Assessment of the Biological Response of the Scleractinian Coral Seriatopora Hystrix to Sunscreen Products
Andrezza DPM Canavez1*, Eloïse Renouf2, Marine Mercy2, Marcio Lorencini1, Carla
Abdo Brohem1, Desiree C Schuck1
1Product Safety Management- Q&PP, Grupo Boticário, São José dos Pinhais, PR, Brazil
2Eurofins Ecotoxicologie France, Maxéville, France
*Corresponding Author: Andrezza DPM Canavez, Product Safety Management- Q&PP, Grupo Boticário, São José dos Pinhais, PR, Brazil
Received: 18 December 2021; Accepted: 23 December 2021; Published: 13 January 2022
Article Information
Citation: Andrezza DPM Canavez, Eloïse Renouf, Marine Mercy, Marcio Lorencini, Carla Abdo Brohem, Desiree C Schuck. Assessment of the Biological Response of the Scleractinian Coral Seriatopora Hystrix to Sunscreen Products. Journal of Environmental Science and Public Health 6 (2022): 015-030.
View / Download Pdf Share at FacebookAbstract
Every second, 0.8 litres of sunscreen enters ocean waters, which corresponds to the release of 25.000 tons per year. UV filters may present substantial threats to marine fauna and flora and have an impact similar to that of other contaminants. Coral reefs play a major role in marine biodiversity, and some publications suggest that they are threatened by the release of sunscreen into the environment, which should cause bleaching. The aim of this study was to evaluate the potential impact of sunscreen products on hard corals. Laboratory experiments in which Seriatopora hystrix coral fragments were exposed to 9 sunscreens at concentrations up to 100 mg/L for 96 hours were conducted, and the biological responses of the fragments were assessed. The examined parameters were coral bleaching and polyp retraction. The results obtained revealed that the 9 tested sunscreens had no effects on S. hystrix, with a recorded NOEC (No Observed Effect Concentration) of 100 mg/L for both tested parameters. This concentration is much higher than those of chemicals in the natural environment, which are on the order of µg/L or ng/L. Under the conditions in this experiment, the absence of toxic effects from the tested sunscreens allows us to argue the absence of potential danger on corals.
Keywords
<p>Hard corals; Sunscreens; Bioassay; Fragments bleaching; Polyp retraction</p>
Article Details
Abbreviations
NOEC: No Observed Effect Concentration; WAF: Water Accommodation Fraction; LOEC: Lowest Observed Effect Concentration
1. Introduction
Coral reefs play a major role in marine biodiversity, as they represent a living habitat for many marine species; therefore, the preservation of coral reefs has been a very important environmental problem in recent decades, as massive coral bleaching, which leads to the loss of symbiotic zooxanthellae hosted within scleractinian corals, has been observed and its extent has expanded quickly [1]. The causes of this phenomenon are diverse, such as excess UV radiation, temperature variation, increasing bacterial pathogens and pollutants [2]. Human activities have a strong impact on oceans and, by extension, on coral reefs, through global warming and ocean acidification, and on a more local scale, urbanization, fishing, pollution and tourism [3]. Among the many supposed threats to marine ecosystems is the use of personal care products.
The consumption of skincare and suncare products is increasing worldwide in association with the expansion of tourism in marine coastal areas. It is estimated that every second, 0.8 litres of suncare product are released into ocean waters [4]. According to previous publications, the UV filters they contain may represent substantial threats to underwater flora and fauna, and the residues of sunscreen products affect all marine ecosystems. Numerous studies have shown that some sunscreen products have hormonal effects that affect the fertility and reproduction of fish [5-9]. These products also impact the activities of marine microorganisms and increase the abundance of viruses present in the water [10]. Sunscreen products, of which 4.000 tons per year are absorbed by coral beds, threaten more than 10% of reefs, and by extension, disturb the biodiversity in all marine ecosystems [11].
In this context, it is essential for the cosmetics industry to develop environmentally friendly products by reducing or eliminating the use of certain controversial ingredients and/or by controlling the effects of products on the marine environment to ensure their environmental safety. This study aims to assess the potential impacts of sunscreen products on the biology of hard corals. Biological studies were conducted by exposing fragments of the coral S. hystrix to different concentrations of sunscreens and assessing its biological responses to identify potential harmful effects.
