Characterization of GATA3 and Mammaglobin in breast tumors from African American Women
Luisel J. Ricks-Santi1, Kristianna Fredenburg2, Moein Rajaei1, Ashwin Esnakula3, Tammey Naab4, J. Tyson McDonald5, Yasmine Kanaan6
1Department of Pharmacotherapy and Translational Research, College of Pharmacy, University of Florida, Gainesville, FL 32610
2Department of Pathology, College of Medicine, University of Florida, Gainesville, FL 32610
3Department of Pathology, The Ohio State University Wexner Medical Center, Columbus, OH 43210
4Department of Pathology, Howard University Hospital, Washington, DC 20060
5Department of Radiation Medicine, Georgetown University School of Medicine, Washington D.C. 20007
6Department of Microbiology, Howard University College of Medicine, Washington, DC 20060
*Corresponding author: Luisel J. Ricks-Santi, Department of Pharmacotherapy and Translational Research, College of Pharmacy, University of Florida, Gainesville, FL 32610
Received: 22 February 2022; Accepted: 02 March 2022; Published: 17 March 2023
Article Information
Citation: Luisel J. Ricks-Santi, Kristianna Fredenburg, Moein Rajaei, Ashwin Esnakula, Tammey Naab, J. Tyson McDonald, Yasmine Kanaan. Characterization of GATA3 and Mammaglobin in breast tumors from African American Women. Archives of Microbiology and Immunology 7 (2023): 18-28
View / Download Pdf Share at FacebookAbstract
GATA3 and Mammaglobin are often used in the clinic to identify metastases of mammary origin due to their robust and diffuse expression in mammary tissue. However, the expression of these markers has not been well characterized in tumors from African American women. The goal of this study was to characterize and evaluate the expression of GATA3 and mammaglobin in breast tumors from African American women and determine their association with clinicopathological outcomes including breast cancer subtypes. Tissue microarrays (TMAs) were constructed from well preserved, morphologically representative tumors in archived formalin-fixed, paraffin-embedded (FFPE) surgical blocks from 202 patients with primary invasive ductal carcinoma. Mammaglobin and GATA3 expression was assessed using immunohistochemistry (IHC). Univariate analysis was carried out to determine the association between expression of GATA3, mammaglobin and clinicopathological characteristics. Kaplan-Meier estimates of overall survival and disease-free survival were also plotted and a log-rank test performed to compare estimates among groups. GATA3 expression showed statistically significant association with lower grade (p<0.001), ER-positivity (p<0.001), PR-positivity (p<0.001), and the luminal subtype (p<0.001). Mammaglobin expression was also significantly associated with lower grade (p=0.031), ER-positivity (p=0.007), and PR-positivity (p=0.022). There was no association with recurrence-free or overall survival. Our results confirm that GATA3 and mammaglobin demonstrate expression predominantly in luminal breast cancers from African American women. Additional markers with improved specificity and sensitivity are warranted for triple negative breast tumors given the high prevalence in women of African descent.
Keywords
<p>breast cancer, GATA3, Mammaglobin, triple negative breast cancer, immunio histochemistry</p>
Article Details
1. Introduction
Tumors originating from breast tissue can be identified through the use of mammary-specific markers such as mammaglobin and estrogen receptor (ER). The aforementioned markers also assist with the identification of tumors of unknown primaries and metastases [1–6]. Mammaglobin is generally positive in normal breast epithelium, as is the Estrogen Receptor. Given their frequent absence in breast cancer metastases and triple negative breast cancer (TNBC) [2–6], additional markers such as GATA Binding Protein 3 (GATA3) are being used to distinguish tumors originating from the breast. GATA3 is a transcription factor with a role in cell proliferation and differentiation of breast luminal epithelial cells. GATA3 and ER are closely associated and are involved in a positive cross-regulatory loop explaining the positive correlation between GATA3 and ER expression in breast cancers. Notably, GATA3 has been found to be more sensitive in detecting metastatic breast tumors in cytologic specimens [7] and several studies have suggested a prognostic or predictive role for GATA3 expression in breast cancer [8–11].
GATA3 is expressed in breast and urothelial carcinomas while mammaglobin may be expressed in breast, salivary gland, and endometrial carcinomas. However, GATA3 expression varies in breast cancer molecular subtypes. For example, GATA3 expression ranges from 93-100% in Luminal tumors, 59-94% in HER2 overexpressing tumors, and 20-44% in TNBCs [12–17]. It is well known that compared to Caucasian women, African American women are almost twice as likely to be diagnosed with TNBC (ER-negative, PR-negative, and HER2-negative) [18–22]. Therefore, GATA3 may have limited clinical utility in the population. Given the higher frequency of metastatic breast cancer and TNBC in the group, identifying improved diagnostic markers, as well markers that can identify the origin of the tumor is paramount for prognostication, determining treatment options, and deploying treatment options in a timely manner. The goal of this study was to characterize and evaluate the expression of GATA3 and mammaglobin in breast tumors from African American women and determine their association with clinicopathological outcomes including breast cancer subtypes. It is hypothesized that their expression will be reduced in TNBC and will be associated with prognostic indicators such as stage, grade, tumor size, and survival.
