Evaluation of a Lateral Flow Assay for Rapid and Simple Detection of IFN-γ for the Diagnosis of Latent Mycobacterium tuberculosis Infection
Hae Yeong Kang1, Su-Bin Seong1, Nari Kim1, Tae Sun Shim2, Sooyeon Chung3, Jeong-Ran Kim1*
1Department of Research and Development, The Korean Institute of Tuberculosis, Cheongju-si, Republic of Korea
2Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
3National Institute of Medical Device Safety Information, Seoul, Republic of Korea
*Corresponding author: Jeong-Ran Kim, Department of Research and Development, The Korean Institute of Tuberculosis, 168-5 Osongsaengmyeong 4-ro, Heungdeok-gu, Cheongju-si, Chungcheongbuk-do, 28158, Republic of Korea.
Received: 27 March 2024; Accepted: 03 April 2024; Published: 17 April 2024
Article Information
Citation: Hae Yeong Kang, Su-Bin Seong, Nari Kim, Tae Sun Shim, Sooyeon Chung, Jeong-Ran Kim. Evaluation of a Lateral Flow Assay for Rapid and Simple Detection of IFN-γ for the Diagnosis of Latent Mycobacterium tuberculosis Infection. Archives of Microbiology and Immunology. 8 (2024): 148-155.
View / Download Pdf Share at FacebookAbstract
Objectives: Interferon-gamma (IFN-γ) release assays (IGRAs) are useful for the diagnosis of Mycobacterium tuberculosis infection. Current IGRAs use either enzyme-linked immunosorbent assay (ELISA) or enzyme-linked immunospot assay, which require complex procedures and techniques to determine IFN-γ secretion. We aimed to compare the usefulness of the easy-to-use lateral flow assay (LFA) with that of the QuantiFERON-TB Gold In-Tube (QFT-GIT) or QuantiFERON-TB Gold Plus (QFT-plus) ELISAs for detecting IFN-γ, produced by the blood T cells stimulated by tuberculosis (TB) antigen.
Design and Methods: Following informed consent, 176 participants, including health care workers such as TB laboratory workers and radiologists, were enrolled for the study from June 2017 to June 2018. Blood samples were collected and tested using QFT-GIT and QFT-plus. The secreted IFN-γ was quantified by LFA, which took approximately 15 min, and ELISA, which took approximately 3 h.
Results: A total of 176 blood samples were screened. The positive rates of QFT-GIT and QFT-plus were 34.1% and 37.5%, respectively. Overall agreement between QFT-GIT and QFT-plus was 93.1% (κ = 0.86). The positive rates of LFA with QFT-GIT tube and QFT-plus tube were 25.6% and 31.3%, respectively, overall agreement of LFA being 90.3% (κ = 0.78) and 89.2% (κ = 0.77), respectively, compared to the QFT-GIT and QFT-plus ELISA.
Conclusions: The ability of LFA to measure IFN-γ was similar to that of ELISA. The current findings suggested that the new LFA could be more conveniently utilized for diagnosing TB infection.
Keywords
<p style="text-align:justify">Lateral flow assay; Latent tuberculosis infection; Interferon-gamma release assays; Enzyme-linked immunosorbent assay</p>
Article Details
Abbreviations:
Mtb: Mycobacterium tuberculosis; TB: Tuberculosis; IFN-g: Interferon-gamma; IGRAs: Interferon-gamma release assays; LFA: Lateral flow assay; QFT-GIT: QuantiFERON-TB Gold In-Tube; QFT-plus: QuantiFERON-TB Gold Plus; ELISA: Enzyme-linked immunosorbent assay; ESAT-6: Early secreted antigenic target 6 kDa; CFP-10: Culture filtrate protein 10 kDa
1. Introduction
One-quarter of the world’s population is estimated to be affected by latent Mycobacterium tuberculosis (Mtb) infection, approximately 5–10% of which eventually develops into active tuberculosis (TB) by the reactivation or re-infection of Mtb [1]. Accurate diagnosis of latent TB infection is crucial to facilitate early treatment of infectious cases and reduction of the spread of TB [2].
Latent TB infection can be diagnosed by either the tuberculin skin test (TST) or interferon-gamma (IFN-g) release assays (IGRAs); while the former uses a purified protein derivative (PPD) from mycobacterial culture fluid, the latter uses blood T cells stimulated by specific TB antigens [3,4]. The mycobacterial antigens used in IGRAs are not found in BCG (which is derived from Mycobacterium bovis) or in most non-tuberculous mycobacteria (NTM) causing human infection [5]. In areas where BCG vaccinations are essential, as in Korea, the specificity of TST is limited [6]. However, unlike the TST, IGRAs used for the diagnosis of TB infection are highly specific, since they are not influenced by BCG vaccination or NTM infection [7,8].