2. Materials and Methods
2.1 Sunscreens assessed
The present study covers 9 sunscreen products produced by the Grupo Boticário (Table 1).
|
Sunscreen product |
Sun protection factor |
Batch number |
Formulation |
|
Sunscreen 1 |
30 |
2019.741.017.03 |
Lotion |
|
Sunscreen 2 |
30 |
2019.741.005.04 |
Lotion |
|
Sunscreen 3 |
50 |
2019.741.018.03 |
Lotion |
|
Sunscreen 4 |
50 |
2019.741.007.12 |
Lotion |
|
Sunscreen 5 |
70 |
2019.741.010.05 |
Lotion |
|
Sunscreen 6 |
70 |
2020.881.001.05 |
Lotion |
|
Sunscreen 7 |
50 |
2020.881.004.05 |
Lotion |
|
Sunscreen 8 |
50 |
2020.881.003.05 |
Lotion |
|
Sunscreen 9 |
50 |
2020.881.002.06 |
Lotion |
Table 1: Description of the sunscreen products included in the biological tests.
2.2 Study organism
The test species used was Seriatopora hystrix (Dana, 1846, Cnidarian, Scleractinian). Fragments of this species measuring approximately 3 cm in length and including more than one hundred polyps each were used. The fragments were obtained from the shop of an aquarist specialized in reef environments (“Animalerie des Nations”) in Vandoeuvre-lès-Nancy in north-eastern France.
The S. hystrix strain, which was grown by the purchaser, dates from 2002. It was cultured in large fish tanks, where lighting was provided, skimming was performed, and optimal water movement was assured by wavemaker pumps. The characteristics of the artificial seawater used by the purchaser are as follows:
- Nitrates: ≤2 mg/L
- Phosphates: ≤0.03 mg/L
- Water hardness: 8.5°F
- Calcium: approximately 400 mg/L
- pH during the day: 8.25
During culturing, the corals were fed every night by the addition of amino acids and living phytoplankton to the tanks. S. hystrix is not considered an animal under the terms of animal experimentation. According to French law [12], animal experimentation concerns "any experiment on a living vertebrate animal, including larval forms that are autonomous and/or capable of reproduction”. In accordance with this decree, experiments on invertebrate animals and on the embryonic forms of oviparous vertebrates are not considered animal experimentation. Corals are invertebrate animals that form symbioses with algae, which are vegetal species. The fragments that were used in the experiment were not taken from the environment; they were specially grown in aquariums to be used for this study. Furthermore, the biological tests were not performed in situ but in a laboratory; therefore, no products were released into the environment to conduct the study.
2.3 Test medium
For the whole study, the test medium was artificial seawater prepared from high-purity salts added to ultrapure water, ensuring full dissolution between each salt in the following order and proportions: NaF, 0.003 g/L; SrCl2,6H2O, 0.02 g/L; H3BO3, 0.03 g/L; KBr, 0.1 g/L; KCl, 0.7 g/L; CaCl2,2H2O, 1.47 g/L; Na2SO4, 4.0 g/L; NaCl, 10.78 g/L; MgCl2,6H2O, 23.5 g/L; Na2SiO3,5H2O, 0.015 g/L; and NaHCO3, 0.2 g/L. Once prepared, the water was filtered through a 1 µm membrane. After 2 weeks of maturation, the seawater was analysed. The following physicochemical characteristics were maintained in the seawater: pH of 8.0 +/- 0.3, salinity between 27 and 35‰ and dissolved oxygen content greater than 80%.
2.4 Choice of the tested concentrations
The test solutions were prepared by using the water accommodation fraction (WAF) method, which is suitable for products that are insoluble or partially soluble in water. The principle of this preparation method is to add a certain amount (called the “loading rate”) of the tested product directly to the test medium and mix it in a closed flask with
orbital shaking for 24 hours at 110 rpm at 20° +/- 2°C. The aqueous fraction of each loading charge is then recuperated. For this study, the tested loading rates for each sunscreen were 100, 56, 32, 18, 10, 5.6 and 3.2 mg/L.
The maximum loading rate corresponds to the maximum concentration (100 mg/L) used in the OECD guidelines for ecotoxicological testing [13, 14] for the CLP classification. The chosen concentrations are much higher than those found in the natural environment for chemicals that come into contact with organisms. Bibliographic data indicate that the concentrations of the main UV filters from sunscreen products that enter coastal waters and other aqueous media is in the range of µg/L or ng/L [15, 16]. The absence of an effect or the observation of a small effect under the conditions in this study, which were "extreme" compared to those in the natural environment, would suggest a lack of danger from the product on the tested organisms and studied parameters.
