Biochemical and Histological Profiling of Heart of Wistar Rat Concurrently Administered with Alcohol, Cadmium and Magnesium
Chidi J. OGHAM*, Jonathan D. DABAK, Kiri. H. JARYUM
Department of Biochemistry, Faculty of Basic Medical Sciences, College of Health Sciences, University of Jos, Nigeria
*Corresponding author: Chidi J. OGHAM. Department of Biochemistry, Faculty of Basic Medical Sciences, College of Health Sciences, University of Jos, Nigeria.
Received: 31 January 2023; Accepted: 13 February 2023; Published: 15 March 2023
Article Information
Citation: Chidi J. OGHAM, Jonathan D. DABAK, Kiri. H. JARYUM. Biochemical and Histological Profiling of Heart of Wistar Rat Concurrently Administered with Alcohol, Cadmium and Magnesium. Fortune Journal of Health Sciences 6 (2023): 88-95
View / Download Pdf Share at FacebookAbstract
There was a health challenge in our study area with no known etiology as result of the consumption of well waters which affected mostly alcoholics. A chronic and complex interplay of cardiovascular disease symptoms which occasionally leads to mortality were witnessed. Water quality assessment in the area, indicated that it is contaminated with cadmium and had high magnesium content. The aim of this work, therefore, was to mimic the co-administration of cadmium and magnesium with graded concentrations of alcohol using a rat model to assess their effects in the heart, in order to give a plausible explanation that could be the cause of this health challenges. To achieve this aim, rats were randomly divided into eight groups of 4 rats per group in cages. Group 1 served as normal control and fed with animal Feed and Water only. Group 2 was treated with feed and 6% Alcohol only (Test control). Group 3 to 8 were treated with the combination of cadmium and magnesium and graded concentrations of alcohol (from 1-6%)(aq). Treatments were done for a period of 21 days, after which the rats were humanely sacrificed, serum was obtained after centrifugation and frozen (-20oC) for cardiac panel analysis and the heart were harvested and preserved in 10% formalin for histopathological examinations. The results revealed that at alcohol concentration above 4%, various degree of cardiac injuries was induced. This suggest that alcoholics have higher risk of cardiovascular diseases caused by cadmium toxicity in areas where the water source is polluted with cadmium as it is the case with our study area.
Keywords
<p>Cadmium, Magnesium, Alcohol, Consumption, Bar village waters, Heart</p>
Article Details
1. Background of the Study
Bar is a land village separated by Bununu river to the north and surrounded by hills both to the east and west with a population of about 1300 people. Narration has it that some mineral resources were once exploited in the area in quantities not viable for commercial purposes. Some years back, a health crisis of grave concern was reported in the aforementioned village, after the community source of water was changed from surface water to well water for villagers’ utilization. The irruption of the unfamiliar disease was characterized by severe clinical manifestations leading to the death of some members of the population. The disease was fingered to have started in October 2003 and was assumed to be caused by the consumption of the hand-dug well water in the area. This occurrence was broadcasted in national dailies and the once active population had been living in fear due to the unknown disease. In respect to the aforementioned, the public health department of the Bauchi State Ministry of Health had conducted an epidemiology study which revealed that the said deaths were mainly drinkers of locally brewed alcoholic beverage produced in the area. However, the study was rather suggestive due to its inability define a clear link between the health effects of the concurrent exposure of toxic metals and alcohol administration.
Environmental pollution is a critical phenomenon in recent times [1]. Environmental pollution and contamination by heavy metals is a menace to the environment with serious consequences [2]. Contamination of the environment by heavy metals has been increased by massive industrialization and urbanization, and their accumulation in the environment have greatly accelerated over the years [3,4]. These metals occur naturally but are introduced into the environment through weathering of rocks containing the metals and volcanic activities, and also through human activities such as industrial emissions, mining, smelting, pesticides and phosphate fertilizers application [5]. Combustion of fossil fuels also contributes to the release of heavy metals such as cadmium (Cd) to the environment [6]. Heavy metals contaminate the food chains, persistent in the environment, and cause different health problems due to their toxicities. Chronic exposure to heavy metals in the environment is a major threat to living organisms [7], because the population living in polluted areas are not even aware of the danger lurking around.