The principle of IGRAs is based on cell-mediated immune responses to TB, similar to TSTs. When Mtb is introduced into the body, T lymphocytes are activated after recognizing the TB antigens displayed by antigen presenting cells; the activated T lymphocytes then secrete cytokines, such as IFN-γ. IFN-g production by T lymphocytes, stimulated by the TB antigens present in IGRAs, is measured and utilized to identify patients infected with TB [9-11]. Although IGRAs have higher sensitivity and specificity than TSTs, these tests are expensive and require specialized equipment and highly skilled individuals to perform the tests and interpret the results [12].
Currently, two major IGRAs, QuantiFERON-TB Gold In-Tube (QFT-GIT; Qiagen, Hilden, Germany) and T-SPOT.TB (T-SPOT; Oxford Immunotec, Abingdon, UK), are commercially available [13,14]. The QFT-GIT consists of three tubes (a Nil or negative control tube, a TB antigen tube, and a Mitogen or positive control tube); the antigen tube contains three TB antigens: early secreted antigenic target 6 kDa (ESAT-6), culture filtrate protein 10 kDa (CFP-10), and TB7.7. A sample of the patient’s blood is added to each of the three tubes and incubated for 16–24 h. The amount of IFN-g released by stimulated T cells is then measured via an enzyme-linked immunosorbent assay (ELISA) [9,15]. In the T-SPOT, mononuclear cells isolated from blood are incubated with two TB antigens (ESAT-6 and CFP-10) for 16–24 h. An enzyme-linked immunospot (ELISPOT) assay is then performed to determine the number of T cells secreting IFN-g after being stimulated by the TB antigens. The TB-sensitized IFN-g-releasing T cells are counted as spot-forming cells (SFCs) and the test is generally considered to be positive if there are more than 8 SFCs/2 × 105 cells [13,16,17]. QuantiFERON-TB Gold Plus (QFT-plus), which is approved as the 4th generation test, contains four tubes (Nil control, TB1, TB2, and Mitogen control). It is also available as a 3rd generation test, QFT-GIT, which has one antigen tube containing ESAT-6, CFP-10, and TB7.7. One of the TB antigen tubes, TB1, contains ESAT-6 and CFP-10 (without TB7.7), and specifically targets CD4+ T lymphocytes; the second TB antigen tube, TB2, targets both CD4+ and CD8+ T lymphocytes. As previously mentioned, one of the major disadvantages of these assays is their high cost [18]. These assays are also very complex; both ELISA and ELISPOT involve multiple steps and require approximately 3 h and 6 h, respectively, to complete.
The lateral flow assay (LFA) is generally a paper-based chromatography assay. It has several advantages, such as ease of use, low cost, and rapid results. With these advantages, LFA has been widely used in resource-limited environments as point-of-care diagnostic tests [19,20]. Compared to ELISA, LFAs are simple and use rapid detection technology, providing results in 5–30 min. Until recently, there was no commercially available LFA for IFN-g detection. Boditech Med Inc. (Chuncheon, Republic of Korea) has recently developed an LFA for IFN-g detection, namely ichroma™ IGRA-TB, for the diagnosis of TB. The feasibility of using IGRA-TB as a diagnostic tool has been addressed, and the test has been shown to be accurate and capable of diagnosing latent TB [21].
The ichroma™ IGRA-TB uses a LFA cartridge to detect IFN-g, which is quantitated by a mobile analyzer (ichroma ™ II reader). It takes 15 min for IFN-γ to react with the anti-IFN-γ antibody, and the entire process, including testing the Nil, TB antigen, and Mitogen tubes, can be completed within 20 min. Thus, the ichroma™ IGRA-TB cartridge is much faster than QFT-Plus IFN-g ELISA, and has the advantage of not requiring any specialized equipment or complex laboratory procedures. Another advantage of the ichroma™ IGRA-TB cartridge is that it uses time-resolved fluorescence-based analyzers to improve detection sensitivity over conventional gold particles or fluorophore-based rapid tests. The ichroma™ IGRA-TB cartridge uses a sandwich assay, with both a capture antibody and a detection antibody, for IFN-g measurement. IFN-g, the analyte in the blood sample, reacts with the europium (III) chelate nanoparticle (EuNP)-conjugated anti-IFN-g (capture) antibody and the biotinylated detection antibody to form a triplet (antibody-analyte-antibody) complex; the triplet complex is immobilized on the solid phase by a biotin-streptavidin system. As the amount of IFN-g antigen in the sample increases, more triplet complexes are formed, leading to an increase in the intensity of fluorescence signal in the detector.
