Effect of Flubendazole, with Jarish-Herxheimer Reactions Followed by Cure, in A Patient with A Polymorphic Persistent Syndrome Suggestive of Chronic Lyme Disease: A Sign of Parasitic Disease?
Alexis Lacout1, Christian Perronne2
1Centre de diagnostic ELSAN, Centre médico-chirurgical 83 avenue Charles de Gaulle 15000 Aurillac, France
2Infectious Diseases, Paris
*Corresponding author: Alexis Lacout. Centre de diagnostic ELSAN, Centre médico-chirurgical 83 avenue Charles de Gaulle 15000 Aurillac, France
Received: 02 February 2023; Accepted: 09 February 2023; Published: 13 March 2024
Article Information
Citation: Alexis Lacout, Christian Perronne. Effect of Flubendazole, with Jarish-Herxheimer Reactions Followed by Cure, in A Patient with A Polymorphic Persistent Syndrome Suggestive of Chronic Lyme Disease: A Sign of Parasitic Disease. Archives of Microbiology and Immunology. 8 (2024): 96-100.
View / Download Pdf Share at FacebookAbstract
This paper discusses the case of a 40-year-old male patient presenting with a "polymorphic persistent syndrome after a possible tick bite" (SPPT), a syndrome officially recognized by the French High Authority for Health (HAS). Anti-infection protocols were implemented, gradually improving the patient's clinical condition until complete remission was achieved. Each time flubendazole was taken, it was accompanied by severe symptoms - not suggestive of adverse reactions but of a Jarisch-Herxheimer reaction. Each administration of flubendazole was followed by a period of remission of symptoms.
Keywords
<p>Lyme; Flubendazole; Borrelia; Jarish-Herxheimer; SPPT</p>
Article Details
1. Background
Borrelia burgdorferi sensu lato (including B. burgdorferi sensu stricto, B. afzelii, B. garinii and B. hermsii) is the bacterium responsible for Lyme disease and is transmitted by tick bites.
Tick bites can transmit numerous other micro-organisms: bacteria (other species of Borrelia, Bartonella spp., Ehrlichia spp., Rickettsia spp....), parasites (Babesia) and viruses (tick-borne encephalitis virus (TBEV)), the so-called co-infections which could cause different signs and symptoms in patients [1-4]. Other parasites could play a role in this disease.
2. History of the disease
This 40-year-old patient, a forestry worker, began presenting symptoms in early 2016. He was regularly bitten by ticks (more than 20 bites per year). In January 2016, abdominal pain appeared. A colonoscopy was normal. In March 2016, the patient experienced lumbago on returning from a trip to Spain, followed by diffuse pain and asthenia associated with decreased motivation, negative thoughts, sadness and depression, prompting visits to his referring physician.
In July 2017, erythematous skin patches appeared on the ankles; the patient reported swimming in the waters of the Tarn River, France, in June. In November, there was an upward trend in the extent of skin lesions, followed by increased asthenia and migratory, abdominal and joint pain affecting the ankles, knees and hips. In 2018, the disease, although fluctuating, progressively became more systemic in nature, associated with abnormal and incapacitating exhaustion, episodes of confusion or mental fog, amnesic disorders, cramps, cold sweats, spinal and joint pain. Skin lesions showed a fluctuating course; they disappeared for a while after PUVA therapy, before reappearing and worsening. His professional activities were affected. The patient's general practitioner had given him doxycycline at a dose of 200 mg per day, which led to total exhaustion and violent headaches after a week, with a resurgence of skin lesions. This was considered as a Jarish-Herxheimer reaction. The patient took activated charcoal as an adsorbent and returned to "normal" the following day.