2. Material & Methods
2.1 Tissue Samples
All data were anonymized and because of the retrospective nature and use of anonymized specimens and clinical data, this study was exempted by the Howard University Institutional Review Board (IRB-10-MED-24). Along with the exemption, the need for written informed consent was waived by the Howard University Institutional Review Board. We also confirm that all methods were performed in accordance with the relevant guidelines and regulations. We analyzed invasive breast ductal carcinomas (IDCs) from 202 African American women diagnosed and treated at the Howard University Hospital between 2000 and 2010. Demographic and clinical information was obtained through the Howard University Cancer Center Tumor Registry.
2.2 Tissue Arrays
A series of tissue microarrays (TMAs) were constructed (Pantomics, Inc., Richmond, CA) consisting of 10 x 16 arrays of 1.0-mm tissue cores from well preserved, morphologically representative tumors in archived formalin-fixed, paraffin-embedded (FFPE) surgical blocks from 202 patients with primary IDCs. A precision tissue arrayer with two separate core needles for punching the donor and recipient blocks was used. The device also had a micrometer-precise coordinate system for tissue assembly on a multi-tissue block. Two separate tissue cores of IDC represented each surgical case in the TMA series. Each separate tissue core was assigned a unique TMA location number, which was subsequently linked to an Institutional Review Board-approved database containing demographic and clinical data. Using a microtome, 5-µm sections were cut from the TMA blocks and mounted onto Superfrost Plus microscope slides.
2.3 Immunohistochemistry
Mammaglobin and GATA3 expression was assessed using immunohistochemistry (IHC), which was performed on TMA sections. Sections were stained with mouse monoclonal antibodies against GATA3 (L50-823, Biocare Medical, Concord, CA) and mammaglobin (304-1A5, Dako Agilent Technologies, SanTMA Clara, CA). IHC stained sections were scored by two independent observers (TN and AE) blinded to the clinical outcome. The sections were evaluated for the intensity of cytoplasmic (mammaglobin) and nuclear (GATA3) reactivity (0-3) and the percentage of reactive cells; an H-score was derived from the product of these measurements. Cases were categorized as having negative/weak (score <=10) or moderate/strong (score >10) expression for all three markers. The results were entered into a secure research database. Breast subtypes were defined using immunohistochemical expression of estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67%. Luminal A was characterized by strong expression of ER or PR (H-score ≥200) and HER2 negativity. Luminal B was characterized by weaker expression of ER or PR (H-score <200) and HER2-positivity, Ki-67 > 14%, or by triple-positive expression of ER, PR, and HER2. The HER2 subtype was hormone receptor-negative with only HER2 positivity. The triple-negative subtype lacked expression of ER, PR, and HER2.
2.4 Statistical Analysis
All immunohistochemical results were analyzed as categorical/bivariate variables (negative/weak and positive/moderate/strong) as described in the immunohistochemistry section. Clinicopathological variables analyzed for this study include ER status, PR status, HER2 status, molecular subtype, stage, grade, tumor size, overall survival and recurrence-free survival. Univariate analysis was utilized to determine the association between IHC markers and clinicopathological variables such as: ER, PR, HER2, subtype, grade, stage, and size. Chi-square c2 test or Fisher’s exact test, as appropriate, was used to examine the association between categorical variables. ANOVA was also utilized to compare H-scores in breast tumor subtypes. Kaplan-Meier estimates of overall survival, and disease-free survival, were plotted and a log-rank test performed to compare estimates among groups. All analyses were carried out using the SPSS 28 statistical program (SPSS Inc., Chicago, IL).
3. Results
3.1 Characteristics of the Study Population
Clinical and pathological characteristics of the study population are summarized in Table 1 and are presented in supplemental file 1. GATA3 and mammaglobin IHC results were available for 189 and 183 patients, respectively. Only patients with IHC results underwent further analysis. Among 189 female patients with invasive ductal carcinomas diagnosed from 2000 to 2010, the luminal A subtype was most frequent constituting 43.8% of the study population. TNBC was the second most common subtype representing 33.3% of the total number and were purposely overrepresented to improve the study of TNBC in African American women. It is noteworthy that 75% of the TNBCs demonstrated basal-like phenotype, which was determined by cytokeratin 5/6 immunohistochemistry. More than two-thirds of the tumors were stage I and II; however, the tumors tended to be of high grade, with Grade 3 tumors comprising 67.3% of the total in the study population. A summary of the patient clinicopathological features, molecular profiles and IHC expression status can be found in figure 1 which shows expression of GATA3 and mammaglobin primarily in luminal A and luminal B tumors.
