Antibiotic Resistance and Associated Factors with Purulent Skin Infections Due to Staphylococcus Aureus
Lalaina Mamenosoa Rakotondraoelina1, Zafindrasoa Domoina Rakotovao-Ravahatra2, Solofo Sarah Rafaramalala3, Andriamiadana Luc Rakotovao4, Andry Rasamindrakotroka5
1Biologist doctor, Laboratory of Anosiala University Hospital Antananarivo, Madagascar
2Biologist doctor, Laboratory of Joseph Raseta Befelatanana University Hospital Antananarivo, Madagascar
3Biologist doctor, Malagasy Medical Analysis Laboratory Androhibe Antananarivo, Madagascar
4Professor in biological hematology, Medical Biology Department of the Faculty of Medicine, University of Antananarivo, Madagascar
5Professor in immunology, Medical Biology Department of the Faculty of Medicine, University of Antananarivo, Madagascar
*Corresponding author: Zafindrasoa Domoina Rakotovao-Ravahatra, Biologist doctor, Laboratory of Joseph Raseta Befelatanana University Hospital Antananarivo, Madagascar.
Received: 09 December 2022; Accepted: 15 December 2022; Published: 24 December 2022
Article Information
Citation: Lalaina Mamenosoa Rakotondraoelina, Zafindrasoa Domoina Rakotovao-Ravahatra, Solofo Sarah Rafaramalala, Andriamiadana Luc Rakotovao, Andry Rasamindrakotroka. Antibiotic Resistance and Associated Factors with Purulent Skin Infections Due to Staphylococcus Aureus. Archives of Microbiology and Immunology 6 (2022): 289-293.
View / Download Pdf Share at FacebookAbstract
Background: Purulent skin infections due to Staphylococcus aureus are common in hospitals and are frequently due to secondary bacterial infections. The aims of this study were to evaluate antibiotic resistance and to describe the factors associated with Staphylococcus aureus purulent skin infections.
Methods: This is a descriptive and cross-sectional study of 179 results of cytobacteriological examinations of pus over a period of 18 months, from January 2021 to June 2022, at the laboratory of the University Hospital of Befelatanana.
Results: Among the 179 cytobacteriological examinations of pus, 131 cases were positive showing a hospital prevalence of 73.2%. Among the germs identified, 46 cases (25.7%) were represented by isolates of Staphylococcus aureus. Regarding the associated factors, subjects aged 60 and over (30%) (p=0.32; NS), women (32.8%) (p=0.11; NS) and patients hospitalized in Internal Medicine departments (39.3%)(p=0 .02) were the most affected by Staphylococcus aureus purulent skin infections. Concerning the results of the antibiograms, the resistances of the isolates of Staphylococcus aureus to Penicillin G (97.8%), to Doxycycline (56.5%) and to Cotrimoxazole (41.3%) were the highest. Methicillin-resistant Staphylococcus aureus isolates were rare (4.3%) and all isolates were susceptible to Vancomycin.
Conclusion: The prevention of cutaneous suppuration in vulnerable people is very important in hospital departments as well as hospital hygiene measures to fight against nosocomial infections. Similarly, it is necessary to limit the use of broad-spectrum antibiotics in hospital departments to control the evolution of Staphylococus aureus isolates towards increasing antibiotic resistance.
Keywords
<p>antibiotic resistance, self-medication, staphylococcus, suppuration</p>
Article Details
1. Introduction
Skin and skin structure infections are among the most common infectious diseases, with an estimated incidence of almost 500 cases per 10 000 person-years [1]. Skin and skin structure infections vary in severity and depth, from mild infections that can be treated with topical antibiotics to life-threatening necrotizing fasciitis requiring surgical intervention. In an immunocompetent host, the vast majority of these infections are due to Staphylococcus aureus and β-hemolytic streptococci, primarily Streptococcus pyogenes [1–2]. Staphylococcus aureus is the predominant pathogen identified in skin infection due to its greater proclivity, compared with streptococci, to form abscesses, which also increases the probability of obtaining a positive culture [1, 3]. The treatment of these purulent skin infections due to Staphylococcus aureus is sometimes difficult because of the presence of multi-resistant isolates such as Methicillin-Resistant Staphylococcus Aureus (MRSA) isolates. Thus, we carried out this study to better understand the current situation of Staphylococcus aureus skin infections in Madagascar. The aims of this study were to evaluate antibiotic resistance and to describe the factors associated with Staphylococcus aureus purulent skin infections.
