [12, 13]. Consistent with our current findings, listeriosis during pregnancy mainly manifested as fever (80%), WBC count elevation (78.6%), abdominal pain (30%), and gastrointestinal symptoms (13%); however, listeriosis in fetuses and newborns was likely associated with more severe symptoms, and consequently leads to fetal death, premature birth, neonatal sepsis, meningitis, and death [10, 14]. Thus, if pregnant women had infection indicators such as fever and elevated WBC and CRP levels before delivery, blood tests as well as blood and cervical secretion cultures and smears should be analyzed on a timely basis. If pregnant women had signs of infection combined with positive culture results, timely termination of pregnancy is recommended, and rapid culturing of multiple organ secretions (e.g., sputum, eye, ear, and vulvar secretions), blood, and CSF of newborns is warranted to improve the L. monocytogenes detection rates.
Among the six non-neonates, two had neoplastic diseases and one had rickets and PDA. The positivity rate of CSF cultures was higher than that of blood cultures in these patients. A previous study reviewed that 23 non-neonatal cases of L. monocytogenes meningitis had normal immune function, and 19 cases had negative blood cultures[15]. Unlike neonatal cases, the number of blood-associated infections was lower in non-neonatal cases.
4.2 Differences in clinical manifestations among patients of different age groups
Here, L. monocytogenes meningitis in neonates principally manifested as poor response, frequent apnea or tachypnea, birth asphyxia, and seizure, but only three neonates had fever. All neonatal cases had critical symptoms including respiratory failure, mechanical ventilation, refractory seizure, and multiple organ dysfunctions. Non-neonatal cases predominantly manifested as fever, vomiting, headache, and other meningitis symptoms (neck stiffness and bulging fontanelle). Only one non-neonatal case had critical symptoms. This result is distinct from that of neonatal cases.
Among the 14 study patients, 2 had a history of diarrhea, including one neonate and one non-neonate patient, thereby suggesting that L. monocytogenes infection is foodborne and likely causes intestinal infections.
4.3 Analysis of predisposing factors for death and prognosis
Three of the study, patients died (21.4%), including two neonates and one non-neonate. Death is likely associated with the following factors. (1) Maternal infection before delivery: the two newborns who died were premature and had maternofetal infection. Pregnant mothers of these patients presented with fever or diarrhea before delivery and elevated WBC counts and CRP levels; the vaginal secretion culture of one of those mothers even tested positive for L. monocytogenes. (2) Antibiotic treatment duration: an insufficient or incomplete antibiotic treatment course can lead to the death of pediatric patients, which was suggested by the outcome of a non-neonatal patient in our study. Although having received antibiotic therapy for 17 days, sudden treatment cessation led to the progressive development of a fulminant disease course with refractory seizure, coma, hernia, and consequent death 7 days later.
4.4 Prognosis
Unlike other forms of bacterial meningitis, infection of the central nervous system by L. monocytogenes can lead to meningitis/meningoencephalitis, ventriculitis, brain and spinal cord abscesses, brainstem encephalitis, and myelitis [16–19]. One of the fourteen pediatric patients in this study had abnormal subcortical and periventricular white matter signals. Even with early and effective antibiotic treatment, Listeria-associated meningitis and meningoencephalitis has an average case fatality rate of 24-26% [20,21].
Among the neonatal cases, two patients died, one discontinued the treatment, and three experienced developmental retardation (two had hydrocephalus). Among the non-neonatal cases, one patient died and one experienced development delays (combined with hydrocephalus). The prognosis of non-neonatal cases was much better than that of neonatal cases. Hydrocephalus was the most common neurological complication in our study because three patients had hydrocephalus.
4.5 Treatments
Early diagnosis and timely treatment are extremely important for meningitis caused by L. monocytogenes commonly causes meningitis in newborns. One hundred and three cases of L. monocytogenes meningitis were reported in England and Wales between 1990 and 2013, and only 7.8% of the patients were infants older than 30 days [22].
Among eight neonates in this study, two neonates were initially treated with meropenem and ampicillin, while one neonate was treated with penicillin plus ceftazidime; their medications were only adjusted after establishing positive L. monocytogenes culture, which might have delayed the treatment and led to death. This suggested little awareness of listeriosis in newborns in Southwest China. Increased awareness of L. monocytogenes infection in high-risk newborns is warranted. If newborns <30 days old are suspected of having bacterial meningitis, administration of ampicillin, rather than third-generation cephalosporins, combined with meropenem is recommended [23]. Although L. monocytogenes infection in infants and young children is rare, L. monocytogenes meningitis should be considered when infants and young children have fever, headache, and vomiting accompanied by diarrhea and elevated monocyte counts in routine blood and CSF test results and when treatment with third-generation cephalosporins remains ineffective.
5. Conclusion
In conclusion, L. monocytogenes meningitis has obvious predisposing factors, and premature infants and children with low immune function are susceptible to the disease. Infection in pregnant mothers before delivery is a predisposing factor for L. monocytogenes infection in neonates, and we recommend monitoring of pregnant women for L. monocytogenes infection, including screening of blood and cervical secretion cultures. If a gravida at the postconceptual age is found with L. monocytogenes infection, timely antibiotic treatment and pregnancy termination, if necessary, are important steps to reduce morbidity in newborns. If neonatal patients have frequent apnea, poor response, and elevated CRP and monocyte counts in blood, L. monocytogenes infection should be considered. Incidence of meningitis in neonatal L. monocytogenes infection can be as high as 90% and is mostly due to sepsis secondary to CNS infection. If infants and young children have fever, headache, or vomiting accompanied by diarrhea and elevated monocyte counts in routine blood and CSF culture tests as well as ineffective outcomes after treatment with third-generation cephalosporins, L. monocytogenes meningitis should be considered.