3. Clinical Presentation
On examination, the patient was found to have abnormal, incapacitating asthenia -sometimes with depressive episodes, great emotional lability and abnormal excitement. The patient was anxious, presented with spatial disorientation, brain fog, concentration and memory problems, and speech disorders with a lack of words. There were sleep disturbances which were non-restorative, and frequent nocturnal awakenings. He had cramps, eyelid twitching, chest tightness, impatience of the lower limbs, and sometimes trembling when extremely tired. He also suffered from hot flushes and photophobia. There were no abnormal sweats. Pains were in the foreground, articular, muscular to a lesser degree, spinal, intercostal, very intense under the left foot, and abdominal. Pain was migratory and fluctuating. Headaches, dizziness and clumsiness (dropping objects) were also noted. Overall, his quality of life and professional activities were affected.
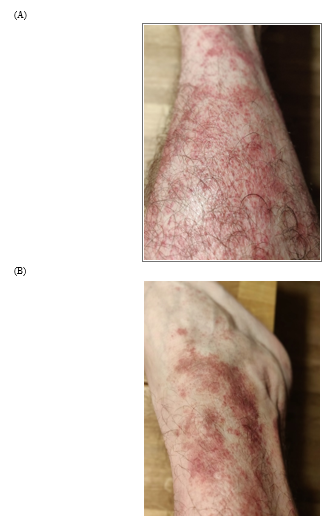
The patient presented with an erythematous, fine scaly skin rash in 2 to 3 cm patches, increasing since November 2017 with an ascending course (Figure 1). Skin biopsy revealed acanthosis with mild irregular papillomatosis and a lymphocytic infiltrate in the dermis: in total, a lichenoid pityriasis was diagnosed, for which a treatment combining topical corticosteroids and emollient was proposed. A diagnosis of "polymorphic persistent syndrome after a possible tick bite" (“syndrome polymorphe après possible piqûre de tique”, SPPT), a syndrome officially recognized by the French High Authority for Health (Haute Autorité de Santé, HAS) was mentioned. This syndrome is rather similar to PTLDS (post-treatment Lyme disease syndrome) (5, 6).
The usual laboratory work-up, including CPK and blood protein electrophoresis, was normal. TSH was normal. Rheumatoid factors were negative. There was no biological inflammatory syndrome. The cardiological work-up was normal (ECG, cardiac ultrasound). Abdominal ultrasound revealed non-dysmorphic hepatomegaly (200 mm maximum long axis) with steatosis. Liver enzyme tests were normal.
Serologies for Mycoplasma pneumoniae, Anaplasma phagocytophilum, Bartonella, Francisella tularensis and Coxiella burnetii were negative. Chlamydia pneumoniae serology was IgG positive, indicating a long-standing infection. Babesia divergens serology was weakly positive. The following viral serologies were performed: HBV in favor of a vaccination profile, EBV and CMV in favor of a long-standing infection. Lyme serology was negative by ELISA. Borrelia ELISPOT was negative (Borrelia burgdorferi s.s., Borrelia garinii, Borrelia afzelii, Borrelia spielmanii). PCR analyses were performed on blood, capillary blood, urine and saliva at two-day intervals. Rickettsia spp. was detected in saliva (CT of 27.42; 6.2 105 /ml) and Mycoplasma spp. was detected in saliva (CT of 27.26; 1.09 106 / ml) (Figure2).
Treatment was carried out by targeting the various possible co-infections, under biological monitoring, weekly observations for adverse effects and looking for a Jarisch-Herxheimer reaction or for a beneficial effect. Overall, treatment was continued until a plateau was reached in which the patient's condition no longer improved. Thus, other protocols were evaluated, until complete remission was finally achieved. Treatment was initiated at the beginning of December 2018, combining doxycycline (200 mg per day) and hydroxychloroquine (200 mg per day) for a few weeks, resulting in a resurgence of symptoms and, ultimately, improvement. An ampoule of 80,000 IU of vitamin D was also prescribed. At the end of this period, the patient self-administered flubendazole at a dose of 100 mg per day at the end of January 2019, which resulted in a very marked temporary recrudescence of the usual symptoms, resembling a Jarisch-Herxheimer reaction.