2.5 Toxicity bioassay
Coral fragments were picked up by the purchaser. On arrival at the laboratory, they were placed in aquariums containing artificial seawater. The fragments were left in the aquariums to allow them to recover from possible stress related to transport or water change. Recovery was determined on the basis of reblooming polyps (after 2 or 3 hours). The test solutions were prepared by recovering the solutions containing the 7 loading rates for each sunscreen, prepared with the WAF method, and placing them in plastic containers, with triplicates of 200 mL for each loading rate, plus a triplicate of the same volume for the control, consisting of the test medium. In parallel, Cu2+, in the form of copper sulfate (CuSO4,5H2O), was used as a reference substance to check fragment sensitivity (three 200 mL solutions with the following concentrations: 100, 82, 64, 48, 33, 23, and 17 µg/L). After recovery, fragments were placed individually in each container. The test containers were not sealed to allow light and CO2 circulation, but they were covered with Petri dish covers to prevent aero-contamination and to reduce the evaporation of water.
The containers were then incubated in a controlled enclosure at 25°C±1°C with a 12 h/12 h day/night cycle. After 48 hours, the test media were renewed, and the first observations were performed. Fragments were observed by binocular loupe to identify retracted polyps, and fragment bleaching was recorded. Photographs were taken to facilitate ulterior data treatment. After 96 hours, observations were performed a second time, and photographs were taken a new. Based on the data from the repeated assays for each loading rate, it was possible to determine the NOEC (“no observed effect concentration”; the highest concentration causing no significant effects on the test organisms) and LOEC (“lowest observed effect concentration”; the lowest concentration of the test range, which causes a significant effect on tested organisms) for both evaluated parameters (polyp retraction and fragment bleaching).
These two parameters allow evaluation of two types of sublethal effects: an early response (polyp retraction) and a delayed response (fragment bleaching, which can lead to fragment death). For the determination of the NOEC and LOEC, a Bonferroni statistical model was constructed with ToxCalc™ 5.0 software. The results obtained in this study were considered valid if the following conditions were met: a) there was no retraction of the polyps and no bleaching of the fragments in the 3 replicates of the control and b) the NOEC (Cu2+)-96 h was between 33 and 64 µg/L (based on previous laboratory results).
3. Results and Discussion
Corals are home to 25% of the world's underwater fauna and flora; therefore, assessing the effects of chemicals on corals is a major topic in environmental protection. Coral reefs result from a photosynthetic symbiosis between fixed animal colonies, called polyps, and algae (zooxanthellae). These algae play a very important role in the metabolism of polyps by providing them with most of their carbonaceous nutrition; in particular, zooxanthellae provide sugars via photosynthesis [17].
Bleaching is a response of corals to external stressors, such as temperature variations, overexposure to light or exposure to chemicals [18-20]. This phenomenon marks the rupture of the symbiosis between the corals and algae, with the partial or total loss of zooxanthellae populations and/or the degradation of the pigments responsible for coral colour within these algae [21-22]. Without symbiotic algae, corals are more vulnerable and do not have a source of energy. If a disturbance is not too intense and/or is not sustained over time, bleaching can be reversed, and corals can re-establish their symbiosis with zooxanthellae. Conversely, if the suffered effect is too strong, the coral dies [23]. Evaluating the effects of chemicals, particularly sunscreen products, on the environment has been a major concern in recent decades. The protection of humans against UV radiation is necessary; however, the use of sunscreen products, which are released into the environment, represents a real threat to ecosystems, especially marine ecosystems and coral reefs. Indeed, due to their lipophilic nature [24], sunscreen products tend to accumulate along the food chain and form a film on the water surface [25]; because of their UV filter, sunscreen products prevent the penetration of solar radiation necessary for underwater life [26]. There are two types of UV filters used in sunscreen products: mineral UV filters and organic UV filters. The use of certain organic filters, such as octocrylene, oxybenzone, and 4-methylbenzylidene camphor, is subject to much controversy, as these filters can represent a danger to the environment [16, 27]. It has been demonstrated that these products can cause hormonal effects that affect the fertility and reproduction of fish [8, 9]. They also impact the activities of marine microorganisms and increase the abundance of viruses present in water [11].