In this study, we aimed to investigate the diagnostic performance of the newly developed LFA (ichroma™ IGRA-TB cartridge; ichroma™ II, Boditech Med Inc., Republic of Korea) for IFN-g detection to diagnose latent TB infection, as well as to compare the results obtained by LFA with those from ELISA, using the QFT-GIT or QFT-plus.
2. Material and Methods
2.1 Ethics and study participants
This study was performed by the Korean Institute of Tuberculosis, with approval from the Institutional Review Board of the Ministry of Health and Welfare, South Korea (approval number: P01-201706-31-002). One hundred and seventy six subjects, mainly health care workers who provided TB diagnostic and treatment services and TB laboratory researchers, were enrolled in this study from June 2017 to June 2018. All participants in this study provided written informed consent and the study followed the Declaration of Helsinki.
2.2 Testing procedures
Blood samples were collected from all subjects for both QFT-GIT and QFT-plus (Qiagen, Hilden, Germany). Peripheral blood was collected in lithium heparin tubes from each subject, and l mL of this blood was aliquoted in each tube. The test consisted of one negative control tube (nil), one positive control tube (mitogen), and three antigen tubes: the 1st antigen tube, from QFT-GIT, contained peptides derived from CFP-10, ESAT-6, and TB7.7; the other two antigen tubes were TB1 and TB2 from QFT-plus, and contained peptides from CFP-10 and ESAT-6. The blood samples were incubated for 18–22 h at 37°C. Thereafter, the tubes were centrifuged at 2,000–3,000 RCF for 15 min and plasma was harvested from the samples for IFN-γ measurement by ELISA and LFA. The QFT ELISAs and LFAs (ichroma™ IGRA-TB cartridge; ichroma™ II, Boditech Med Inc., Republic of Korea) were conducted according to each manufacturer’s instruction manual. For performing the QFT ELISAs, 50 μl of the harvested plasma sample was analyzed. The optical density value of each ELISA well was measured at 450 nm in a microplate spectrophotometer (BioTek Instruments Inc.) and used to calculate the result. The concentration of IFN-g (IU/mL) was determined from the standard curve. For performing the LFAs, 100 μl of the harvested plasma sample was analyzed. LFA (ichroma™ IGRA-TB cartridge) was performed at a wavelength of 613 nm using the ichroma™ II reader (Boditech Med Inc.) and the IFN-g concentration was directly calculated in terms of IU/mL. The test results of ELISAs and LFAs were determined as negative, indeterminate, or positive. The ELISA and LFA results were interpreted as recommended by the manufacturer. Results were considered positive if the amount of IFN-γ, measured after stimulation with TB antigens, minus the amount in the negative control (nil), was ≥ 0.35 IU/mL and ≥ 25% of the nil. Results were considered negative if the IFN-γ level was < 0.35 IU/mL or < 25% of the nil. Results were considered indeterminate if the IFN-γ level of the nil was > 8.0 IU/mL or antigen minus the nil was ≥ 0.35 IU/mL and < 25% of the nil when the nil was ≤ 8.0 IU/mL and the mitogen was < 0.5 IU/mL.
2.3 Statistical analysis
Statistical analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA). Concordance between dichotomized QFT-GIT and QFT-plus, each by ELISA and ichroma™ IGRA-TB cartridge, was assessed by kappa (κ) coefficients, taking into account the probability of agreement between two groups occurring by chance. Results were defined as ‘poor’ for κ ≤ 0.20, ‘fair’ for 0.20 < κ ≤ 0.40, ‘moderate’ for 0.40 < κ ≤ 0.60, ‘good’ for 0.60 < κ ≤ 0.80, and ‘excellent’ for 0.80 < κ ≤ 1.00. Indeterminate test results were excluded from the analysis of overall agreement. Fisher’s exact test examined the significance of this association. McNemar’s test and Bowker’s test were used for the detection of linkage disequilibrium. The analysis outcome was considered statistically significant if p-value < 0.05. The receiver operating characteristic (ROC) curve was drawn using Analyse-it software (Analyse-it Software Ltd. Leeds, UK).
3. Results
3.1 Demographics of the participants
A total of 176 volunteers were recruited for the study, most of whom were health care workers (i.e., clinical technicians, radiologists, doctors, and nurses examining and/or treating patients with TB) and laboratory researchers studying TB. The participation rate was 100% since all 176 volunteers were enrolled. The study population was composed of 86 females (48.86%) and 90 males (51.14%), the mean age being 38.5 years (standard deviation (SD) 12.05 years) and median age being 34 years (range 22–69 years).