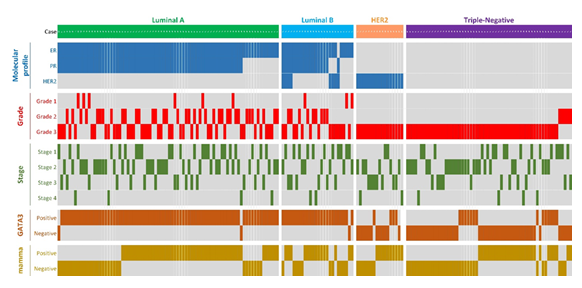
Figure 1: Summary of clinicopathological features, molecular profiles and IHC expression status in each patient
Table 1: Clinical and pathological characteristics of study population
|
Parameter |
Category |
Frequency |
% |
|
Age (years) |
|||
|
<50 |
57 |
28.2 |
|
|
≥50 |
145 |
71.8 |
|
|
ER status (n=201) |
|||
|
Positive |
116 |
57.7 |
|
|
Negative |
85 |
42.3 |
|
|
PR status |
|||
|
Positive |
90 |
47.1 |
|
|
Negative |
101 |
52.9 |
|
|
HER2 status |
|||
|
Positive |
28 |
13.9 |
|
|
Negative |
173 |
86.1 |
|
|
Subtype* |
|||
|
Luminal A |
88 |
43.8 |
|
|
Luminal B |
29 |
14.4 |
|
|
Her2 |
17 |
8.5 |
|
|
Triple-negative |
67 |
33.3 |
|
|
Pathologic stage (n=197) |
|||
|
Stage 1 |
62 |
31.5 |
|
|
Stage 2 |
82 |
41.6 |
|
|
Stage 3 |
41 |
20.8 |
|
|
Stage 4 |
12 |
6.1 |
|
|
Grade |
|||
|
Grade I |
9 |
4.5 |
|
|
Grade II |
57 |
28.2 |
|
|
Grade III |
136 |
67.3 |
|
|
Recurrence |
|||
|
None |
136 |
67.3 |
|
|
Loco-regional |
11 |
5.4 |
|
|
Distant |
21 |
10.4 |
|
|
Never disease-free |
18 |
8.9 |
|
|
Unknown |
16 |
7.9 |
|
ER: estrogen receptor; PR: progesterone receptor; HER2: Human Epidermal Growth Factor Receptor 2, Luminal A: ER+ or PR+, HER2-; luminal B: ER+ or PR+, HER2+; triple-negative: ER-, PR-, HER2-; HER2+: ER-, PR-, HER2+
3.2 GATA3
Figure 2 demonstrates GATA3 staining intensities where 2a, 2c, 2e, and 2g demonstrate no, weak, moderate, and strong immunoreactivity. 2a shows a TNBC with no GATA3 immunoreactivity (Reactivity score=0, H-score =0). 2c shows weak GATA3 immunoreactivity (Reactivity score = 1, H-score=60) in a luminal A patient. 2e shows moderate diffuse immunoreactivity in a luminal B patient (Reactivity score = 2, H-score=270) and 2g shows strong diffuse immunoreactivity in a luminal A patient (Reactivity score = 3, H-score=300). Figure 3 demonstrates mean H-scores by subtype. GATA3 was expressed in 67.7% (128/189) of all tumors. However, GATA3 had the highest H-score expression in Luminal A (mean=218.88 ± 77.34 SD) and Luminal B (mean=212.12 ± 75.62 SD) tumors, whereas mean GATA3 H-scores were lowest in HER2 (mean=33.82 ± 71.71 SD) overexpressing and TNBCs (mean=33.20 ± 68.56 SD) (ANOVA p<0.001).
