Anti-Inflammatory Properties of Glucosamine Used in Combination with Plants Extracts on Adjuvant Arthritis Rat
Amélie St-Pierre, Véronique Desrosiers, France Dufresne, Pierre U. Blier*
Université du Québec à Rimouski, Département de biologie, Rimouski, Québec, G5L3A1, Canada.
*Corresponding author: Pierre U. Blier, Université du Québec à Rimouski, Département de biologie, Rimouski, Québec, G5L3A1, Canada
Received: 02 November 2023 Accepted: 10 November 2023 Published: 06 December 2023
Article Information
Citation: Amélie St-Pierre, Véronique Desrosiers, France Dufresne, Pierre U. Blier. Anti-Inflammatory Properties of Glucosamine Used in Combination with Plants Extracts on Adjuvant Arthritis Rat. Archives of Microbiology and Immunology. 7 (2023): 362-371.
View / Download Pdf Share at FacebookAbstract
Rheumatoid arthritis has increased significantly these past years. There is a major interest in the development of treatments with drugs derived from plants or other natural sources with little adverse effects as an alternative to current treatments who have limited efficiency due to their undesirable effects on patient health. The present study evaluates the therapeutic effects of glucosamine in combination with hyaluronic acid, resin extract of Boswellia serrata, and bark extract of Salix alba on an animal model. We suggest that combinations with plants could improve the attenuation of arthritis symptoms and articular inflammation. We used Freund’s complete adjuvant on rats as models of rheumatoid arthritis. Individuals were separated into eight experimental groups: a control group without arthritis, one with arthritis and without treatment, and six other groups receiving a daily therapeutic treatment. Hind-paw thickness and arthritis scores were measured at different days. At the end of the treatment, the mRNA content of three pro-inflammatory cytokines from cartilage was measured using real-time PCR. The total antioxidant activity was assessed with an Antioxidant Assay Kit. Treatments with Boswellia serrata and Salix alba (Glu+Hyal A+Bosw, Glu+Bosw+Sal, Glu+Bosw and Glu+Hyal A+Sal) resulted in significant reductions in hind-paw thickness and arthritis scores as compared to the untreated group. Expression of pro-inflammatory gene IL 17A was also reduced, but only the Glu+Hyal A+Sal combination significantly decreased the expression of IL-1β and TNF-α. The total antioxidant activity in blood plasma significantly increased in groups treated with plant extracts.
Keywords
<p>adjuvant arthritis; glucosamine; Boswellia serrata; Salix alba; clinical signs</p>
Article Details
Abbreviations
BS: Boswellia serrata; CFA: Freund’s complete adjuvant; CIA: collagen-induced arthritis; FDA: Food and Drugs Administration; GAPDH: glyceraldehyde 3-phosphate; GS: Glucosamine sulfate; HA: Hyaluronic acid; IL-1β: interleukin-1; IL-17A: interleukin-17; NNHPD: Natural and Non-prescription Health Products Directorate; NSAIDs: nonsteroidal anti-inflammatory drugs; RA: Rheumatoid arthritis; ROS: Reactive oxygen species; SA: Salix alba; TNF-α: tumor necrosis factor
1. Introduction
Rheumatoid arthritis (RA) is an inflammatory autoimmune disorder. Its incidence and prevalence are increasing worldwide. In North America and North-European countries, its incidence varies between 20 and 50 per 100,000 population [1]. RA causes inflammation in joints that leads to pain, stiffness, swelling and cartilage damage [2]. Current treatments do not usually regenerate damaged cartilage or slow the degeneration, but relieve symptoms [2]. Treatments using steroids, nonsteroidal anti-inflammatory drugs (NSAIDs), topical anti-inflammatories, biological agents (TNF-α and IL-1 antagonists), acetaminophen and injection of corticosteroids and hyaluronic acid are used against joint diseases but show limited efficiency due to their undesirable adverse effects on patient health [3, 4]. These pharmaceutical drugs can provoke gastrointestinal disturbances (ulcers and perforations), cardiovascular complications, reproductive toxicity, loss of bone mass, and topical applications can be of no benefit when the target joints are too deep [3-5]. Due to these limitations, there is an important incentive for the development of biomolecules derived from plants or natural sources without adverse effects as an alternative to NSAIDs and other treatments. Glucosamine sulfate (GS), hyaluronic acid (HA), resin extracts of indian frankincense (Boswellia serrata Roxb. Ex Colebr., BS) and bark extracts of white willow (Salix alba L., SA) are four natural ingredients that are individually considered efficient against arthritis by regulatory agencies (for example see the monograph of Natural and Non-prescription Health Products Directorate (NNHPD) in Canada [6]).