3.2 Concordance between QFT-GIT and QFT-plus
Samples were prepared using QFT-GIT and QFT-plus and then analyzed using ELISA and the ichroma™ IGRA-TB cartridge. ELISA results from QFT-GIT and QFT-plus were directly compared. There were 57 concordant positive (32.39%), 105 concordant negative (59.66%), 2 indeterminate (1.14%), and 12 discordant results (6.82%). Agreement between the results from QFT-GIT and QFT-plus by ELISA was 93.18% (95% confidence interval (CI): 89.46–96.9%), with a κ value of 0.856 (95% CI: 0.778–0.935). The p-value obtained by Bowker's test was 0.3916 (Table 1). In case of the ichroma™ IGRA-TB cartridge, there were 43 concordant positive (24.43%), 117 concordant negative (66.48%), 2 indeterminate (1.14%), and 14 discordant results (7.95%). Agreement between the results from QFT-GIT and QFT-plus using the ichroma™ IGRA-TB cartridge was 92.05% (95% CI: 88.05–96.04%), with a κ value of 0.8126 (95% CI: 0.719–0.906). The p-value obtained by Bowker's test was 0.0675 (Table 2). There was no statistically significant difference between the QFT-GIT and QFT-Plus results obtained by either ELISA or ichroma™ IGRA-TB cartridge.
|
ELISA |
QFT-GIT |
P* |
Overall agreement |
Kappa |
||||
|
Positive |
Negative |
ID |
Total |
|||||
|
QFT-plus |
Positive |
57 |
9 |
0 |
66 |
0.3916 |
||
|
Negative |
3 |
105 |
0 |
108 |
0.9318 |
0.8563 |
||
|
ID |
0 |
0 |
2 |
2 |
(0.8946–0.9691) |
(0.7779–0.9348) |
||
|
Total |
60 |
114 |
2 |
176 |
||||
ELISA enzyme-linked immunosorbent assay, QFT-GIT QuantiFERON-TB Gold In-Tube, QFT-plus QuantiFERON-TB Gold Plus, ID indeterminate. * p-value obtained by Bowker's test.
Table 1: Results of QFT-GIT and QFT-Plus measured by ELISA.
|
iCHROMA |
QFT-GIT |
P* |
Overall agreement |
Kappa |
||||
|
Positive |
Negative |
ID |
Total |
|||||
|
QFT-plus |
Positive |
43 |
12 |
0 |
55 |
0.0675 |
||
|
Negative |
2 |
117 |
0 |
119 |
0.9205 |
0.8126 |
||
|
ID |
0 |
0 |
2 |
2 |
(0.8805–0.9604) |
(0.719–0.9061) |
||
|
Total |
45 |
129 |
2 |
176 |
||||
QFT-GIT QuantiFERON-TB Gold In-Tube, QFT-plus QuantiFERON-TB Gold Plus, ID indeterminate.
* p-value obtained by Bowker's test.
Table 2: Results of QFT-GIT and QFT-Plus measured by ichroma™ IGRA-TB cartridge.
3.3 Agreement between ELISA and lateral flow assay
There were 174 valid test results that could be used to compare the ELISA with ichroma™ IGRA-TB cartridge, after excluding the two indeterminate results. The same QFT-GIT samples were analyzed by ELISA and ichroma™ IGRA-TB and results from the two IFN-g assays were compared. The number of positive results obtained by ELISA (34.48%) differed slightly from that obtained using the ichroma™ IGRA-TB cartridge (25.86%). Agreement between ELISA and ichroma™ IGRA-TB was 90.23% (95% CI: 85.82–94.64%), with a κ value of 0.7702 (95% CI: 0.6686–0.8717). The p-value obtained by Bowker's test was 0.0003, which indicated very good agreement between the two assays (Table 3). Similar results were obtained using the QFT-plus samples: 37.93% of the ELISA results and 31.61% of the ichroma™ IGRA-TB results were positive. For the QFT-plus samples, agreement between ELISA and ichroma™ IGRA-TB cartridge results was 89.08% (95% CI: 84.45–93.71%), κ = 0.7603 (95% CI: 0.6598–0.8609). The p-value was 0.0116, indicating good agreement (Table 3). The QFT-plus samples were analyzed further by comparing the results from TB1 with those from TB2. The response correlation between the two IFN-g assays using the QFT-plus TB1 tube is presented in Table 4. Agreement between the TB1 results by ELISA and that by ichroma™ IGRA-TB was 88.51% (95% CI: 83.77–93.24%), κ = 0.7416 (95% CI: 0.6375–0.8457). The number of positive results from TB2 samples was higher than that from TB1 samples. Overall agreement between the two IFN-g assays using TB2 samples was also higher than that from TB1 samples, and so was the κ value (Table 4).