2. Materials and Methods
2.1 Study design
This is a descriptive and cross-sectional study of 179 results of cytobacteriological examinations of pus over a period of 18 months, from January 2021 to June 2022 at the University Hospital Center Joseph Raseta Befelatanana (CHUJRB) in Antananarivo, Madagascar.
2.2 Study setting
This study was carried out in the laboratory of the CHUJRB which is located in Antananarivo city in the Analamanga, region in Madagascar. This laboratory is a general-purpose medical analysis laboratory open 24 hours a day, 7 days a week. This laboratory receives biological samples from patients hospitalized in Antananarivo hospitals or outpatients. The biological analyzes carried out in this laboratory are represented by hematological, biochemical, immunological, virological, parasitological and bacteriological analyses.
2.3 Participants
All bacteria identified by the cytobacteriological examinations of pus during the study period were include in this study. All bacteria identified in other samples were exclude from the study. Similarly, all bacteria identified in cytobacteriological examinations of pus outside the study period were also exclude from the study.
2.4 Variables
The dependent variable was represented by the positivity of the CBEU showing Staphylococcus aureus. The independent variables were represented by the age, the gender, the clinical information, the departments and the results of antibiogram of each Staphylococcus aureus isolates.
2.5 Procedures in laboratory
The pus sample is collected in a swab and quickly transported to the laboratory at room temperature. Arrived at the laboratory, the swab is inoculated into 4 types of culture media (brain heart broth, blood agar, chocolate agar and uriselect). After culture, the technician performs direct examinations (between slide and coverslip and after Gram staining) to identify germs or other elements. The next day, the technician identifies the different bacterial colonies that are present on the culture media by carrying out different bacterial identification tests. Regarding Staphylococus aureus isolates, they are Gram-positive cocci, arranged in clusters, catalase positive and coagulase positive. After identifying the Staphylococcus aureus isolates, the technician performs the antibiogram. The antibiotic discs tested were glycopeptides (vancomycin), aminoglycosides (gentamycin, tobramycin), sulphonamides (cotrimoxazole), cyclins (tetracyclines), quinolones (ciprofloxacin), lincosamides (lincomycin), macrolides (erythromycin), penicillins (penicillin G) and methicillin (oxacillin). The interpretative reading of the results of the antibiograms made it possible to identify the resistance and the sensitivities of the PGCs to the discs of antibiotics tested.
2.6 Data collection
Data are collected from register notebooks and antibiogram result sheets.
2.7 Data analysis
The entry and processing of data were performed on Epi-info 3.5.2 software. Proportions are presented as numbers (percent) and were compared using the chi-square test. Results were considered significant if p was 0.05 or less, with a 95% confidence interval.
2.8 Ethical considerations
The authorization of this study by an ethics committee was not necessary because we analyzed isolates of bacteria. Nevertheless, the authorization of the director of the establishment was obtained before the data were collected in the registers. Likewise, the seizure was done anonymously to maintain confidentiality.
3. Results
3.1 Hospital prevalence of bacterial purulent skin infections
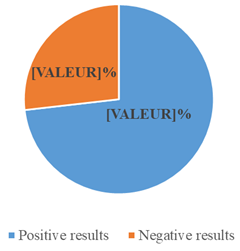
Among the 179 cytobacteriological examinations of pus, 131 cases were positive showing a hospital prevalence of 73.2% (figure 1). Among the germs identified, 46 cases (25.7%) were represented by isolates of Staphylococcus aureus.
3.2 Factors associated with Staphylococcus aureus purulent skin infections
Regarding the associated factors, subjects aged 60 and over (30%) (p=0.32; NS), women (32.8%) (p=0.11; NS) and patients hospitalized in Internal Medicine departments (39.3%)(p=0 .02) were the most affected by Staphylococcus aureus purulent skin infections (table 1).