GS is an important component of cartilage and is naturally synthesized in the body. This amino monosaccharide stimulates the biosynthesis of glycosaminoglycan chains, giving the cartilage its strength, flexibility and elasticity, all the while possessing anti-inflammatory properties [7]. HA is a large viscoelastic glycosaminoglycan present in the synovial fluid, and is responsible for its viscoelastic properties [8]. It also confers good protective properties including shock absorption, protective coating of the articular cartilage surface, and lubrication. BS and SA have both anti-inflammatory and analgesic properties due to the presence of boswellic acid and salicin respectively [9, 10]. Boswellic acid reduces pain and swelling, has antioxidant and free radical-scavenging properties, and appears as a potential new treatment of inflammatory disorders like rheumatoid arthritis and osteoarthritis [5]. It reduces glycosaminoglycan degradation, keeping the cartilage in good condition unlike NSAIDs that can induce the disruption of the glycosaminoglycan synthesis, accelerating articular damage [9]. Salicin has anti-inflammatory and anabolic effects, as shown in canine joints [10]. Benefits of these natural ingredients have so far only been studied separately, and their potential synergistic effects need to be assessed, as a combination of ingredients can improve their therapeutic effects at the low doses recommended by the health regulation agencies. The aim of this study was to examine the potency of different combinations of natural ingredients to limit arthritis symptoms and articular inflammation on an animal model of rheumatoid arthritis. In this context, we used rats previously injected with Freund’s complete adjuvant and quantified the modulation of inflammation when hyaluronic acid, Boswellia serrata, and Salix alba extract were combined to glucosamine to help determine if these extracts can improve the therapeutic efficiency of glucosamine.
2. Materials and Methods
2.1 Animals
Adult female Lewis rats (10 weeks old) were obtained from Charles River Laboratories (Montreal, QC, Canada). Animals were kept at the Université du Québec à Rimouski (UQAR) in controlled experimental conditions (23 ± 1°C, relative humidity 40-60%, 12h light/dark cycles, water and LabDiet 5002 ad libitum). They were acclimated during 1 week before the experiment. Animal manipulation was conducted in accordance with the Institutional Animal Care Committee of Université du Québec à Rimouski (protocol #CPA-66-16-178).
2.2 Adjuvant Induction
Arthritis was induced by subcutaneous injection of 60 µl of Freund’s adjuvant, a solution of Mycobacterium tuberculosis inactivated by heat (Chondrex, Inc. Redmond, WA, USA, 10 mg/ml), at the base of the tail. First symptoms of arthritis appeared 12 days after induction.
2.3 Evaluation of Clinical Signs of Arthritis
Arthritis symptoms were examined at days 0, 3, 6 and 9 post-induction, and then every day from days 12 to 29. Hind-paw thickness was measured with a digital caliper. Arthritis scores were determined by a score system: for each of hind paw, a scale of 0-4; 0, no macroscopic sign; 1, irritation (swelling and redness) at one joint; 2, irritation at more than one joint and/or ankles; 3, irritation at many joints and moderate swelling at the ankle; 4, irritation at many joints and severe swelling at the ankle. For each forepaw, a scale of 0-3 was used; 0, no macroscopic sign; 1, irritation at one joint; 2, irritation at many joints and/or wrist; 3, irritation at all joints and moderate to severe swelling at the wrist. The final score was calculated by adding the individual score of each paw for a maximal result of 14 [11].
2.4 Therapeutic Ingredient Administration
Rats were separated randomly in eight groups: a control group without arthritis (control), one with arthritis and no treatment (CFA, Freund’s complete adjuvant) and six other groups receiving a daily therapeutic treatment from days 14 to 29. The temporal experimentation plan of animal manipulations is presented in Fig 1. Six therapeutic treatments were administered to the rats: GS (Glu); GS and HA (Glu+Hyal A); GS, HA and BS (Glu+Hyal A+Bosw); GS, HA and SA (Glu+Hyal A+Sal); GS and BS (Glu+Bosw); and GS, BS and SA (Glu+Bosw+Sal).
Each ingredient was administered individually, even when the therapeutic treatment had many compounds, and orally (back of the mouth) with a pipette. For each ingredient, the daily dose corresponded to the maximal recommended dosage for humans by Natural and Non-prescription Health Products Directorate (NNHPD) of Health Canada (2014) in its monograph titled «Multiple ingredient joint health products». The dosages for rats were calculated considering an average human weight of 60 kg (weight approved by Food and Drugs Administration (FDA) for safety studies) and a normalization that takes into account the body surface [12]. The daily dosages are presented in Table 1. This formula represents the allometric conversion:
Animal equivalent dose (mg/kg) = human equivalent dose (mg/kg) / [Factor km rat/Factor km human]
where Factor km = body mass (kg) / total body surface (m2)
and km rat = 6 km human = 37
Table 1: Daily doses of therapeutic ingredients recommended for human and equivalent doses for rat.