|
ELISA |
|||||||
|
QFT-GIT Tubes |
QFT-plus Tubes |
||||||
|
Positive |
Negative |
Total |
Positive |
Negative |
Total |
||
|
iCHROMA |
Positive |
44 |
1 |
45 |
51 |
4 |
55 |
|
Negative |
16 |
113 |
129 |
15 |
104 |
119 |
|
|
Total |
60 |
114 |
174 |
66 |
108 |
174 |
|
|
P* |
0.0003 |
0.0116 |
|||||
|
Overall agreement |
0.9023 |
0.8908 |
|||||
|
(0.8582–0.9464) |
(0.8445–0.9371) |
||||||
|
Kappa |
0.7702 |
0.7603 |
|||||
|
(0.6686–0.8717) |
(0.6598–0.8609) |
||||||
ELISA enzyme-linked immunosorbent assay, QFT-GIT QuantiFERON-TB Gold In-Tube, QFT-plus QuantiFERON-TB Gold Plus. *p-value obtained by McNemar’s test.
Table 3: Comparison between ELISA and ichroma™ IGRA-TB cartridge using QFT-GIT and QFT-plus tubes.
|
ELISA |
|||||||
|
QFT-plus TB1Tubes |
QFT-plus TB2 Tubes |
||||||
|
Positive |
Negative |
Total |
Positive |
Negative |
Total |
||
|
iCHROMA |
Positive |
47 |
2 |
49 |
50 |
3 |
53 |
|
Negative |
18 |
107 |
125 |
13 |
108 |
121 |
|
|
Total |
65 |
109 |
174 |
63 |
111 |
174 |
|
|
P* |
0.0003 |
0.0124 |
|||||
|
Overall agreement |
0.8851 |
0.908 |
|||||
|
(0.8377–0.9324) |
(0.8651–0.951) |
||||||
|
Kappa |
0.7416 |
0.7939 |
|||||
|
(0.6375–0.8457) |
(0.6986–0.8892) |
||||||
Abbreviations: ELISA enzyme-linked immunosorbent assay, QFT-plus QuantiFERON-TB Gold Plus
*p-value obtained by McNemar’s test.
Table 4: Comparison between ELISA and ichroma™ IGRA-TB cartridge using QFT-plus TB1 tubes and QFT-plus TB2 tubes.
3.4 ROC curve analysis
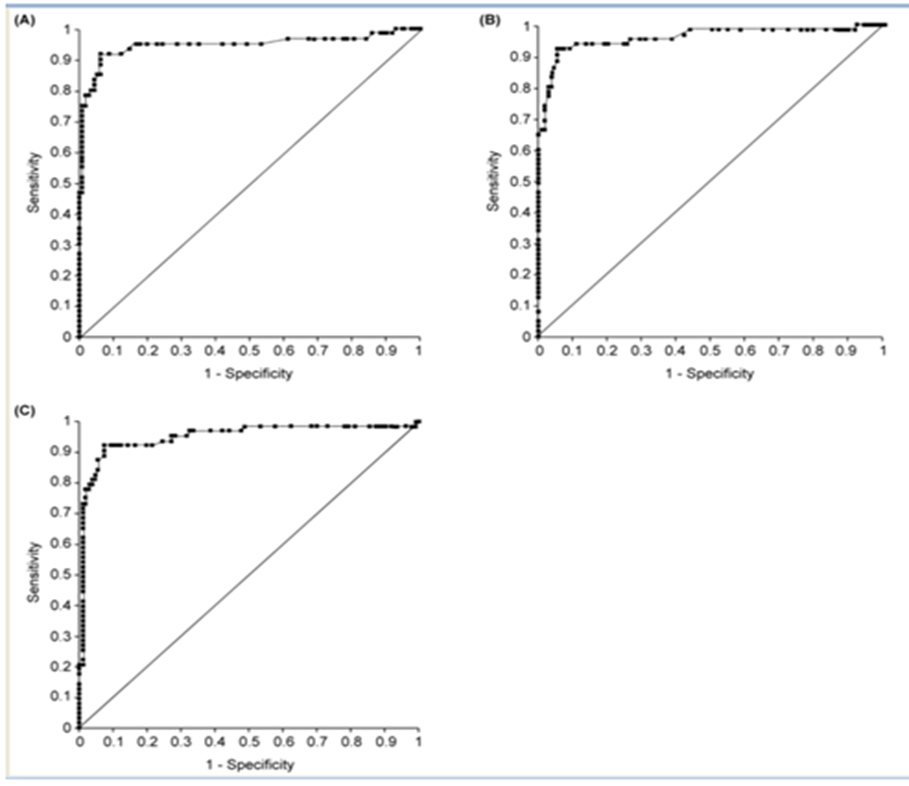
Diagnostic value of the ichroma™ IGRA-TB cartridge was evaluated by ROC curve analysis. Area under the curve (AUC) was measured for all 174 samples to determine the usefulness of the ichroma™ IGRA-TB cartridge in differentiating between positive and negative samples. AUC value for the QFT-GIT samples, evaluated using the ichroma™ IGRA-TB cartridge, was 0.946 (95% CI: 0.900 to 0.992). The ROC curve is shown in Figure 1A and the data are summarized in Table 5. ROC curve analysis was also performed to evaluate the diagnostic value of ichroma™ IGRA-TB cartridge, when it was used to study the TB1 and TB2 samples. AUC value for the TB1 samples was 0.959 (95% CI: 0.924 to 0.994) and that for the TB2 samples was 0.951 (95% CI: 0.911 to 0.990), as shown in Figure 1B and 1C, and Table 5.