As a result of this growing problem, a few countries have banned some of these organic filters to preserve the environment [28]. In this context and considering the demonstrated or suspected harmful effects of some organic filters, the use of mineral filters, i.e., titanium dioxide (TiO2) or zinc oxide (ZnO), is developing in the cosmetics industry. Applying ecolabels to suncare products with mineral filters tends to promote their use, and these filters are reputed to be safer for the marine environment based on their larger particle size and lower solubility in seawater [29, 30]. However, there are studies that did not conclude that these mineral filters, tested individually, are harmless to marine organisms such as green algae, corals or crustaceans [31-33]. Given the uncertainty about the potential harmful effects of both types of UV filters, it is necessary consider the results of biological tests performed with finished products, in which these filters are integrated into mixtures with other cosmetic ingredients. The different compounds in finished products may interact, especially with the UV filters, potentially causing synergistic activities [8]. Studying finished products allows a more refined evaluation of the biological effects, allowing the determination of the potential danger from the whole product to the environment, especially to corals. To study the effects of the 9 Grupo Boticário sunscreens, 3 different analytical series (AS1, AS2 and AS3) were performed. The results of the observations of polyp retraction and fragment bleaching after 48 hours and 96 hours of exposure are shown in Table 2 and Figures 1-3. Bleaching was observed visually and defined by a bleaching gradient: 2=no bleaching, 1=partial bleaching and 0=complete bleaching.
*Bleaching being observed visually, it has been chosen to define a bleaching gradient; -2=No bleaching, 1=Partial bleaching and 0=Complete bleaching.
Table 2: Observations of polyp retraction and fragment bleaching.
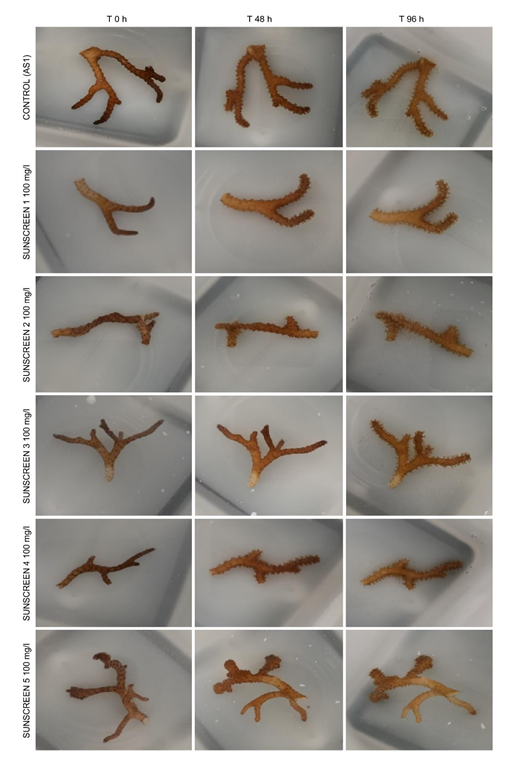
Figure 1: Effect of 100 mg/L of sunscreen on Seriatopora hystrix fragments after 48 and 96 h of exposure in AS1.

Figure 2: Effect of 100 mg/L of sunscreen on Seriatopora hystrix fragments after 48 and 96 h of exposure in AS2.

Figure 3: Effect of 100 mg/L of sunscreen on Seriatopora hystrix fragments after 48 and 96 h of exposure in AS3.
The results obtained were validated by the assessment of the following criteria: retraction of the polyps and bleaching of the fragments were not observed in the 3 replicates of the control for any of the analytical series, and the NOEC (Cu2+)-96 h values were between 33 and 64 µg/L (AS1=48 µg/L; AS2=33 µg/L; AS3=33 µg/L). No toxic effects on either tested parameter were observed at any of the tested loading rates or for any of the tested sunscreens. Table 2 show results observed at only the highest tested loading rate, as there was no difference in the effects between the 7 tested loading rates.
In this study, the coral response to sunscreen exposure was not dose dependent, as the same effects were observed at low and high sunscreen loading rates. For all the tested sunscreens, the NOEC was defined as 100 mg/L, and the LOEC was > 100 mg/L. The effects of UV filters on living organisms inhabiting marine ecosystems are quite well documented in the scientific literature. Some studies carried out on other species of hard corals (tropical and well-represented species such as S. hystrix) have revealed harmful effects of UV filters, especially ZnO, with short exposure times. ZnO was found to cause strong negative effects in terms of the loss of zooxanthellae by Acropora spp. after as little as 48 hours of exposure to a ZnO concentration of only 6.3 mg/L [34].