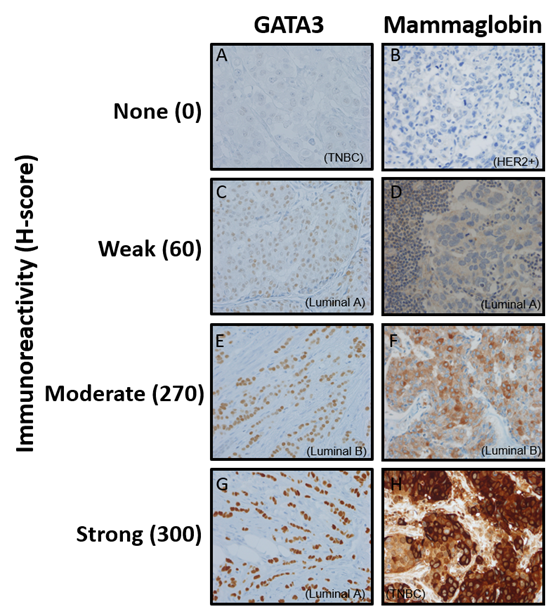
Figure 2: Representative images of immunohistochemical staining of GATA3 and mammaglobin
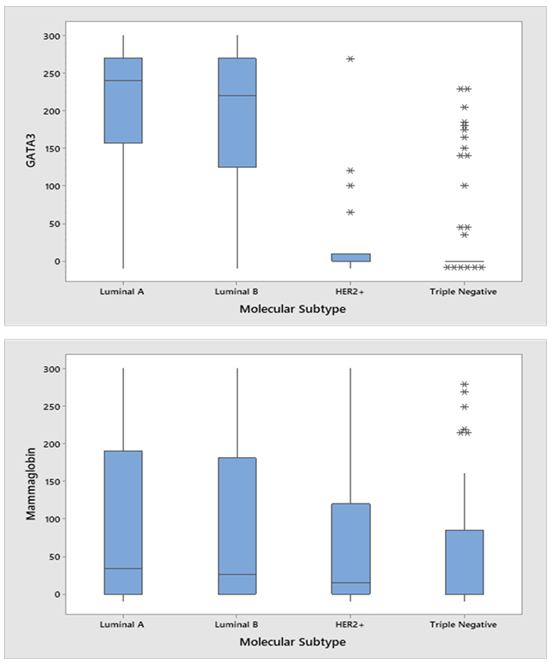
Figure 3: H-scores for GATA3 (A) and Mammaglobin (B) by molecular subtype
Following the dichotomization of H-scores, the frequency of positive and negative expression of GATA3 was determined for clinicopathological characteristics. GATA3 expression showed statistically significant association with a lower grade (p<0.001), ER positivity (p<0.001), or PR positivity (p<0.001) (Table 2). GATA3 was expressed in 97% and 98% and 58% of ER, PR, and HER2 positive tumors, respectively (Table 2). Among the molecular breast cancer subtypes, GATA3 was expressed in 98%, 96%, 35%, and 23% of Luminal A, Luminal B, HER2 overexpressing and TNBC subtypes, respectively, and was found to be associated with the luminal subtype (p<0.001).
Table 2: Summary of staining results for GATA3 and mammaglobin and their association with molecular and clinicopathological features
*Missing Data not included
IHC expression of GATA3 was not associated with overall survival or disease-free survival (Figure 4). However, after grouping all luminal subtypes with GATA3 positive tumors and comparing them non-luminal and GATA3 negative tumors, there was a marginal significance with overall survival (4A, log rank p=0.073); mean months survival for the luminal and GATA3 positives was 142.55 months compared to 89.15 months. However, there was no association with recurrence-free survival (4B, log rank p=0.19).
3.3 Mammaglobin
Figure 2 demonstrates mammaglobin staining intensities where 2b, 2d, 2e, and 2h demonstrate no, weak, moderate, and strong immunoreactivity. 2b shows a HER2 overexpressing subtype patients with no mammaglobin immunoreactivity (Reactivity score=0, H-score =0). 2d shows weak mammaglobin immunoreactivity (Reactivity score = 1, H-score=60) in a luminal A patient. 2f shows moderate immunoreactivity in a luminal B patient (Reactivity score = 2, H-score=270) and 2h shows strong immunoreactivity in a TNBC patient (Reactivity score = 3, H-score=300). Mammaglobin was expressed in 56.8% (104/183). Mean mammaglobin H-scores were 102.06 (±110.62 SD), 78.92 (±106.55 SD), 69.82 (± 101.71), and 53.53 (±78.40 SD) for the luminal A, luminal B, HER2+, and TNBC subtypes, respectively (ANOVA p=0.23).
Mammaglobin expression was also significantly associated with a lower grade (p=0.031), ER positivity (p=0.07), or PR positivity (p=0.022). The frequency of positive and negative expression of mammaglobin was determined for clinicopathological characteristics. Mammaglobin was expressed in 62%, 64%, and 54% of ER, PR, and HER2 positive tumors, respectively (Table 2). Positive mammaglobin expression was found in 63%, 58%, 59%, and 48% of Luminal A, Luminal B, HER2 overexpressing and TNBC subtypes, respectively, and was found to be associated with the non-TNBC subtype (p<0.043). IHC expression of mammaglobin was not associated with overall survival or disease-free survival even after grouping the luminal subtypes with mammaglobin positive tumors and comparing to non-luminals= subtypes with negative mammaglobin expression (Figure 4C, D).