Table 1: Factors associated with Staphylococcus aureus purulent skin infections
|
Associated factors |
Negative results or other infections (N=133) |
Staphylococcus aureus purulent skin infections (N=46) |
Total (N=179) |
p-value |
||
|
n |
% |
n |
% |
|||
|
Age (years) |
||||||
|
20-39 |
30 |
71.4 |
12 |
28.6 |
42 |
0.32 (NS*) |
|
40-59 |
40 |
71.4 |
16 |
28.6 |
56 |
|
|
≥60 |
28 |
70 |
12 |
30 |
40 |
|
|
<20 |
35 |
85.4 |
6 |
14.6 |
41 |
|
|
Gender |
||||||
|
Female |
41 |
67.2 |
20 |
32.8 |
61 |
0.11 (NS) |
|
Male |
92 |
78 |
26 |
22 |
118 |
|
|
Departments |
||||||
|
Other departments |
36 |
78.3 |
10 |
21.7 |
46 |
0.02 |
|
Surgery |
17 |
94.4 |
1 |
5.6 |
18 |
|
|
Internal Medicine |
37 |
60.7 |
24 |
39.3 |
61 |
|
|
Traumatology |
12 |
85.7 |
2 |
14.3 |
14 |
|
|
Emergency |
31 |
77.5 |
9 |
22.5 |
40 |
|
3.3 Antibiotic resistance of Staphylococcus aureus isolates responsible for purulent skin infections
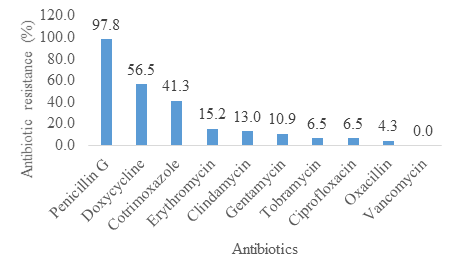
Concerning the results of the antibiograms, the resistances of the isolates of Staphylococcus aureus to Penicillin G (97.8%), to Doxycycline (56.5%) and to Cotrimoxazole (41.3%) were the highest. MRSA isolates were rare (4.3%) and all isolates were susceptible to Vancomycin (figure2).
4. Discussion
4.1 Hospital prevalence of bacterial purulent skin infections
In this study, 73.2% of cytobacteriological examination of pus were positive showing bacterial infections. Other studies have also shown the high frequency of bacterial infections in suppuration [4-5]. Indeed, other causes of skin suppuration are rare, such as parasitic, viral and mycological infections, traumatic causes or cancers. In this study, 25.7% of cases were represented by isolates of Staphylococcus aureus. Indeed, the literature confirms that Staphylococcus aureus is the most common bacteria causing purulent skin and soft tissue infections [6]. Other studies have also found that the most commonly implicated pathogens in purulent skin infections include gram-positive bacteria, mainly Staphylococcu aureus, and coagulase-negative staphylococci (CoNS) [7-8].
According to the literature, the isolates of CoNS, that constitute the skin microbiota, play a pivotal role in the orchestration of cutaneous homeostasis and immune competence. This balance can be promptly offset by the expansion of the opportunistic pathogen Staphylococcus aureus, which is responsible for the majority of bacterial skin infections. Staphylococcus aureus carriage is also known to be a precondition for its transmission and pathogenesis. Recent reports suggest that skin-dwelling CoNS can prime the skin immune system to limit the colonization potential of invaders, and they can directly compete through production of antimicrobial molecules or through signaling antagonism [9]. Nevertheless, the immune deficiency or the weakening of the host will cause the multiplication of isolates of Staphylococcus aureus resulting in a purulent skin infection despite of presence of CoNS isolates.
4.2 Factors associated with Staphylococcus aureus skin infections
Regarding the associated factors, subjects aged 60 and over women were the most affected by Staphylococcus aureus purulent skin infections but no significant difference. Nevertheless, these subjects are part of the vulnerable personnel. Elderly people have many age-related diseases (arterial hypertension, diabetes, etc.) which make them more fragile. Likewise, women are more fragile because of their physiological state such as pregnancy, menstruation or breastfeeding. So, the immune deficiency of these subjects cause the multiplication of isolates of Staphylococcus aureus resulting in a purulent skin infection [9].
Regarding the departments, patients hospitalized in Internal Medicine departments were the most affected by Staphylococcus aureus purulent skin infections with significant difference. Indeed, the majority of patients hospitalized in the infectious diseases department show signs of infections such as fever, hyperleukocytosis, explaining the increase in patients with purulent skin infections in this department. Among these services, the dermatology service is the most concerned because dermatological diseases are sometimes complicated by skin bacterial superinfection.