Subsequently, in early January 2019, an anti-Babesia protocol was administered due to positive serology and symptoms suggestive of parasitosis (unexplained dyspnea, hot flashes, some chills): atovaquone (250 mg) and proguanil (100 mg) 3 tablets per day, then 6 tablets per day, combined with azithromycin (250 mg) once a day, for 15 days. Treatment was well tolerated, with no Jarisch-Herxheimer-type reactions. Fatigue diminished; brain fog and concentration problems disappeared. Joint pain remained, mainly in the feet, knees and elbows. Skin lesions, on the other hand, reappeared. Because of the incomplete improvement, and the chronicity of probable Lyme disease, several courses of anti-infective treatments were implemented from early April 2019, including azithromycin, tinidazole (7), doxycycline and hydroxychloroquine, followed by ceftriaxone infusions.
Each treatment resulted in Jarisch-Herxheimer-type reactions, pointing to treatment efficacy and partial improvement in the patient's clinical condition, followed by stabilization. The skin lesions eventually disappeared. In September 2023, a relapse was effectively treated with 10 days of doxycycline (200mg daily). Sometime later another self-administered dose of flubendazole resulted in intense return of all clinical signs, just as pronounced as at the start of treatment: pain and, above all, confusion and brain fog. A short course of corticosteroids rapidly and completely resolved these symptoms. Every dose of flubendazole since the onset of the disease produced the same reactions in the patient. The hypothesis of sequestration of bacteria, in particular Borrelia, inside the parasites was put forward. Other courses of low doses of flubendazole (25 to 50 mg per day for a few days) were each time followed by Jarisch-Herxheimer reactions. Finally, at the time of writing (end of 2023), the patient is in complete remission (he has stopped taking flubendazole), has been able to devote himself fully to his work once again, and has set up several companies in the forestry sector.
4. Discussion
We suspected that this patient had Lyme disease (caused by infection with a bacterium of the genus Borrelia) despite negative serology, as it is published that seronegative cases are possible (8-10). His clinical pattern was compatible with SPTT/PTLDS, a syndrome that may be due to other factors than borreliosis, including co-infections, such as babesiosis (11, 12). The term chronic Lyme disease has long been controversial, and we might have referred to it as "persistent" Lyme disease. Nonetheless, published case reports and observations in everyday practice point to a succession of remissions and relapses when antibiotics are introduced and stopped in patients with Lyme disease. In addition, mechanisms of chronicity are known, such as biofilms and round forms, and have been observed in vivo. The Centers for Disease Control and Prevention (CDC) which is the national public health agency of the United States under the Department of Health and Human Services in Atlanta now recognizes the existence of chronic manifestations of Lyme disease (13). There should therefore no longer be any scientific controversy on this subject.
Jarisch-Herxheimer reactions are well known exacerbations of the symptoms of patients with some chronic infectious diseases, when effective treatment is initiated. These reactions, first described in the treatment of syphilis, reveal the treatment activity. They must be distinguished from possible adverse drug reactions. Jarisch-Herxheimer reactions may be severe. They result from the destruction of infectious agents, possibly through the release of endotoxins, and are mediated by TNF alpha, along with other inflammatory cytokines (14). These reactions are usually a sign of the effectiveness of the treatment. Indeed, the patient’s symptoms decreased after each exacerbation observed with flubendazole, providing transient recovery and finally long-term remission. This favorable course had not been achieved with antibiotics or antiprotozoal drugs alone. Particularly striking were the severe reactions to flubendazole. These were not adverse reactions, since they vanished despite the continuation of treatment. It is unclear why flubendazole, which is an antiparasitic drug, with no known direct effect on Borrelia, is able to trigger such reactions and then to improve the patient’s condition. The question could be raised of a possible sequestration of Borrelia inside undetected helminths, as it has been well described for schistosomes and Salmonella typhi (15, 16). The destruction of parasites could release bacteria, in particular Borrelia, which would explain the temporary exacerbation of clinical signs. This hypothetical mechanism could also explain some chronic forms of Lyme disease in addition to other known mechanisms such as sequestration of bacteria in biofilms or transformation of spirochetes in round bodies, allowing Borrelia persistence despite antibiotic therapy. Further investigations should be conducted to corroborate this hypothesis.