Other work showed that ZnO was responsible for rapid and severe coral bleaching by altering the established symbiosis [35], and studies on another hard coral species (Stylophora pistillata) showed a decrease in photosynthetic efficiency at 100 µg/L and bleaching at 1 mg/L [31]. Concerning the effects of TiO2 on hard corals, it has been determined that this mineral filter may interfere with the symbiotic relationship between corals and zooxanthellae by reducing algal populations within symbioses, without leading to coral death, at a concentration of 10 mg/L over an exposure period of 17 days [36]. Some organic filters, such as benzophenone 2, benzophenone 3 (oxybenzone), avobenzone, 4-methylbenzylidene camphor and ethylhexylmetho-xycinnamate, have impacts on corals.
Avobenzone can induce a significant decrease in photosynthetic efficiency at a concentration of 1 mg/L [31], and benzophenone 2 and oxybenzone are toxic to corals, causing damage to zooxanthellae and DNA damage to coral cells [27, 37]. Ethylhexyl-methoxycinnamate and 4- methylbenzylidene camphor induce rapid bleaching at a concentration of 33 µg/L [11]. In this last study, finished sunscreen products from three different brands (containing diverse UV filters) were tested and the results revealed significant adverse effects on coral bleaching after only 24 hours of exposure to concentrations much lower than those in our study (between 10 and 100 µg/L).
In conclusion, the two mineral filters currently authorized, TiO2 and ZnO, and the main organic filters that were previously assessed have been shown to have harmful effects on corals, sometimes at very low concentrations. However, previous studies were based on the evaluation of only the filters, not the filters integrated into cosmetic formulations. Moreover, these individually tested filters have physicochemical characteristics (coating, size, etc.) that may differ from those of filters present in cosmetic formulations. The performance of ecotoxicological tests on finished products, such as those carried out in this study, allow the effects of the interactions between filters and the other constituent ingredients of sunscreen products to be assessed and enable the prediction of the potential threat from a product.
In our study, we were thus able to observe that the tested finished products, which contained filters in combination with other ingredients, do not have any substantial ecotoxicological effects on corals, in contrast to other finished products tested previously.
The results of this study, which was conducted under "extreme" conditions compared to those in the natural environment (i.e., at product concentrations of 100 mg/L, which is much higher than those measured in coastal waters (on the order of ng/L or µg/L), allow us to argue for the absence of potential danger from the tested products to corals.
Acknowledgements
In Memoriam of Dr. Marcio Lorencini, a co-author of this work, an excellent researcher in the field of cosmetics and devoted to promote cosmetic research in Brazil.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
This study was supported by Grupo Boticário, which also manufactured the sunscreens.
E.R. and M.M. are employed by Eurofins Ecotoxicologie, where the experiments was conducted. D.C.S., C.A.B. and A.D.P.M.C. are researchers employed by Boticario Group.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
- Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Freshw. Res 50 (1999): 839-866.
- Hughes TP, Baird AH, Bellwood DR, et al. Climate change, human impacts, and the resilience of coral reefs. Science 301 (2003): 929-933.
- Duprey NN, Yasuhara M, Baker DM. Reefs of tomorrow: eutrophication reduces coral biodiversity in an urbanized seascape. Glob. Chang. Biol 22 (2016): 3550-3565.
- Global statistics in real time (2021).
- Blüthgen N, Zucchi S, Fent K. Effects of the UV filter benzophenone-3 (oxybenzone) at low concentrations in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol 263 (2012): 184-194.
- Coronado M, De Haro H, Deng X, et al. Estrogenic activity and reproductive effects of the UV-filter oxybenzone (2- hydroxy-4-methoxyphenyl-methanone) in fish. Aquat Toxicol 90 (2008): 182-187.
- Cosnefroy A, Brion F, Maillot-Maréchal E, et al. Selective activation of zebrafish estrogen receptor subtypes by chemicals by using stable reporter gene assay developed in a zebrafish liver cell line. Toxico. Sci 125 (2012): 439-449.
- Fent K, Kunz P, Gomez E. UV filters in the aquatic environment induce hormonal effects and affect fertility and reproduction in fish. CHIM. Int. J. Chem 62 (2008): 368-375.
- Weisbrod CJ, Kunz PY, Zenker AK, et al. Effects of the UV filter benzophenone-2 on reproduction in fish. Toxicol. Appl. Pharmacol 225 (2007): 255-266.
- Danovaro R, Corinaldesi C. Sunscreen products increase virus production through prophage induction in marine bacterioplankton. Microb. Ecol 45 (2003): 109-118.
- Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect 116 (2008): 441-447.