|
Therapeutic Ingredients |
Maximal Recommended Daily Dose for Human (mg) |
Equivalent Daily Dose for Rat (mg/kg Corporeal) |
|
Glucosamine (sulphated form) |
1500 |
154 |
|
Hyaluronic acid (from bacterial fermentation) |
200 |
21 |
|
Extract of Boswellia serrata (normalized at 40% of boswellic acid) |
1000 |
103 |
|
Salicine (active ingredient of Salix alba extract) |
240 |
25 |
Solutions of therapeutic ingredients were made daily as follows: GS (Novel Ingredients, NJ, USA), 200 mg/mL of water; HA from bacterial fermentation (A&A Pharmachem Inc, Ontario, Canada), 10 mg/mL of water; BS (40% of boswellic acid) (Dolcas Biotech, NJ, USA), 200 mg/mL of organic canola oil; SA (25% of salicin) (Novel Ingredients, NJ, USA), 50 mg/ml of water. Due to the specificity of BS’s solvent, all rats in groups not receiving treatment with BS received an equivalent daily volume of canola oil (100 µL). At the end of the experiment, all rats were euthanized by injection of a lethal dose of pentobarbital. A blood sample and knee cartilage of the two hind paws were collected. Plasma was extracted, samples were rapidly frozen by liquid nitrogen and preserved at -80°C for future assays.
2.5 Expression of Pro-Inflammatory Genes and Cartilage Degradation
Cartilage samples were reduced to powder with liquid nitrogen. RNA was extracted with Pure LinkRNA Mini Kit (Life Technologies; protocol with Trizol and DNase). Extraction purity was validated using spectrophotometry (absorbance ratio 260/280 nm). Reverse transcription was carried out on 400 ng of RNA for each extract using the high capacity cDNA reverse transcription kit method (Applied Biosystems). Real-time PCR was performed with SensiFAST SYBR No-ROX kit from Bioline and with a LightCycler 480 from Roche (Mississauga, Canada). Three cytokines responsible for pro-inflammatory processes were targeted: interleukin-1 (IL-1β), interleukin-17 (IL-17A) and tumor necrosis factor (TNF-α).
Primer sequences used for amplification were purchased from Sigma Aldrich (Oakville, Ontario, Canada) (table 2). Gene expression was quantified by Cycle Treshold method (Ct). Amplification standard curves of each gene was performed and the specific amplification efficiency was verified with a minimal threshold of 1,8 (maximum 2). In order to standardize and compare the different assays, a pool of cDNAs of all groups was used as an internal calibrator. Gene quantification values were expressed relative to the gene quantification of two endogenous references, β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [13]. Ratio expressions for both references were similar; only results with the expression of β-actin are shown in this study. The specificity of PCR products was confirmed by migration on electrophoresis gel and melting curve analysis.
Table 2: Primer sequences used for real-time PCR analysis
|
Gene |
Forward Primer (5'-3') |
Reverse Primer (5'-3') |
Fragment Length (Base Pair) |
|
IL-17A 1 |
GTGAGCCGGCAGAAGCAGGA |
GGCTCCGCCCAACCCAAGAT |
107 |
|
IL1-β 1 |
GGGATTTTGTCGTTGCTTGTC |
TGCAGGCTTCGAGATGAAC |
147 |
|
TNF-α 1 |
CTTCTGTCTACTGAACTTCGGG |
GCTACGGGCTTGTCACTC |
146 |
|
β-actin 1,2 |
TCACTATCGGCAATGAGCG |
GGCATAGAGGTCTTTACGGATG |
143 |
|
GAPDH 1,2 |
GCCAAGGCTGTGGGCAAGGT |
GCAGGTTTCTCCAGGCGGCAT |
119 |
1Amplification cycle: 1) initial activation at 95°C for 2 minutes, 2) 2-step: [95°C for 15 sec followed by 60°C for 30 sec] X 40 amplification cycles.
2Amplification cycle: 1) initial activation at 95°C for 2 minutes, 2) 2-step: [95°C for 15 sec followed by
63°C for 30 sec] X 40 amplification cycles.
2.6 Total Antioxidant Activity
Total antioxidant activity of plasma was measured with the Antioxidant Assay Kit from Cayman Chemical (Ann Arbor, MI, USA; cat# 709001). This assay is based on the capacity of antioxidants to inhibit the oxidation of ABTS (2,2’-azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS•+ by metmyoglobin. The amount of ABTS•+ can be measure by reading the absorbance at 750 nm or 405 nm. Values were compared with that of Trolox and expressed in equivalent values of Trolox. All antioxidant constituents are assessed in this assay including vitamins, proteins, lipids, glutathione, uric acid, etc.
2.7 Statistical Analysis
Results were analyzed using JMP Pro (SAS, Cary, NC, USA). One-way ANOVAs followed by a Tukey’s test were used to test for differences between groups. The normality of data and homogeneity of variance were tested using a Shapiro-Wilk and Bartlett’s test respectively. Quantitative data are expressed as mean ± standard error of the mean. p values <0.05 were considered statistically significant.