|
Result of ELISA |
Diagnostic accuracy of ichroma™ IGRA-TB cartridge |
||||
|
Positive |
Negative |
Total |
AUC (95% CI) |
Standard Error |
|
|
TB antigen tube in QFT-GIT |
60 |
114 |
174 |
0.946 (0.900–0.992) |
0.023 |
|
TB1 antigen tube in QFT-Plus |
65 |
109 |
174 |
0.959 (0.924–0.994) |
0.018 |
|
TB2 antigen tube in QFT-Plus |
63 |
111 |
174 |
0.951 (0.911–0.990) |
0.02 |
AUC area under the curve, CI confidence interval, ELISA enzyme-linked immunosorbent assay, QFT-GIT QuantiFERON-TB Gold In-Tube, QFT-plus QuantiFERON-TB Gold Plus.
Table 5: Comparison of diagnostic accuracy for latent tuberculosis by area under the curve (AUC) based on ELISA and ichroma™ IGRA-TB cartridge.
Figure 1: Receiver operating characteristic (ROC) curves for ELISA and ichroma™ IGRA-TB cartridge. (A) ROC curves for ELISA and ichroma™ IGRA-TB cartridge with TB antigen tube of QFT-GIT; (B) ROC curves for ELISA and ichroma™ IGRA-TB cartridge with TB1 antigen tube of QFT-plus; (C) ROC curves for ELISA and ichroma™ IGRA-TB cartridge with TB2 antigen tube of QFT-plus. ELISA: enzyme-linked immunosorbent assay; QFT-GIT: QuantiFERON-TB Gold In-Tube; QFT-plus: QuantiFERON-TB.
4. Discussion
In 2001, the QuantiFERON test, which used non-specific PPD as an antigen to stimulate Mtb-sensitized T lymphocytes, was approved by the US Food and Drug Administration (FDA) for the diagnosis of latent TB infection. Subsequently, a 2nd generation test, QuantiFERON-TB Gold (QFT-G, Cellestis Limited, Carnegie, Victoria, Australia), was developed using ESAT-6 and CFP-10, instead of PPD, and was approved by the FDA in 2005. Early versions of ex-vivo IFN- γ tests used only ESAT-6 as the antigen; newer versions began using ESAT-6 and CFP-10 together [8,9]. The combination of antigens increased the sensitivity [10], since human leukocyte antigen (HLA) type is different for each individual, and response to the antigen is also different [11]. This implied that addition of other antigens, specific for Mtb, may enhance sensitivity of the ex-vivo IFN-γ detection method. The QFT-Plus assay, rather than QFT-GIT, was developed to increase the sensitivity to active TB through the use of two antigen tubes, TB1 and TB2, in order to induce an IFN-g response in CD4+ and CD8+ T lymphocytes [16,22,23]. This assay reflected the results as positive when any of the analytical values of TB1 or TB2 reached the criterion value [24,25].