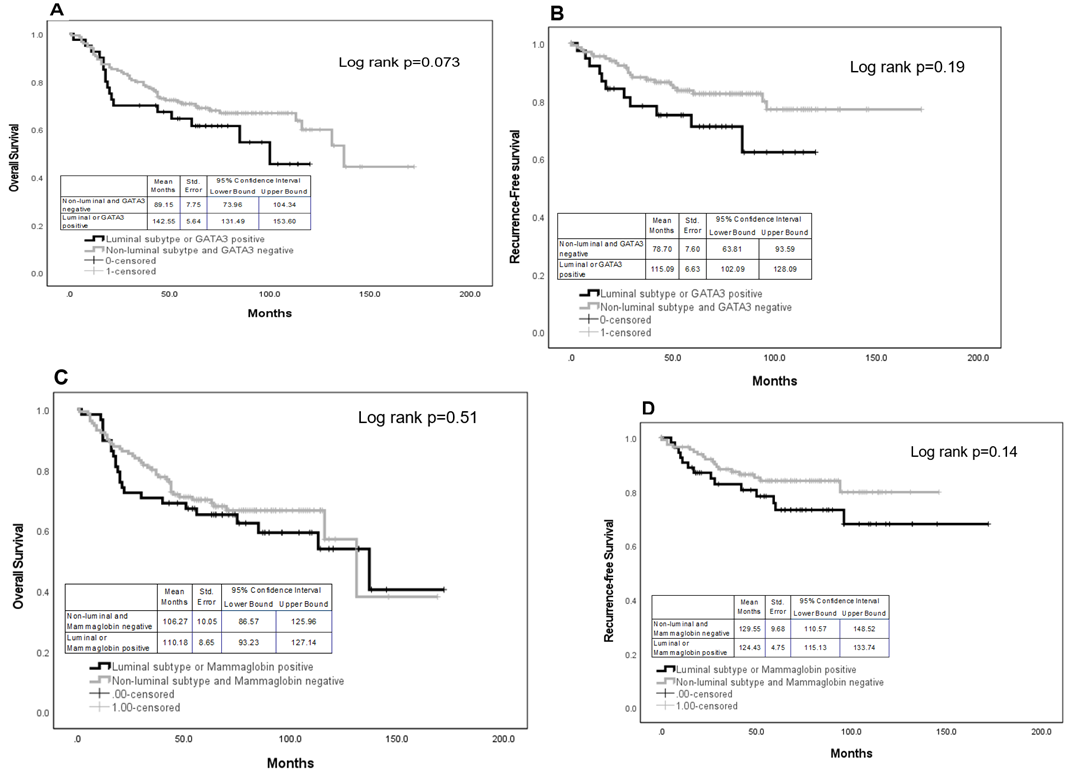
Figure 4: Overall and recurrence-free survival for GATA3 (A and B) and mammaglobin (C and D), respectively
3.4 GATA3 and Mammaglobin co-expression
There was a significant correlation between GATA3 and mammaglobin expression (Pearson correlation= 0.17; p=0.022). Therefore, the sensitivity of GATA3 and mammaglobin by subtype were analyzed alone and in combination. GATA3 could detect 97% of luminal tumors and 23% of TNBCs (Table 3). However, mammaglobin had 64% sensitivity in luminal tumors. Co-expression of both markers decreased the overall detection sensitivity of luminal tumors from 97% to 86%. The expression of at least one marker was found in 43% (34/80) of TNBCs. However, co-expression of GATA3 and mammaglobin reduced the sensitivity of detecting TNBCs to 7%. In fact, 76% of TNBCs were GATA3 and mammaglobin negative.