It should be emphasized that exposure to these toxic metals most often involves exposure to a combination of toxic metals rather than to just a single metal; thus, accurate assessment of health risk due to drinking metal-contaminated groundwater must consider potential interactions between the metals on the chemical, biochemical, and physiological levels. When subjects are exposed to metals in combination, the metals may each have their own typical health effects; either synergistically or antagonistically. In estimating the risks due to drinking water contaminants, it is vital to consider not only the health risks due to individual contaminants, but also those risks due to combinations of contaminants. Ingestion of metals in combination may increase or decrease the absorption and distribution of the individual metals in the digestive tract and circulatory system as well as also affecting the excretion of other metals [8,9].
Absorption of metals through drinking water can be affected by certain diets, behaviours and addiction. Interactions with dietary components and the general nutritional levels also affect the outcomes of exposure to toxic metals [10]. Health effects due to co-exposures to toxic metals are further affected by behavioural and cultural practices (e.g. smoking) and dietary habits (alcoholism). Particularly interesting are interactions between xenobiotics to which exposure is not often common. Examples of such substances are cadmium and ethanol. Interactions between cadmium and ethanol is an important problem in the field of modern toxicology, as both substances pose a risk to human and animal health [11]. Dabak et al. (2016) as also reported that magnesium possesses some level of protective effect against cadmium toxicity [12].
Alcoholism is a serious problem in almost in most societies. The excessive consumption of ethanol in the form of alcoholic beverages may be common among some industrial workers exposed to cadmium, including smokers [11]. Ethanol has been reported to increase the permeability of biological membranes to cadmium [13], which can make alcoholics more susceptible to the effect of cadmium toxicity. Findings suggest that with typical patterns of exposure, multiple mechanisms probably contribute to the uptake of Cd in the proximal tubule in vivo [14]. Regardless of the uptake mechanisms that are involved, it is clear that over time Cd can accumulate in the epithelial cells of the proximal tubule. The traditional view has been that when the tissue levels of Cd exceed a critical concentration of about 150 μg/g tissue, intracellular defenses such as metallothionine (MT) and glutathione (GSH) are overwhelmed and the cells undergo injury and begin to die [15,16].
Based on the above-mentioned studies, the dose, the duration, the chemical form of Cd in the kidney, liver and intestinal tissue, heart and the exposure route of Cd must be considered as an important factor in evaluating the chronic effects of long-term and short-term Cd administration. The important organs of metabolism, detoxification, storage and xenobiotics excretion and their metabolites are the Liver and kidney, and these organs vulnerable to damage. The important target organ for ethanol metabolism is the liver [17], while the kidney is essentially for Cd toxicity [18]. The development of CVD is a result of a chronic and complex interplay between genetic and environmental factors. Whereas genetic makeup is a critical determinant (related to a set of non-modifiable risk factors such as age, sex, family history, height, and postmenopausal status in women), large changes in the incidence of CVD over the last century indicate that environmental influences are also as important. Multiple studies show that non-genetic factors such as diet, smoking, physical activity, and alcohol intake significantly modify CVD risk [19]. Particulate matter exposures lead to the delivery of metals to multiple extra-pulmonary sites where they form reactive centers that continually catalyze the generation of reactive oxygen species and induce oxidative stress. Heart failure deaths, which make up 10% of all cardiovascular deaths, accounted for 30% of cardiovascular deaths related to metal toxicity [20]. Based on the above-mentioned studies, the dose, the duration, the chemical form of Cd in the heart, and the exposure route of Cd must be considered as an important factor in evaluating the chronic effects of long-term Cd administration. This work is therefore designed to investigate the possible effects the concurrent administration of water containing cadmium and magnesium with graded concentrations of alcohol will exert on the biochemical and histological assessments of the heart in order to establish and document the plausible explanation on the cause(s) of this health challenges leading to deaths of mostly alcoholics in our study area. This is an in vivo study using rat models that employed methods towards estimating the interactions between parameters in subject to support relevant in vitro data, findings and correlations.