3. Results
3.1 Effect on Clinical Signs of Arthritis
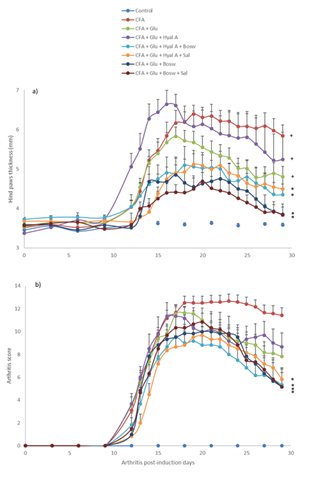
The first signs of arthritis appeared on day 12 after the injection of Freund’s adjuvant (Fig. 2). On day 19, hind-paw thickness of the CFA group increased significantly (6.39 ± 0.16 mm) compared to the control group (3.59 ± 0.16 mm, F(1,22) = 142.17, p < 0.0001). Treatments with combinations of three ingredients (Glu+Hyal A+Bosw; Glu+Hyal A+Sal; Glu+Bosw+Sal) and Glu+Bosw limited articular swelling and significantly reduced hind-paw thickness during the treatment. At the end of the experiment (day 29), hind-paw thickness of these groups was significantly inferior to the CFA group (Fig. 2A) (Glu+Hyal A+Sal: 4.50 ± 0.32 mm; Glu+Hyal A+Bosw: 4.35 ± 0.27 mm; Glu+Bosw: 3.85 ± 0.25 mm; Glu+Bosw+Sal: 3.84 ± 0.19 mm; CFA: 5.84 ± 0.28 mm, F(7,57) = 8.04, p < 0.0001). Arthritis scores increased from days 12 to 18 post-induction. At day 18, the maximal score was reached for the CFA group (Fig. 2B).
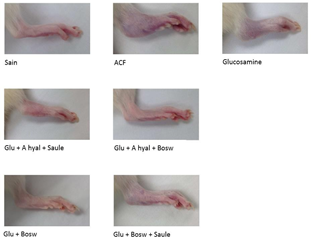
A significant decrease of arthritis scores was observed for Glu+Hyal A+Bosw, Glu+Bosw+Sal, Glu+Bosw and Glu+Hyal A+Sal treatments in comparison to the CFA group at days 23 (F(4,31) = 3.61, p = 0.0157), 25 (F(4,31) = 4.41, p = 0.0062), 26 (F(4,31) = 5.42, p = 0.0020) and 27 (F(4,31) = 5.45, p = 0.0019) respectively. At day 29, arthritis scores of these groups were significantly inferior to the CFA group (Glu+Hyal A+Bosw: 5.33 ± 1.48; Glu+Bosw+Sal: 1.66 ± 4.07; Glu+Bosw: 5.17 ± 1.25; Glu+Hyal A+Sal: 5.83 ± 0.87; CFA: 11.42 ± 0.66, F(7,57) = 17.07, p < 0.0001). Treatments with only glucosamine (Glu) and both glucosamine and hyaluronic acid (Glu+Hyal A) yielded no significant improvement of the clinical symptoms. The hind-paw conditions of each group at the end of the experiment are shown in Fig. 3.
Figure 2: Effect of treatments on severity of arthritis. a) Hind paws thickness (mm) and b) arthritis score in time (number of arthritis post-induction days). Treatments were administered daily starting at day 14 to day 29. Each circle is a mean + SEM (control and CFA groups: n = 12; Glu: n = 11; Glu+Hyal A, Glu+Hyal A+Bosw, Glu+Hyal A+Sal, Glu+Bosw and Glu+Bosw+Sal: n = 6). Significant differences with CFA group (*) and control group (+) at day 29 are shown in a) p <0.05 and in b) p < 0.01.
3.2 Effect on Expression of Pro-inflammatory and Cartilage Degradation Genes
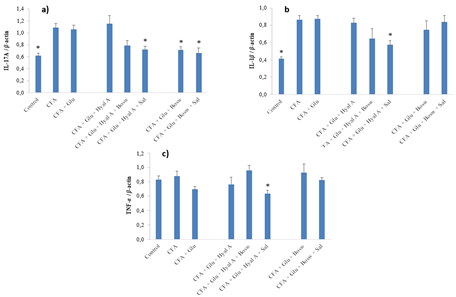
At day 29, the expressions of cytokines IL-17A and IL-1β of CFA group were significantly greater than the control group (Fig. 4A-B) (F(7,55) = 8.15, p < 0.0001 and F(7,50) = 9.87, p < 0.0001, respectively). Glu+Hyal A+Sal treatment significantly reduced the expression of IL-17A and IL-1β compared to the CFA group. A decrease in the expression of IL-17A by Glu+Bosw and Glu+Bosw+Sal was also noticed. Glu+Hyal A+Bosw tended to limit IL-17A and IL-1β expressions, but the difference with the CFA group was not significant. TNF-α expression of CFA group was not superior to the control group (Fig 4C). Glu+Hyal A+Sal treatment inhibited TNF-α compared to both the control and the CFA group (F(7,50) = 2.91, p = 0.0123). Compared to Glu treatment, multi-ingredient treatments Glu+Bosw and Glu+Bosw+Sal was efficient to reduce IL-17A expression and Glu+Hyal A+Sal was efficient to reduce IL-1β.