Table 3: Combined expression of GATA3 and mammaglobin in luminal and triple negative breast tumors from African American women
|
Luminal Tumors |
Triple negative tumors |
|||||
|
+ |
- |
Sensitivity |
+ |
- |
Sensitivity |
|
|
GATA3 positive |
108 |
3 |
97% |
14 |
48 |
23% |
|
Mammaglobin positive |
70 |
40 |
64% |
30 |
33 |
48% |
|
GATA3 negative and mammaglobin negative |
1 |
28 |
3% |
22 |
7 |
76% |
|
GATA3 positive and mammaglobin positive |
63 |
10 |
86% |
5 |
68 |
7% |
|
Either GATA3 or mammaglobin positive |
42 |
39 |
52% |
34 |
46 |
43% |
4. Discussion
The objective of this study was to characterize and evaluate the expression of GATA3 and mammaglobin in breast tumors from African American women and to determine their association with clinicopathological outcomes including breast cancer subtypes. GATA3 and mammaglobin are currently used to identify tumors of unknown primaries and metastases [1–6]. Our results confirm that GATA3 and mammaglobin demonstrate expression predominantly in luminal breast cancers, but that mammaglobin is superior to GATA3 when utilized to identify triple negative tumors in African American women, as GATA3 was found to be less frequently expressed in TNBC cases (23%) compared to mammaglobin (47%). The high frequency of GATA3 positivity in luminal tumors aligns with its pivotal role in the differentiation of luminal progenitors to mature luminal cells [23]. Along with FOXA1 and ER-alpha, they form a hormone responsive signaling network in the normal breast that maintain epithelial differentiation by activating genes responsible for luminal features while blocking genes associated dedifferentiation or with basal or mesenchymal phenotypes [24]. GATA’s estrogen dependence greatly hinders its ability to serve as a biomarker for hormone-independent molecular breast cancer subtypes such as TNBC. Moreover, GATA3 has been found to be altered in approximately 10% of breast tumors [25]. The lack of expression may also be influenced by mutations in the gene which have been found to be overrepresented in women of African descent compared to white women with European ancestry [26]. Nakashatri et al. suggest that hormonal- and differentiation-signaling networks show genetic ancestry-dependent differences and it is likely that ERa:GATA3- dependent transcriptional program is more active in the normal breast of whites compared with African American women [27]. Gardner et al. [28] also showed that luminal differentiators are differentially expressed in African American women potentially contributing to more triple negative tumors.
Mammaglobin has also been previously utilized as immunohistochemical markers for identifying metastatic breast tumors, with reported overall sensitivities ranging from 50% to 87% and 10% to 79%, respectively [29]. While others have found greater sensitivities using GATA3, this study demonstrated mammaglobin’s increased ability to identify non-luminal tumors from 23% to 48%, which is much higher than previously reported by Liu et al. [30] (35% of ER negative tumors), Ordonez and Sahin [31] (18% of TNBCs), and Krings et al. [31] (26% of TNBCs). Still, given that either GATA3 or mammaglobin fail to identify more than 50% of TNBCs these markers should be supplemented with markers specific for TNBC, such as Sry-related HMG box (SOX) 10, which is found in 40%-70% of TNBCs and appears to be expressed in tumors that are negative for GATA3 [32-34]. More recently, it was demonstrated that adding SOX10 improved the sensitivity of the markers in metastatic breast cancer (sensitivity = 0.89), metastatic TNBC (0.78), and primary TNBC (0.78) [35]. Another study found that 95% of metastatic breast tumors were positive for GATA3 or SOX10 confirming SOX10’s role in identifying TNBC tumors [36]. Another interesting finding in our study is that GATA3 and mammaglobin lacked prognostic value although an association was demonstrated by others [11, 37–39]. While the markers were associated with grade, there was no association with stage, tumor size (not shown), or survival. Identifying prognostic markers still remains a priority in the field of breast oncology.
The development of a TMA made up of tumors from African American women is a strength in this study. Clinical data was abstracted from the tumor registry and survival data acquired from the social security death index. Ki67 IHC was also performed to differentiate the luminal A tumors from the luminal B tumors. The overrepresentation of triple negative tumors also aids in its improved understanding as African American women with TNBC continue to have worse clinical outcomes than women of European descent even after adjusting for disparities in access to health-care treatment, comorbidities and other socioeconomic factors, such as income [40, 41]. The use of the TMA in this study allowed assessment of the expression of proposed diagnostic and prognostic markers and allowed for the improved characterization of tumors from African American women. It is paramount that as clinical markers are developed, that there is clinical validity and utility across groups or that the limitations are acknowledged when used in clinical practice. In conclusion, GATA3 and mammaglobin still have limited utility in detecting non-luminal tumors and should be potentially used together to identify tumors that originate in the breast.
Ethics approval and consent to participate
All data were anonymized and because of the retrospective nature and use of anonymized specimens and clinical data, this study was exempted by the Howard University Institutional Review Board (IRB-10-MED-24). Along with the exemption, the need for written informed consent was waived by the Howard University Institutional Review Board. We also confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Availability of data and materials
The data generated and analyzed during this study are included in this published article as supplemental file 1.
Competing interests
The authors declare no conflict of interest.
Funding
R15CA239100, Functional analysis of genetic variants in African Americans with Breast Cancer.
Authors' contributions
Luisel J. Ricks-Santi, PhD – conceive study, data analysis, wrote manuscript
Kristianna Fredenburg, MD, PhD - data analysis
Moein Rajaei, PhD - data analysis
Ashwin Esnakula, MBBS, MS - conceive study, data collection
Tammey Naab, MD - conceive study, data collection
Tyson McDonald, PhD- data analysis
Yasmine Kanaan, PhD - conceive study, data collection
References
- Fleming TP, Watson MA. Mammaglobin, a breast-specific gene, and its utility as a marker for breast cancer. Annals of the New York Academy of Sciences 923 (2000): 78-9.
- Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. American journal of clinical pathology 127 (2007): 103-13.
- Chia SY, Thike AA, Cheok PY, Tan PH. Utility of mammaglobin and gross cystic disease fluid protein-15 (GCDFP-15) in confirming a breast origin for recurrent tumors. The Breast 19 (2010): 355-9.
- Rollins-Raval M, Chivukula M, Tseng GC, Jukic D, Dabbs DJ. An immunohistochemical panel to differentiate metastatic breast carcinoma to skin from primary sweat gland carcinomas with a review of the literature. Archives of pathology & laboratory medicine 135 (2011): 975-83.
- Lewis GH, Subhawong AP, Nassar H, Vang R, Illei PB, Park BH, et al. Relationship between molecular subtype of invasive breast carcinoma and expression of gross cystic disease fluid protein 15 and mammaglobin. American journal of clinical pathology 135 (2011): 587-91.
- Huo L, Zhang J, Gilcrease MZ, Gong Y, Wu Y, Zhang H, et al. Gross cystic disease fluid protein-15 and mammaglobin A expression determined by immunohistochemistry is of limited utility in triple-negative breast cancer. Histopathology 62 (2013): 267-74.
- Dyhdalo KS, Booth CN, Brainard JA, Croyle MC, Kolosiwsky AM, Goyal A, et al. Utility of GATA3, mammaglobin, GCDFP-15, and ER in the detection of intrathoracic metastatic breast carcinoma. Journal of the American Society of Cytopathology 4 (2015): 218-24.
- Tominaga N, Naoi Y, Shimazu K, Nakayama T, Maruyama N, Shimomura A, et al. Clinicopathological analysis of GATA3-positive breast cancers with special reference to response to neoadjuvant chemotherapy. Annals of oncology 23 (2012): 3051-7.
- Albergaria A, Paredes J, Sousa B, Milanezi F, Carneiro V, Bastos J, Costa S, Vieira D, Lopes N, Lam EW, Lunet N. Expression of FOXA1 and GATA-3 in breast cancer: the prognostic significance in hormone receptor-negative tumours. Breast cancer research 11 (2009): 1-5.
- Parikh P, Palazzo JP, Rose LJ, Daskalakis C, Weigel RJ. GATA-3 expression as a predictor of hormone response in breast cancer. Journal of the American College of Surgeons 200 (2005): 705-10.
- Mehra R, Varambally S, Ding L, Shen R, Sabel MS, Ghosh D, Chinnaiyan AM, Kleer CG. Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer research 65 (2005): 11259-64.
- Shaoxian T, Baohua Y, Xiaoli X, Yufan C, Xiaoyu T, Hongfen L, et al. Characterisation of GATA3 expression in invasive breast cancer: differences in histological subtypes and immunohistochemically defined molecular subtypes. Journal of Clinical Pathology 70 (2017): 926-34.
- Huo L, Gong Y, Guo M, Gilcrease MZ, Wu Y, Zhang H, et al. GATA-binding protein 3 enhances the utility of gross cystic disease fluid protein-15 and mammaglobin A in triple-negative breast cancer by immunohistochemistry. Histopathology 67 (2015): 245-54.
- Yang Y, Lu S, Zeng W, Xie S, Xiao S. GATA3 expression in clinically useful groups of breast carcinoma: a comparison with GCDFP15 and mammaglobin for identifying paired primary and metastatic tumors. Annals of diagnostic pathology 26 (2017): 1-5.
- Deftereos G, Sanguino Ramirez AM, Silverman JF, Krishnamurti U. GATA3 immunohistochemistry expression in histologic subtypes of primary breast carcinoma and metastatic breast carcinoma cytology. The American Journal of Surgical Pathology 39 (2015): 1282-9.
- Braxton DR, Cohen C, Siddiqui MT. Utility of GATA3 immunohistochemistry for diagnosis of metastatic breast carcinoma in cytology specimens. Diagnostic Cytopathology 43 (2015): 271-7.
- Ni YB, Tsang J, Shao MM, Chan SK, Cheung SY, Tong J, et al. GATA-3 is superior to GCDFP-15 and mammaglobin to identify primary and metastatic breast cancer. Breast cancer research and treatment 69 (2018): 25-32.
- Kwan ML, Kushi LH, Weltzien E, Maring B, Kutner SE, Fulton RS, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Research 11 (2009): 1-3.
- Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast cancer research and treatment 113 (2009): 357-70.
- Sineshaw HM, Gaudet M, Ward EM, Flanders WD, Desantis C, Lin CC, et al. Association of race/ethnicity, socioeconomic status, and breast cancer subtypes in the National Cancer Data Base (2010–2011). Breast cancer research and treatment 145 (2014): 753-63.