2. Materials and Methods
2.1 Experimental Animals
Thirty-two (32) 10-week-old white albino rats with an average initial body weights of 230g were obtained from the animal house of the University of Jos, Nigeria and were fed commercial feed (Vital Feed) and provided with various treatment water ad libitum. They were housed in ventilated cages and maintained on a regular diurnal lighting cycle (12:12 light: dark). Chopped corn cob was used as bedding. They were also left to acclimatized for 7 days under standard environmental conditions before treatments.
2.2 Ethical Clearance
All experimental procedures were approved by the Ethical Committee on Animal Experimental unit of the University of Jos, Faculty of Pharmaceutical Sciences Reference number: F17-00379, in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
2.2 Chemicals/Reagents
The feed used was vital feed (growers mash) produced by Grand Cereal and Oil Mills Ltd, Jos Plateau state. The cadmium chloride used was a product of May and Baker (M&B) Ltd, Gagenhan, England while ethanol was a product of British Drug House (BDH), England. Magnesium chloride used was a product of Sigma-Aldrich. Other chemicals used were of analytical grade purchased by Ministry of Education, Bauchi state for Government Science Secondary School Misau and also from NanaRich Medical Laboratories Bauchi and Prestige Laboratories Jos. All chemical preparations were done with distilled water which was distilled from pyrex apparatus. Standards of Cadmium were obtained from Water and Sanitation Agency (WATSAN) laboratory established by United Nations Children Fund (UNICEF) at Bauchi. The RapidCardiac Panel test is an immunochromatography based one step in vitro test. It is designed for qualitative determination of cardiac troponin I (cTnI), CK-MB and Myoglobin in human serum specimens as an aid in the diagnosis of myocardial infarction. The 1J04C2 RapidCardiac Panel test kit of Xiamen Boson Biotech Co., Ltd, China was used for the assay.
2.3 Grouping and Treatment of Experimental Rats
Rats were randomly divided into eight groups of 4 rats per group in cages and all the groups drank the solution meant for each group ad libitum. The administration of the ethanol at a dose of 5 g of 96% ethanol/kg body wt/24 h was done through intragastric intubation at intervals of 12 hours all through the period of the experiment. During the whole course of the experiment, the animals were kept under identical conditions and had unlimited access to feed. As a case study for events taking place in Bar/Bula village, we have used cadmium and magnesium exposure level to correspond to those which occur in the ground water sampled in the area. Preparations of aqueous solutions containing 0.16mg/L of CdCl2 and 105mg/L of MgCl2 were served as representative water samples administered to the rats.
Group 1 (Normal control) – This group was placed on redistilled water to drink for the whole course of the experiment. Group 2 (Test control) was treated with 6% (v/v) aqueous solution of ethanol only. Group 3 was treated with 1% (v/v) aqueous solution of ethanol and drinking aqueous solution of the combination of 0.16mg/L of CdCl2 and 105mg/L of MgCl. Group 4 treated with 2% (v/v) aqueous solution of ethanol and drinking aqueous solution of 0.16mg/L of CdCl2 and 105mg/L of MgCl. Group 5 was treated with 3% (v/v) aqueous solution of ethanol and drinking aqueous solution of 0.16mg/L of CdCl2 and 105mg/L of MgCl. Group 6 was treated with 4% (v/v) aqueous solution of ethanol and drinking aqueous solution of 0.16mg/L of CdCl2 and 105mg/L of MgCl. Group 7 was treated with received 5% (v/v) aqueous solution of ethanol and drinking aqueous solution of 0.16mg/L of CdCl2 and 105mg/L of MgCl. Group 8 was treated with 6% (v/v) aqueous solution of ethanol and drinking aqueous solution of 0.16mg/L of CdCl2 and 105mg/L of MgCl. The animals were included in the study if they underwent successful intragastric intubation throughout the study period. The animals were excluded if they died prematurely before collection of analytical specimens.