Figure 4: Effect of treatments on gene expression of pro-inflammatory cytokines. Gene expression of a) IL-17A, b) IL-1β and c) TNF-α were measured in the articular cartilage of hind paws at day 29 (15e day of treatment). Each circle is a mean + SEM (control and CFA groups: n = 10-12; Glu: n = 9-11; Glu+Hyal A, Glu+Hyal A+Bosw, Glu+Hyal A+Sal, Glu+Bosw and Glu+Bosw+Sal: n = 5-6). Significant differences with CFA group (*) at day 29 are shown (p < 0.05).
3.3 Effect on Total Antioxidant Activity of Plasma
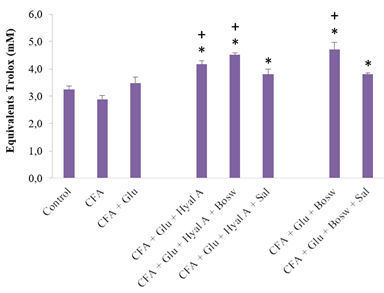
All treatments with combinations of two or three ingredients significantly increased the antioxidant capacity of blood, compared to the CFA group and control group (Glu+Hyal A: 4,16 ± 0,14; Glu+Hyal A+Bosw: 4,51 ± 0,07; Glu+Hyal A+Sal: 3,81 ± 0,18; Glu+Bosw: 4,71 ± 0,27; Glu+Bosw+Sal: 3,80 ± 0,06; CFA: 2,87 ± 0,16; Control: 3,24 ± 0,13, (F(7,43) = 11.80, p < 0.0001)) (Fig. 5). Some multi-ingredient treatments were also more effective than the treatment with Glu alone to increase antioxidant activity (Gly+Hyal A+Bosw and Glu+Bosw).
Figure 5: Effect of treatments on plasma content as total antioxidants. Antioxidant contents were measured in blood plasma at day 29 (15e day of treatment). Each circle is a mean + SEM (control group: n = 8; CFA groups: n = 10-11; Glu: n = 9-10; Glu+Hyal A, Glu+Hyal A+Bosw, Glu+Hyal A+Sal, Glu+Bosw and Glu+Bosw+Sal: n = 5-6). Significant differences with CFA group (*) and control group (+) at day 29 are shown (p < 0.05).
4. Discussion
The aim of this study was to evaluate if glucosamine sulfates therapeutic effects against rheumatoid arthritis could be enhanced through a combination of hyaluronic acid, Boswellia serrata extract or Salix alba extract. The combinations with BS and/or SA significantly reduced hind-paw thickness and arthritis scores during the treatment. The inhibition of collagen-induced arthritis has also been observed when using Salix nigra bark methanol extract (100 mg/kg/day) [14]. In other studies, boswellic acid extract (total acid content: 93 ± 3%) from BS (250 mg/kg) was found to be more efficient than glucosamine (250 mg/kg) to reduce inflammation in Mycobacterium-induced arthritis in acute and chronic model of inflammation in rats [7]. Furthermore, in the same study, the combination of these two ingredients had a significant synergistic effect on chronic inflammation with a dose of 125 mg/kg for boswellic acid and 125 mg/kg for glucosamine. We suspect that the synergistic effect results from the combination of the different metabolic targets by which the bioactive molecules reduce inflammation. Anti-arthritic properties of each ingredient might have amplified the therapeutic effect of treatments. Glucosamine and hyaluronic acid have major structural roles in articular cartilage. Glucosamine stimulates the production of glycosaminoglycans that provide strength and elasticity to cartilage and connective tissues by holding joint tissue together and giving shock-absorbing properties [7]. HA is a component of the synovial fluid and confers viscosity, as well as shock-absorbing and lubricating abilities [8]. Boswellia spp. and Salix spp. do indeed produce active compounds like boswellic acid and salicin that show anti-inflammatory activities [9, 10].