5. Conclusion
In conclusion, patients with chronic Lyme disease can sometimes be multiple-infected: with bacteria, parasites and viruses. In such patients, an exacerbation of clinical signs (Jarisch-Herxheimer reaction) on flubendazole could be indicative of an associated parasitic infection. Borrelia could be sequestered within the parasites that could be destroyed by flubendazole. This mechanism could explain certain chronic forms of Lyme disease.
Acknowledgement
We would like to thank the endowment fund “Lyme Support”. We would like to thank David Cowley,
University of Lincoln, Brayford Pool, Lincoln, Lincolnshire, LN6 7TS, United Kingdom for his help.
References
- Cisak E, Wójcik-Fatla A, Zajac V, Dutkiewicz J. Prevalence of tick-borne pathogens at various workplaces in forest exploitation environment. Med Pr 65 (2014): 575-81.
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, Tokarz R. Polymicrobial Nature of Tick-Borne Diseases. mBio 10 (2019): e02055-19.
- Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, et al. High-throughput screening of tick-borne pathogens in Europe. Front Cell Infect Microbiol 29 (2014): 103.
- Nelder MP, Russell CB, Sheehan NJ, Sander B, Moore S, Li Y, et al. Human pathogens associated with the blacklegged tick Ixodes scapularis: a systematic review. Parasit Vectors 9 (2016): 265.
- Rebman AW, Bechtold KT, Yang T, Mihm EA, Soloski MJ, Novak CB, et al. The Clinical, Symptom, and Quality-of-Life Characterization of a Well-Defined Group of Patients with Posttreatment Lyme Disease Syndrome. Front Med (Lausanne) 14 (2017): 224.
- https://www.has-sante.fr/jcms/c_2857558/fr/borreliose-de-lyme-et-autres-maladies-vectorielles-a-tiques
- Brorson O, Brorson SH. An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to tinidazole. Int Microbiol 7 (2004): 139-42.
- Schutzer SE, Coyle PK, Belman AL, Golightly MG, Drulle J. Sequestration of antibody to Borrelia burgdorferi in immune complexes in seronegative Lyme disease. Lancet 335 (1990): 312-5.
- Perronne C, Lacout A, Marcy PY, El Hajjam M. Errancy on Lyme Diagnosis. Am J Med 130 (2017): e219.
- Alexis Lacout, Marie Mas, Michel Franck, Véronique Perronne, Julie Pajaud, Pierre Yves Marcy, et al. Serological and PCR evidence of Infection in 105 Patients with SPPT. Archives of Microbiology & Immunology 5 (2021): 139-150.
- Lacout A, Mas M, Pajaud J, Perronne V, Lequette Y, Franck M, et al. Real time micro-organisms PCR in 104 patients with polymorphic signs and symptoms that may be related to a tick bite. Eur J Microbiol Immunol (Bp) 11 (2021): 62–75.
- Mas M, Lacout A, Perronne V, Lequette Y, Gadiolet Y, Rambeaud B, et al. Multi-Matrix Real Time PCR in 108 Patients with Polymorphic Signs Suggestive of Fibromyalgia or Related to A Tick Bite. Archives of Microbiology and Immunology 7 (2023): 250-270.
- https://www.cdc.gov/ncezid/what-we-do/our-topics/chronic-symptoms.html
- Pound MW, May DB. Proposed mechanisms and preventative options of Jarisch-Herxheimer reactions. J Clin Pharm Ther 30 (2005): 291-5.
- Bajinka O, Qi M, Barrow A, Touray AO, Yang L, Tan Y. Pathogenicity of Salmonella During Schistosoma-Salmonella Co-infections and the Importance of the Gut Microbiota. Curr Microbiol 79 (2021): 26.
- Hsiao A, Toy T, Seo HJ, Marks F. Interaction between Salmonella and Schistosomiasis: A Review. PLoS Pathog 12 (2016): e1005928.



 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