4.3 Antibiotic resistance of Staphylococcus aureus isolates responsible for purulent skin infections
Concerning the antibiotic resistance, 97.8% of isolates of Staphylococcus aureus were resistant to penicillin G. Indeed, in Madagascar, the population consumes this class of antibiotics excessively without a medical prescription. In this study, tetracyclines and cotrimoxazole also begin to show ineffectiveness. Indeed, these drugs are sold in small grocery stores and the population can buy them easily. The literature confirms that the self-medication practiced by the population favors the increase in the resistance of germs to antibiotics [10]. Similarly, doctors in Basic Health Centers tend to give empirical treatments when the patient does not have enough money for bacteriological analyses. These treatments lead to excessive and inappropriate use of antibiotics. The literature confirms that the selection pressure of germs linked to their frequent exposure to a class of antibiotic promotes their genetic mutation leading to the emergence of new multi-resistant strains [11]. For the other classes of antibiotics, resistance has been low but should still be controlled by reducing their use in the community and in hospitals as much as possible. In this study, no resistance to vancomycin was found. Vancomycin is a broad-spectrum antibiotic and is the standard treatment for the multi-resistant strains of staphylococci [12]. Thus, it is very important to control the hospital use of vancomycin to maintain its effectiveness. Indeed, the increase in the use of this molecule could lead to the appearance of resistant strains leading to a therapeutic impasse which can be fatal for the patient.
5. Conclusion
This study showed that Staphylococcus aureus purulent skin infections are common in hospitals. These germs are opportunistic flora of the skin and cause skin infections in vulnerable patients. Thus, the prevention of purulent skin infections in vulnerable people is very important in hospital departments as well as hospital hygiene measures to fight against nosocomial infections. Similarly, it is necessary to limit the use of broad-spectrum antibiotics in hospital departments to control the evolution of Staphylococus aureus isolates towards increasing antibiotic resistance.
Acknowledgements
We extend our sincere thanks to the director of the CHUJRB establishment, to the head of the CHUJRB laboratory department and to the laboratory technicians who participated in the realization of this study.
Conflicts of Interest
The authors do not declare any conflict of interest
References
- Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a US population: a retrospective population-based study. BMC Infectious Diseases 13 (2013): 252.
- Zervos MJ, Freeman K, Vo L, Haque N, Pokharna H, et al. Epidemiology and outcomes of complicated skin and soft tissue infections in hospitalized patients. Journal of Clinical Microbioly50 (2012): 238–245.
- Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJC, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clinical Infectious Diseases59 (2014): 147–159.
- Shimazaki T, Taniguchi T, Saludar NRD, Gustilo LM, Kato T, et al. Bacterial co-infection and early mortality among pulmonary tuberculosis patients in Manila, The Philippines. International Journal of Tuberculosis and Lung Diseases 22 (2018): 65-72.
- Poor AP, Moreno LZ, Monteiro MS, Matajira CEC, Dutra MC, et al. Vaginal microbiota signatures in healthy and purulent vulvar discharge sows. Scientific Reports 12 (2022): 9106.
- Hatlen TJ, Miller LG. Staphylococcal Skin and Soft Tissue Infections. Infectious Diseases in Clinic North America 35 (2021): 81-105.
- Azoury S, Farrow N, Hu Q, Soares K, Hicks C, et al. Postoperative abdominal wound infection – epidemiology, risk factors, identification, and management. Chronic Wound Care Management Researches 2 (2015): 137-138.
- Kalra L, Camacho F, Whitener CJ, Du P, Miller M, et al. Risk of methicillin-resistant Staphylococcus aureus surgical site infection in patients with nasal MRSA colonization. American Journal of Infection Control 41 (2013): 1253–1257.
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureusand are deficient in atopic dermatitis. Science Translational Medicine 9 (2017): eaah4680.
- Randriatsarafara FM, Ralamboson J, Rakotoarivelo R, Raherinandrasana A, Andrianasolo R. Antibiotic consumption at Antananarivo University Hospital: prevalence and strategic challenges. Santé Publique 27 (2015): 249- 255.
- Tello A, Austin B, Telfer TC. Selective pressure of antibiotic pollution on bacteria of importance to public health. Environnemental Health Perspectives 120 (2012): 1100-1106.
- Diaz R, Afreixo V, Ramalheira E, Rodrigues C, Gago B. Evaluation of vancomycin MIC creep in methicillin-resistant Staphylococcus aureus infections-a systematic review and meta-analysis. Clinical Microbiology and Infectious diseases 24 (2018): 97-104.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