- Plasilova ML, Hayse B, Killelea BK, Horowitz NR, Chagpar AB, Lannin DR. Features of triple-negative breast cancer: Analysis of 38,813 cases from the national cancer database. Medicine 95 (2016).
- Ricks-Santi LJ, Barley B, Winchester D, Sultan D, McDonald J, Kanaan Y, et al. Affluence does not influence breast cancer outcomes in African American women. Journal of Health Care for the Poor and Underserved 29 (2018): 509-29.
- Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127 (2006): 1041-55.
- Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M. Positive cross-regulatory loop ties GATA-3 to estrogen receptor α expression in breast cancer. Cancer research 67 (2007): 6477-83.
- Takaku M, Grimm SA, Roberts JD, Chrysovergis K, Bennett BD, Myers P, et al. GATA3 zinc finger 2 mutations reprogram the breast cancer transcriptional network. Nature communications 9 (2018): 1-4.
- Pitt JJ, Riester M, Zheng Y, Yoshimatsu TF, Sanni A, Oluwasola O, et al. Characterization of Nigerian breast cancer reveals prevalent homologous recombination deficiency and aggressive molecular features. Nature communications 9 (2018): 1-2.
- Nakshatri H, Kumar B, Burney HN, Cox ML, Jacobsen M, Sandusky GE, et al. Genetic Ancestry–dependent Differences in Breast Cancer–induced Field Defects in the Tumor-adjacent Normal BreastEarly Markers of Breast Cancer. Clinical Cancer Research 25 (2019): 2848-59.
- Byun JS, Singhal SK, Park S, Yi DI, Yan T, Caban A, et al. Racial Differences in the Association Between Luminal Master Regulator Gene Expression Levels and Breast Cancer SurvivalRacial Differences Linked to Breast Cancer Master Regulators. Clinical Cancer Research 26 (2020): 1905-14.
- Ordóñez NG. Value of GATA3 immunostaining in tumor diagnosis: a review. Advances in anatomic pathology 20 (2013): 352-60.
- Liu H, Shi J, Prichard JW, Gong Y, Lin F. Immunohistochemical evaluation of GATA-3 expression in ER-negative breast carcinomas. American journal of clinical pathology 141 (2014): 648-55.
- Ordóñez NG, Sahin AA. Diagnostic utility of immunohistochemistry in distinguishing between epithelioid pleural mesotheliomas and breast carcinomas: a comparative study. Human pathology 45 (2014): 1529-40.
- Sejben A, Vörös A, Golan A, Zombori T, Cserni G. The added value of SOX10 immunohistochemistry to other breast markers in identifying cytokeratin 5-positive triple negative breast cancers as of mammary origin. Pathobiology 88 (2021): 228-33.
- Chiu K, Ionescu DN, Hayes M. SOX10 expression in mammary invasive ductal carcinomas and benign breast tissue. Virchows Archiv 474 (2019): 667-72.
- Kriegsmann K, Flechtenmacher C, Heil J, Kriegsmann J, Mechtersheimer G, Aulmann S, et al. Immunohistological expression of SOX-10 in triple-negative breast cancer: A descriptive analysis of 113 samples. International journal of molecular sciences 21 (2020): 6407.
- Na K, Woo HY, Do SI, Kim SW. Combination of GATA3, SOX-10, and PAX8 in a Comprehensive Panel to Diagnose Breast Cancer Metastases. in vivo 36 (2022): 473-81.
- Tozbikian GH, Zynger DL. A combination of GATA3 and SOX10 is useful for the diagnosis of metastatic triple-negative breast cancer. Human pathology 85 (2019): 221-7.
- Jacquemier J, Charafe-Jauffret E, Monville F, Esterni B, Extra JM, Houvenaeghel G, et al. Association of GATA3, P53, Ki67 status and vascular peritumoral invasion are strongly prognostic in luminal breast cancer. Breast Cancer Research 11 (2009): 1-1.
- Ciocca V, Daskalakis C, Ciocca RM, Ruiz-Orrico A, Palazzo JP. The significance of GATA3 expression in breast cancer: a 10-year follow-up study. Human pathology 40 (2009): 489-95.
- Zehentner BK, Dillon DC, Jiang Y, Xu J, Bennington A, Molesh DA, et al. Application of a multigene reverse transcription-PCR assay for detection of mammaglobin and complementary transcribed genes in breast cancer lymph nodes. Clinical chemistry 48 (2002): 1225-31.
- Dietze EC, Sistrunk C, Miranda-Carboni G, O'regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nature Reviews Cancer 15 (2015): 248-54.
- Cho B, Han Y, Lian M, Colditz GA, Weber JD, Ma C, et al. Evaluation of racial/ethnic differences in treatment and mortality among women with triple-negative breast cancer. JAMA oncology 7 (2021): 1016-23.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