In a previous study, our results had shown that LFA could detect INF-g and be used for diagnosing latent TB [21]. This study was intended to evaluate the performance of the ichroma™ IGRA-TB cartridge (Boditech Med Inc.) and to compare it with that of the existing commercial ELISA (QIAGEN) currently used for IFN-g measurement. To compare the IFN-g measurement capabilities of ELISA and ichroma™ IGRA-TB cartridge, TB antigen tubes from QFT-GIT and QFT-plus were used. First, we evaluated the concordance between results from samples prepared using QFT-GIT versus QFT-Plus and measured by ELISA and the ichroma™ IGRA-TB cartridge. The concordance rate between the results from samples prepared by QFT-GIT and QFT-Plus and measured by ELISA was 93.18%. When the ichroma™ IGRA-TB cartridge was used, the concordance rate was 92.05%. In Table 4, QFT-Plus analysis showed a slightly higher positive rate in TB2 (28.74%) than in TB1 (27.01%). However, in Table 5, ROC value was slightly higher in TB1 than in TB2. Since the difference was not statistically significant, future studies including a larger study population with greater statistical power would be recommended to determine whether there is a difference in agreement between QFT-GIT and QFT-Plus when measured by LFA.
The following issues were noted in IGRA results. The results may be read as indeterminate due to technical problems in sample preparation, for example, delay in sample analysis, inability to operate antigens, immune degradation, and increased basal interferon gamma responses [12]. Discordant results were found in a small number of samples; majority of samples with discordance had quantitative results close to the cutoff value (0.2 to 0.7 IU/mL) [22,24]. Our data also showed discordant results, which were mainly at the border of the assay cutoff. If the amount of IFN-γ secreted is less, the sample may be detected as negative, instead of positive, when measured by ELISA or Rapid kit. We had two indeterminate cases with IFN-g levels of over 8.0 IU/mL at the Nil Control of QFT-GIT and QFT-plus, as analyzed by ELISA and LFA.
ELISA is a common method for measuring IFN-g. Although it can analyze multiple samples simultaneously, it is difficult to perform in a laboratory that does not have a specialist familiar with the ELISA process, including repeated incubation, washing, and activating the enzyme reactions to generate the signal. LFA is one of the simplest point-of care tests. It has several advantages in terms of speed, ease of use, and cost effectiveness. Thus, the use of LFA technology would be beneficial for the diagnosis of TB infection in resource-constrained environments [26,27]. There are a few areas for improvement in the current LFA. To improve accuracy, IFN-g detection capability close to the cut-off value would need to be enhanced. In ELISA of the QFT-GIT, results close to the cut-off value show test variability [28]. In the present QFT-GIT and QFT-plus ELISA results, even in the same sample, positive or negative results were obtained when the cut-off value was close to 0.35 IU. In this study, LFA has been successfully implemented in resource-limited environments and has shown that it can be applied to current LFA-based latent TB diagnosis.
5. Conclusion
Our study evaluated the applicability of the newly developed and easy-to-use LFA, and compared it with ELISA of QFT-GIT or QFT-plus. Agreement between ELISA and ichroma™ IGRA-TB cartridge was 90.23% using QFT-GIT and 89.08% using QFT-plus. In the ROC curve analysis, AUC value of ichroma™ IGRA-TB cartridge did not show any significant difference from that of conventional ELISA. These results indicated strong agreement between ELISA and ichroma™ IGRA-TB cartridge results. Our study demonstrated the diagnostic performance of ichroma™ IGRA-TB cartridge and showed its feasibility as a substitute for ELISA.
Acknowledgements
The authors would like to thank Jin-A Lee of the Korean Institute of Tuberculosis for technical support and members of the Korea National TB Association and the Korean Institute of Tuberculosis for their contribution to the achievement of this study and Dong Hwan Choi, employee of Boditech Med Inc. for explaining the principles and experimental procedures of LFA.
Funding
This study was funded by the Korea Health Technology R&D Project [HI17C1000], through the Korea Health Industry Development Institute (KHIDI), from the Ministry of Health & Welfare, Republic of Korea.
Declarations of interests
The authors declare that Boditech Med Inc. provided ichroma™ IGRA-TB cartridges and ichroma™ II to carry our research. This project was carried out in cooperation with Boditech Med Inc. and Asan Medical Center after receiving the research fund (HI17C1000) from the Ministry of Health & Welfare, Republic of Korea.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Ministry of Health and Welfare (https://public.irb.or.kr/), South Korea (approval number: P01-201706-31-002). All participants signed a formal written consent form.
References
- World Health Organization. Global tuberculosis report (2023).
- World Health Organization. Latent tuberculosis Infection: Updated and consolidated guidelines for programmatic management (2018).
- Kahwati LC, Feltner C, Halpern M, et al. Primary care screening and treatment for latent tuberculosis infection in adults: Evidence report and systematic review for the US Preventive Services Task Force. JAMA 316 (2016): 970-983.
- Kim EY, Lim JE, Jung JY, et al. Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population, BMC Infect Dis 9 (2009): 207.
- Riazi S, Zeligs B, Yeager H, et al. Rapid diagnosis of Mycobacterium tuberculosis infection in children using interferon-gamma release assays (IGRAs). Allergy Asthma Proc 33 (2012): 217-226.