During the course of the study, four different teams of investigators were involved; a first investigator was responsible for treatments preparation. A second investigator was responsible for administration of the treatment based on the groupings. A third investigator was responsible for anaesthetic procedure and performance of the surgical procedure whereas a fourth group of investigators (unaware of treatment) assessed the biochemical and histopathological examinations.
2.4 Sample Collection and Preparation
After 21 days’ treatments, the rats were sacrificed by cervical dislocation. Serum was obtained after centrifugation and kept frozen (-20oC) until needed for immunochromatography assays. The hearts were harvested and preserved in 10% formalin for histopathological examinations.
2.5 Cardiac Panel Test
The RapidCardiac Panel test is an immunochromatography based one step in vitro test. It is designed for qualitative determination of cardiac troponin I (cTnI), CK-MB and Myoglobin in serum specimens as an aid in the diagnosis of myocardial infarction. When serum sample is added to sample pad, it moves through the conjugate pad and mobilizes gold antibody conjugate that is coated on the conjugate pad. The mixture moves along the membrane by capillary action and reacts with anti-cardiac marker antibodies that is coated on the test region. If cardiac markers are present at levels of cut-off level or greater, the result is the formation of a colored band in the test region. If there are no cardiac markers in the sample, the area will remain colorless. The sample continues to move to the control area and forms a pink to purple color, indicating the test is working and the result is valid. Below are the cut-off concentrations for each cardiac marker used in the test.
Troponin I 1.5 ng/mL (Abbott AxSYM)
CK-MB 7.0 ng/mL (Abbott AxSYM)
Myoglobin 100 ng/mL (Abbott AxSYM)
2.6 Histopathological studies: Slices of the heart were fixed in 10% formalin for 24 h, and were embedded in paraffin; 5–6 µm sections were routinely stained with haematoxylin and eosin (H&E) and assessed in a light microscope (Nikon Eclipse E400). All alterations from the normal structure were registered.
3. Results
3.1 Radical Cardiac Panel Test
The effects of treatments on the cardiac panel test carried out are depicted in table 1.
The result shows CK-MB marker to be present in group 8 at a cut-off level indicated above.
The result also shows Troponin markers present in groups 7 and 8 at a cut-off level indicated above.
Table 1: Effect of the co-administration of cadmium, magnesium and alcohol on some cardiac panel biomarkers
|
Control Group |
Group 2 |
Group 3 |
Group 4 |
Group 5 |
Group 6 |
Group 7 |
Group 8 |
|
|
Myoglobin |
-ve |
-ve |
-ve |
-ve |
-ve |
-ve |
-ve |
-ve |
|
CK-MB |
-ve |
-ve |
-ve |
-ve |
-ve |
-ve |
-ve |
+ve |
|
Troponin |
-ve |
-ve |
-ve |
-ve |
-ve |
-ve |
+ve |
+ve |
3.2 Histopathological Studies
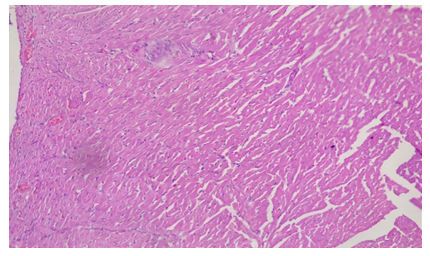
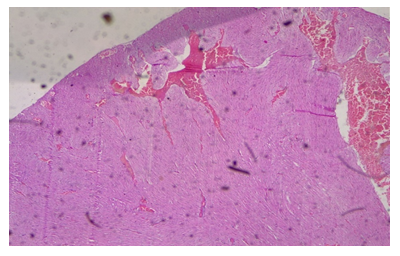
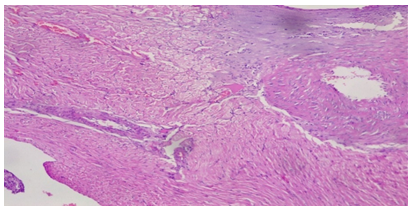
Plate 3: Micrograph of representative Rat Heart section of Group 8 treated concurrently with 6% APV and 0.16mg/l cadmium/185mg/l magnesium preparation shows embolism caused by leukocyte infiltration and haemorrhage (black arrow) and inflammation of the myocardium (oval shape). Cardiac hypertrophy and fibrosis.