They directly target the inflammatory mediators such as interleukins and metalloproteinases [5, 14]. BS extracts inhibit the 5-lipoxygenase which contributes to the progression of chronic inflammation through greater recruitment of white blood cells at inflammatory sites [9]. BS and SA also have ROS-scavenging properties and can have a certain control on antioxidant enzymes [5, 14]. Many studies, including ours, have concluded that combinations of glucosamine with either BS and/or SA are a promising strategy for limiting clinical signs of arthritis [5, 9, 14]. In our study, GS alone or in combination with HA did not result in any improvement of the arthritis symptoms. A previous study has shown that glucosamine can inhibit swelling in joints and reduce arthritic scores in rat adjuvant arthritis [15]. However, the dose used was much higher than the equivalent dose usually administered to humans. Many studies on animals do not adjust the human dose to the size of the animals. Such doses could not be applied to humans according to the severe legislation in different countries. Maximal dosages of natural products are highly regulated to avoid adverse effects. We can therefore suspect that maximal recommended dosages for humans may not result in significant inhibition of the clinical signs of arthritis in both rats and humans. For example, a meta-analysis including 10 trials with an average of at least 100 patients concluded that glucosamine (1500 mg/kg/day: recommended dosage) was not effective against osteoarthritis, having no relevant clinical effect on pain or structure of affected joints [16]. It would be clearly advantageous to use combinations of ingredients to compensate for the small effect of a single ingredient (at recommended doses), and to rely on the synergetic effect of combinations without exceeding the recommended dosages of individual biomolecules.
Inhibition of pro-inflammatory cytokines can reduce clinical signs of arthritis. IL-1β and TNF-α, two major pro-inflammatory cytokines, are both known to be present at high concentrations in serum and synovial fluid in patients with RA [5]. IL-1β and TNF-α stimulate their own production and the production of other cytokines, amplifying the inflammation process [8] and contribute synergistically to produce the inflammasome [17]. In our study, TNF-α and IL-1β expression were significantly reduced only by Glu+Hyal A+Sal. A previous study on synovial-cell cultures from patients with RA, showed that blocking the activity of TNF-α significantly reduced the production of interleukin-1, interleukin-6 and interleukin-8 [18]. Thus, blocking TNF-α may have a greater overall effect on inflammation than only blocking IL-1. Moreover, we suspect that this reduction of TNF-α partly results from SA activity. [10] came to the conclusion that the anti-inflammatory activity of SA was associated with down regulation of the pro-inflammatory effect of TNF-α. In addition to salicin, SA has other active compounds such as polyphenols and flavonoids which may also play a role in the therapeutic action of SA [19]. On the other hand, HA did not seem to have any impact on the level of TNF-α in RA rats. Injection of intra-articular HA in rat antigen-induced arthritis showed no significant changes in the level of TNF-α in short-term and in long-term experiments [20]. Thus, we suggest that SA has an important role in the anti-inflammatory properties of a Glu+Hyal A+Sal treatment.
At the end of the experiment, three treatments containing BS and/or SA were able to inhibit IL-17A expression compared to TNF-α that was only inhibited by Glu+Hyal A+Sal. Furthermore, TNF-α expression did not differ between the control and CFA group. These results suggest that TNF-α had a weaker role in chronic inflammation than IL-17A, at least at the sampling periods of our experimentation. IL-17A is also over-expressed in RA [21]. TNF-α may be important in the onset of the arthritis induction but gradually loses its dominance with the progression of the inflammation [22]. Anti-TNFα treatment was efficient shortly after the collagen-induced arthritis (CIA) in DBA/1 mice, reducing cartilage destruction, but had little effect when CIA was fully established [22]. Measurements should have been performed at the beginning of the inflammatory phase to notice a difference in TNF-α levels. We suggest that TNF-α played a lesser role in the late phase of our experiment and had lower involvement in inflammatory modulation than IL-1β and IL-17A. As mentioned previously, our treatments had a stronger impact on IL-17A expression than in the expression of the two other cytokines. IL-17 is involved in inflammation by stimulating other pro-inflammatory cytokines and metalloproteinases in synoviocytes and chondrocytes [21]. For example, it stimulates secretion of IL-1β and TNF-α by macrophages [23]. Two studies came to the conclusion that arthritis treatments involving inhibition of IL-17 could be as efficient as blocking IL-1 and TNF-α. [24, 25] showed that IL-17 expression and activity was partly independent of these two cytokines under arthritis conditions. They can, for example, aggravate joint inflammation and cartilage destruction on their own without the increase of IL-1 or TNF-α. Also, blocking IL-1 in TNF-deficient mice was not sufficient to reduce IL-17 effects in streptococcal cell wall-induced (SCW) arthritis model [25]. IL-17 has the capacity to partly supplant the functions of IL-1 since these two have many overlapping responses and functions even if they are not from the same cytokine family, as shown in SCW-induced arthritis and IL-1-deficient mice [24]. We conclude that IL-17A might partly lead the inflammatory process at the end of our experimental arthritis and that treatments with plants were effective to decrease the expression of this cytokine. Anti-inflammatory properties of BS and SA successfully reduced the IL-17A effect according to our results.