- Arias GM. Advances in the diagnosis of tuberculosis infection. Arch Bronconeumol 47 (2011): 521-530.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 149 (2008): 177-184.
- Zwerling A, Benedetti A, Cojocariu M, et al. Repeat IGRA testing in Canadian health workers: conversions or unexplained variability?. PLoS One 8 (2013): e54748.
- Petruccioli E, Vanini V, Chiacchio T, et al. G. Analytical evaluation of QuantiFERON-Plus and QuantiFERON-Gold In-tube assays in subjects with or without tuberculosis, Tuberculosis (Edinb) 106 (2017): 38-43.
- Knierer J, Gallegos Morales EN, Schablon A, et al. QFT-Plus: a plus in variability?- Evaluation of new generation IGRA in serial testing of students with a migration background in Germany. J Occup Med Toxicol 12 (2017): 1.
- van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One 4 (2009): e8517.
- Zellweger JP, Zellweger A, Ansermet S, et al. Contact tracing using a new T-cell based test: better correlation with tuberculosis exposure than the tuberculin skin test. Int J Tuberc Lung Dis 9 (2005): 1242-1247.
- Xuan WX, Lu TT, Wang Z, et al. Diagnostic significance of Mycobacterium tuberculosis T-cell assays for active tuberculosis. Chin Med J (Engl) 130 (2017): 811-816.
- Lempp JM, Zajdowicz MJ, Hankinson AL, et al. Assessment of the QuantiFERON-TB Gold In-Tube test for the detection of Mycobacterium tuberculosis infection in United States Navy recruits. PLoS One 12 (2017): e0177752.
- Barcellini L, Borroni E, Brown J, et al. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Respir J 48 (2016): 1411-1419.
- Yi L, Sasaki Y, Nagai H, et al. Evaluation of QuantiFERON-TB Gold Plus for detection of Mycobacterium tuberculosis infection in Japan. Sci Rep 6 (2016): 30617.
- Movahedi B, Mokarram P, Hemmati M, et al. IFN-γ and IL-2 responses to recombinant AlaDH against ESAT-6/CFP-10 fusion antigens in the diagnosis of latent versus active tuberculosis infection. Iran J Med Sci 42 (2017): 275-283.
- Mukai S, Shigemura K, Yamamichi F, et al. Comparison of cost-effectiveness between the quantiFERON-TB Gold-In-Tube and T-Spot tests for screening health-care workers for latent tuberculosis infection. Int J Mycobacteriol 6 (2017): 83-86.
- Hsieh HV, Dantzler JL, Weigl BH. Analytical tools to improve optimization procedures for lateral flow assays. Diagnostics (Basel) 7 (2017): E29.
- Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem 60 (2016): 111-120.
- Hur YG, Hong JY, Choi DH, et al. A feasibility study for diagnosis of latent tuberculosis infection using an IGRA point-of-care platform in South Korea. Yonsei Med J 60 (2019): 375-380.
- Theel ES, Hilgart H, Breen-Lyles M, et al. Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube interferon gamma release assays in patients at risk for tuberculosis and in health care workers. J Clin Microbiol 56 (2018): e00614-18.
- Zhang H, Xin H, Wang D, et al. Serial testing of Mycobacterium tuberculosis infection in Chinese village doctors by QuantiFERON-TB Gold Plus, QuantiFERON-TB Gold in-Tube and T-SPOT.TB. J Infect 78 (2019): 305-310.
- Moon HW, Gaur RL, Tien SS, et al. Evaluation of QuantiFERON-TB Gold-Plus in health care workers in a low-incidence setting. J Clin Microbiol 55 (2017): 1650-1657.
- Petruccioli E, Vanini V, Chiacchio T, et al. Analytical evaluation of QuantiFERON-Plus and QuantiFERON-Gold In-tube assays in subjects with or without tuberculosis. Tuberculosis (Edinb) 106 (2017): 38-43.
- Hu LM, Luo K, Xia J, et al. Advantages of time-resolved fluorescent nanobeads compared with fluorescent submicrospheres, quantum dots, and colloidal gold as label in lateral flow assays for detection of ractopamine. Biosens Bioelectron 91 (2017): 95-103.
- Juntunen E, Myyryläinen T, Salminen T, et al. Performance of fluorescent europium (III) nanoparticles and colloidal gold reporters in lateral flow bioaffinity assay. Anal Biochem 428 (2012): 31-38.
- Metcalfe JZ, Cattamanchi A, McCulloch CE, et al. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med 187 (2013): 206-211.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