- Decree no 2001-464 of May 29, 2001 modifying the decree no 87-848 of October 19, 1987 taken for the application of article 454 of the penal code and of the third paragraph of article 276 of the rural code and relating to the experiments carried out on animals (2001).
- Organisation for Economic Co-operation and Development. OECD guidelines for the test no. 202: Daphnia sp. acute immobilisation test. OECD Publishing, Pairs (2004).
- Organisation for Economic Co-operation and Development. Freshwater alga and cyanobacteria, growth inhibition test. OECD guidelines for the testing of chemicals. 2; test no. 201. OECD, Paris, France (2011).
- Ruszkiewicz JA, Pinkas A, Ferrer B, et al. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep 4 (2017): 245-259.
- Tovar-Sánchez A, Sánchez-Quiles D, Basterretxea G. Sunscreen products as emerging pollutants to coastal waters. PLoS One 8 (2013): e65451.
- United States Environmental Protection Agency (US EPA). Basic information about coral reefs (2018).
- Hoegh-Guldberg O, Poloczanska ES, Skirving W, et al. Coral reef ecosystems under climate change and ocean acidification. Front. Mar. Sci 4 (2017): 158.
- Hughes TP, Kerry JT, Álvarez-Noriega M, et al. Global warming and recurrent mass bleaching of corals. Nature 543 (2017): 373-377.
- McCoshum SM, Schlarb AM, Baum KA. Direct and indirect effects of sunscreen exposure for reef biota. Hydrobiologia 776 (2016): 139-146.
- Douglas AE. Coral bleaching--how and why? Mar. Pollut. Bull 46 (2003): 385-392.
- Kinzie RA, Takayama M, Santos SR, et al. The adaptive bleaching hypothesis: experimental tests of critical assumptions. Biol. Bull 200 (2001): 51-58.
- Muller-Parker G, D’Elia CF, Cook CB. Interactions between corals and their symbiotic algae, in: Birkeland C (Ed.), Coral Reefs in the Anthropocene. Springer Netherlands, Dordrecht (2015): 1-271.
- Santos AJ, Miranda MS, Esteves da Silva JC. The degradation products of UV filters in aqueous and chlorinated aqueous solutions. Water Res 46 (2012): 3167-3176.
- Giokas DL, Salvador A, Chisvert A. UV filters: from sunscreens to human body and the environment. TrAC Trends Anal. Chem 26 (2007): 360-374.
- Botta C, Labille J, Auffan M, et al. TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: structures and quantities. Environ. Pollut 159 (2011): 1543-1550.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (Benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. virgin Islands. Arch. Environ. Contam. Toxicol 70 (2016): 265-288.
- State of Hawaii. A bill for an act. s.b. no. 2571 (2018).
- Manzo S, Miglietta ML, Rametta G, et al. Toxic effects of ZnO nanoparticles towards marine algae Dunaliella tertiolecta. Sci. Total Environ 445-446 (2013): 371-376.
- Spisni E, Seo S, Joo SH, et al. Release and toxicity comparison between industrial- and sunscreen-derived nano-ZnO particles. Int. J. Environ. Sci. Technol 13 (2016): 485-2494.
- Fel JP, Lacherez C, Bensetra A, et al. Photochemical response of the scleractinian coral Stylophora pistillata to some sunscreen ingredients. Coral Reefs 38 (2019): 109-122.
- Miller RJ, Bennett S, Keller AA, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PLoS One 7 (2012): e30321.
- Yung MMN, Mouneyrac C, Leung KMY. Ecotoxicity of zinc oxide nanoparticles in the marine environment in: Bhushan, B. (Ed.), Encyclopedia of Nanotechnolog. Springer Netherlands, Dordrecht (2014): 1-17.
- Corinaldesi C, Marcellini F, Nepote E, et al. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ 637-638 (2018): 1279-1285.
- Wismer S, Tebbett SB, Streit RP, et al. Spatial mismatch in fish and coral loss following 2016 mass coral bleaching. Sci. Total Environ 650 (2019): 1487-1498.
- Jovanovic B, Guzmán HM. Effects of titanium dioxide (TiO2) nanoparticles on caribbean reef-building coral (Montastraea faveolata). Environ. Toxicol. Chem 33 (2014): 1346-1353.
- Downs CA, Kramarsky-Winter E, Fauth JE, et al. Toxicological effects of the sunscreen UV filter, benzophenone-2, on planulae and in vitro cells of the coral, Stylophora pistillata. Ecotoxicology 23 (2014): 175-191.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 