Reactive oxygen species (ROS) can also contribute to matrix component degradation [26]. ROS are generated at high rates in synovial neutrophils from RA patients [27]. Synovial fluid and HA are respectively susceptible to degradation and depolymerization by a high level of ROS [27]. These processes promote loss of viscosity in the joint as well as osteoclast activation [28]. Different antioxidants (polyphenols, tannins, etc.) are found in natural ingredients [27] which have been reported to partly protect and limit damage to cartilage [29]. All combinations with HA, BS and/or SA significantly increased the total antioxidant level in the plasma of our experimental rats. Combinations without SA appear to have more impact on antioxidant levels than those with SA. [14] have shown that CIA in rats increased the activity of three important enzymes involved in oxidative stress management in plasma: superoxide dismutase, glutathione peroxidase and catalase. These enzymes responded naturally to a higher concentration of ROS after arthritis induction, increasing their activities to protect tissues in the joint. The antioxidant properties of biomolecules in the present study may have helped attenuate oxidative stress. [30] further showed that GS reduced superoxide radicals in a dose-concentration manner, partly explaining its antioxidant activity. Synovial fluid and endogenous HA usually protect the articular tissues from oxidative damage. Excessive ROS decrease the HA content of articulation while addition of exogenous HA can decrease ROS levels in synovial cells of RA and buffer the impact on HA oxidation and decline [27]. It has also been observed that extract of BS improved the antioxidant level in CIA rat models, significantly decreasing ROS [5]. Salix nigra bark methanol extract has contributed to attenuate oxidative stress in CIA rats [14]. BS and SA also have a phenolic compound showing antioxidant activities [19, 31]. We suggest that a combination of antioxidant properties of these natural ingredients increases the antioxidant capacity in plasma of our experimental rats. We also suggest that this rise in antioxidant capacity can be partly responsible for the reduction of the clinical signs of arthritis.
6. Conclusion
We conclude that the addition of Boswellia serrata and/or Salix alba to glucosamine attenuates the clinical signs of rheumatoid arthritis in Freund’s complete adjuvant-induced arthritis in rats likely due to both their anti-inflammatory and antioxidant properties. Combinations with these plants have decreased hind-paw swelling and improved arthritis scores. Treatments with BS and/or SA helped reduce inflammation and cartilage degradation by reducing effectively IL-17A expression and to a lesser extent, the expression of IL-1β. BS and SA have helped to create a redox status that might buffer oxidative stress through higher antioxidant capacity in plasma. This capacity may be partly responsible for the amelioration of clinical symptoms of arthritis.
Acknowledgements
We are thankful to Samuel Fortin (Ph.D.) and Caroline Morin (Ph.D.) for their help on the arthritic model of Freund’s complete adjuvant.
Funding
This work was supported by the Industrial Research Assistance Program (IRAP) of National Research Council Canada (NRCC).
References
- Fazal SA, Khan M, Nishi SE, Alam F, Zarin N, Bari MT, et al. A clinical update and global economic burden of rheumatoid arthritis.Endocr Metab Immune Disord Drug Tagets 18 (2018): 98-109.
- Arthritis Society. Arthritis Type – Arthrose, Arthritis Society (2018).
- Fan AY, Lao L, Zhang RX, Zhou AN, Wang LB, Moudgil KD, et al. Effects of an acetone extract of Boswellia carterii Birdw. (Burseraceae) gum resin on adjuvant-induced arthritis in lewis rats. J Ethnopharmacol 101 (2005): 104-109.
- Zheng CJ, Zhao XX, Ai HW, Lin B, Han T, Jiang YP, et al. Therapeutic Effects of Standardized Vitex negundo Seeds Extract on Complete Freund's Adjuvant Induced Arthritis in Rats.Phytomedicine21 (2014): 838-846.
- Umar S, Umar K, Sarwar AHMG, Khan A, Ahmad N, Ahmad S, et al. Boswellia Serrata Extract Attenuates Inflammatory Mediators and Oxidative Stress in Collagen Induced Arthritis.Phytomedicine21 (2014): 847-856.
- Health Canada. Multiple ingredient joint health products, Health Canada (2018).
- Singh S, Khajuria A, Taneja SC, Khajuria RK, Singh J, Qazi GN. Boswellic Acids and Glucosamine Show Synergistic Effect in Preclinical Anti-Inflammatory Study in Rats.Bioorg Med Chem Lett 17 (2007): 3706-3711.
- Moreland LW. Intra-Articular Hyaluronan (Hyaluronic Acid) and Hylans for the Treatment of Osteoarthritis: Mechanisms of Action.Arthritis Res Ther 5 (2003): 54-67.
- Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee - A randomized double blind placebo controlled trial.Phytomedicine10 (2003): 3-7.
- Shara M, Stohs SJ. Efficacy and Safety of White Willow Bark (Salix alba) Extracts.Phytother Res 29 (2015): 1112-1116.