List of abbreviations: MI, Mother infection; PRM, premature rupture of membrane; AFC, amniotic fluid contamination; IA, intrauterine asphyxia; MV, mechanical ventilation; RS, refractory seizure; MOD, multiple organ dysfunction; BA, birth asphyxia; RF, respiratory failure; MA, metabolic acidosis; HA, hyperlactic academia; NHL, non-hodgkin’s lymphoma; ALL, Acute lymphoblastic leukemia; IH, Intracranial hemorrhage; PWM, periventricular white matter; CC, corpus callosum; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; CT, Computer tomography; WBC, white blood cell; N%, neutrophilic granulocyte percentage; M%, monocyte percentage; d, day(s); w, week; m, month(s); y, year(s); h, hour(s); min, minute(s); F, female; M, male; AMP: Ampicillin; AMK, Amikacin; MER, Meropenem; CTX, ceftriaxone; P/T, piperacillin/tazobactam; PNC, penicillin; CZD, ceftazidime; Cep/S, Cefperazone-Sulbactam; VAN, vancomycin.
Funding
Key research and development project of Sichuan Provincial Scinece and Technology Department (2018FZ0054, 2017SZD0153) and National Science foundation of China (81170607).
References
- Swaminathan B & Gerner-Smidt P. The epidemiology of human listeriosis. Microbes and Infection 9 (2007): 1236–1243.
- Anonymous, Risk assessment of Listeria monocytogenes in ready-to eat foods. Microbiological Risk Assessment Series, No. 5, Technical Report, WHO, Rome, 2004.
- de Noordhout CM, Devleesschauwer B, Angulo FJ et al. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis 14 (2014): 1073-1082.
- Posfay-Barbe KM & Wald ER. Listeriosis. Seminars in Fetal and Neonatal Medicine 14 (2009): 228–233.
- Ulloa-Gutierrez R, Avila-Aguero ML, Huertas E. Fulminant Listeria monocytogenes meningitis complicated with acute hydrocephalus in healthy children beyond the newborn period. Pediatr Emerg Care 20 (2004): 233-237.
- Clauss HE, Lorber B. Central nervous system infection with Listeria monocytogenes. Curr Infect Dis Rep 10 (2008): 300-306.
- Koopmans MM, Brouwer MC, Bijlsma MW, et al.Listeria monocytogenes sequence type 6 and increased rate of unfavorable outcome in meningitis: epidemiologic cohort study. Clin Infect Dis 57 (2013): 247-253.
- Feng Y, Wu S, Varma JK, Klena JD, Angulo FJ, Ran L. Systematic review of human listeriosis in china, 1964-2010. Trop med int health 18 (2013): 1248-1256.
- Hsieh WS, Tsai LY, Jeng SF et al. Neonatal listeriosis in Taiwan, 1990-2007. Int J Infect Dis 13 (2009): 193-195.
- Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. Management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol 124 (2014): 1241-1244.
- Lv J, Qin Z, Xu Y, Xie Q. Listeria infection in Chinese pregnant women and neonates from Shandong. Int J Clin Exp Med 7 (2014): 2730-2734.
- Wang P, Chen Y, Wang H, Yang S, Xu Y, Li T. A clinical analysis of 16 patients with maternal listeriosis. Zhonghua Nei Ke Za Zhi 54 (2015): 763-767.
- Wu L, Zhang XH, Chen H, Yin XL. Neonatal septicemia caused by Listeria monocytogenes report of 6 cases. Zhonghua er ke za zhi 46 (2008): 22-25.
- Jiao Y, Zhang W, Ma J, Wen C, Wang P, Wang Y, Xing J, Liu W, Yang L, He J. Early onset of neonatal listeriosis. Pediatr Int 53 (2011): 1034-1037.
- Ben Shimol S, Einhorn M, Greenberg D. Listeria meningitis and ventriculitis in an immunocompetent child: case report and literature review. Infection 40 (2012): 207-211.
- Dhiwakar M, Basu S, Ramaswamy R, Mallucci C. Neurolisteriosis causing hydrocephalus, trapped fourth ventricle, hindbrain herniation and syringomyelia. Br J Neurosurg 18 (2004): 367-370.
- Castro A, Hernandez OH, Uribe CS, Guerra A, Uruena P. Brainstem encephalitis and myelitis due to Listeria monocytogenes: a case report and literature review. Biomedica 33 (2013): 343-349.
- Abbs A, Nandakumar T, Bose P, Mooraby D. Listeria rhomboencephalitis. Pract Neurol 12 (2012): 131-132.
- Horta-Baas G, Guerrero-Soto O, Barile-Fabris L. Central nervous system infection by Listeria monocytogenes in patients with systemic lupus erythematosus: analysis of 26 cases, including the report of a new case. Reumatol Clin 9 (2013): 340-347.
- Pelegrín I, Moragas M, Suárez C et al. Listeria monocytogenes meningoencephalitis in adults: analysis of factors related to unfavourable outcome. Infection 42 (2014): 817-827.
- Mylonakis E, Hohmann EL, Calderwood SB. Central nervous system infection with Listeria monocytogenes. 33 years' experience at a general hospital and review of 776 episodes from the literature. Medicine 77 (1998): 313-336.
- Thønnings S, Knudsen JD, Schønheyder HC, Søgaard M, Arpi M, Gradel KO, Østergaard C; Danish Collaborative Bacteraemia Network (DACOBAN). Antibiotic treatment and mortality in patients with listeria monocytogenes meningitis or bacteraemia. Clin Microbiol Infect 22 (2016): 725-730.
- Okike IO, Awofisayo A, Adak B, Heath PT. Empirical antibiotic cover for Listeria monocytogenes infection beyond the neonatal period: a time for change. Arch Dis Child 100 (2015): 423-425.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