- Aghazadeh-Habashi A, Gilzad Kohan MH, Asghar W, Jamali F. Glucosamine Dose/Concentration-Effect Correlation in the Rat with Adjuvant Arthritis. J Pharm Sci 103 (2014): 760-767.
- Reagan-Shaw S, Nihal M, Ahmad N. Dose Translation from Animal to Human Studies Revisited. FASEB J 22 (2007): 659–661.
- Kozera B, Rapacz M. Reference genes in real-time PCR.J Appl Genetics 54 (2013): 391-406.
- Sharma S, Sahu D, Das HR, Sharma D. Amelioration of Collagen-Induced Arthritis by Salix nigra Bark Extract via Suppression of Pro-Inflammatory Cytokines and Oxidative Stress.Food Chem Toxicol 49 (2011): 3395-3406.
- Hua J, Suguro S, Hirano S, Sakamoto K, Nagaoka I. Preventive actions of a high dose of glucosamine on adjuvant arthritis in rats.Inflamm Res 54 (2005): 127-132.
- Wandel S, Jüni P, Tendal B, Nüesch E, Villiger PM, Welton NJ, et al. Effects of Glucosamine, Chondroitin, or Placebo in Patients with Osteoarthritis of Hip or Knee: Network Meta-Analysis.Brit Med J 341 (2010): c4675.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep11 (2009): 365-370.
- Butler DM, Maini RN, Feldmann M, Brennan FM. Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures: comparison of monoclonal anti TNF-a antibody with interleukin1 receptor antagonist. Eur Cytokine Netw 6 (1995): 225-30.
- Dragos D, Gilca M, Gaman L, Vlad A, Iosif L, Stoian I, et al. Phytomedicine in joint disorders.Nutrients9 (2017): 70.
- Roth A, Mollenhauer J, Wagner A, Fuhrmann R, Straub A, Venbrocks RA, et al. Intra-Articular Injections of High-Molecular-Weight Hyaluronic Acid Have Biphasic Effects on Joint Inflammation and Destruction in Rat Antigen-Induced Arthritis.Arthritis Res Ther 7 (2005): R677-R686.
- Dudler J, Renggli-Zulliger N, Busso N, Lotz M, So A. Effect of interleukin 17 on proteoglycan degradation in murine knee joints.Ann Rheum Dis59 (2000): 529-532.
- Joosten LA, Helsen M, van de Loo FA, van den Berg WB. Anticytokine treatment of established type II collagen–induced arthritis in DBA/1 mice: A comparative study using anti-TNFα, anti–IL-1α/β, and IL-1Ra.Arthritis Rheumatol 39 (1996): 797-809.
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J Immunol 160 (1998): 3513–3521.
- Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Kolls JK, et al. Induction of cartilage damage by overexpression of T cell interleukin-17A in experimental arthritis in mice deficient in interleukin-1. Arthritis Rheumatol 52 (2005): 975–983.
- Koenders MI, Lubberts E, van de Loo FA, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, et al. Interleukin-17 acts independently of TNF-α under arthritic conditions.J Immunol 176 (2006): 6262-6269.
- Campo GM, Avenoso A, Campo S, D'Ascola A, Traina P, Calatroni A. Chondroitin-4-sulphate inhibits NF-kB translocation and caspase activation in collagen-induced arthritis in mice.Osteoarthr Cartilage 16 (2008): 1474-1483.
- Sato H, Takahashi T, Ide H, Fukushima T, Tabata M, Sekine F, et al. Antioxidant Activity of Synovial Fluid, Hyaluronic Acid, and Two Subcomponents of Hyaluronic Acid: Synovial Fluid Scavenging Effect is Enhanced in Rheumatoid Arthritis Patients.Arthritis Rheumatol 31 (1988): 63-71.
- Filippin LI, Vercelino R, Marroni NP, Xavier RM. Redox signalling and the inflammatory response in rheumatoid arthritis.Clin Exp Immunol 152 (2008): 415-422.
- Venkatesha SH, Berman BM, Moudgil KD. Herbal Medicinal Products Target Defined Biochemical and Molecular Mediators of Inflammatory Autoimmune Arthritis.Bioorgan Med Chem 19 (2011): 21-29.
- Xing R, Liu S, Wang L, Cai S, Yu H, Feng J, et al. The Preparation and Antioxidant Activity of Glucosamine Sulfate.Chin J of Oceanol Limn 27 (2009): 283-287.
- Kokkiripati PK, Bhakshu LM, Marri S, Padmasree K, Row AT, Raghavendra AS, et al. Gum resin of Boswellia serrata inhibited human monocytic (THP-1) cell activation and platelet aggregation.J Ethnopharmacol 137 (2011): 893-901.
- Kaur A, Nain P, Nain J. Herbal plants used in treatment of rheumatoid arthritis: a review.Int J Pharm Pharm Sci 4 (2012): 44-57.



 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