4. Discussion
Our results as shown that the treatments significantly increased CK-MB and troponin levels at higher alcohol concentration level in a dose dependent manner when compared with the control. The high CK-MB and troponin levels suggests that there is disease or damage to the heart and skeletal muscle (table 1). For the investigation, if cardiac markers are present in levels of cut-off level, there is an indication of cardiac distress. Compared to other organs, the heart is particularly venerable to damage caused by reactive oxygen species (ROS) due to its highly oxidative metabolism and relatively limited antioxidant defenses. Serum myoglobin, CK-MB and troponin levels are considered important markers of early and late cardiac injury [21]. The present study shows the fact that the treatment groups with the highest concentration of alcohol administration had caused an increase in CK-MB and troponin activities in serum and heart. Since, CK-MB and troponin are released during tissue injury, an increased activity of CK-MB and troponin in serum in the present study reveals damage in the heart and hence got accumulated in the serum. The detection level of CK-MB is 7.0 ng/ml and that of troponin is 1.0 ng/ml with groups 7 and 8 showing positive and thus may indicate damage to the heart and skeletal muscles.
Blood levels of CK-MB begin to rise within 4-8 hours at the advent of myocardial injury [22]. The reliable diagnosis of acute myocardial infarction can be made for up to 48-72 hours after the onset of chest pain by analyzing serum CK and CK-MB levels [22]. Determination of the cardiac-specific isoenzyme CK-MB in patients with acute MI (100% and 98%, respectively) are quite highly sensitive and highly specific. Troponin T is a protein that is unique to cardiac muscle due to its specific structure within the contractile apparatus. Katus et al examined the precision and usefulness of measuring serum levels of Troponin T for detecting acute myocardial infarction in a separate study. In patients with acute myocardial infarction, the serum levels of Troponin T become elevated within 3.5 hours of chest pain onset and continue to remain elevated for at least 5 days [23].
As a result, determining troponin T levels is particularly valuable in individuals who do not seek medical attention within the 48–72-hour window during which total CK and CK-MB levels are raised. The accuracy of troponin T level assessment is considered to be high due to the protein’s normally low baseline levels, which increase substantially during acute infarction [24]. Elevated troponin T levels indicate the presence of irreversible cardiac damage in groups 7 and 8 treatments. It serves as a highly sensitive marker for detecting even small amounts of cardiac muscle necrosis [25]. Piper et al., (1984) also suggested that elevated serum troponin levels could be an indication of both reversible cell ischemia and irreversible myocardial damage [25]. Higher ethanol administered in the later groups of the cadmium/magnesium treatment may have exacerbated this heavy metal’s accumulation in the heart, independently of the level of cadmium treatment. In the histological study, the treated groups of 7 & 8 demonstrated evidences of injuries and syncytial giant cells and also pronounced embolism in some of the cardiocytes. Some focal cells also showed cytoplasmic vacuolation (Plate 2 & 3).
Studies had shown that organs that had the highest cadmium accumulation when ingested are the kidney and liver, whereas the lowest levels were noted in the heart (Brzóska et al, 2013). Cadmium once ingested, either through drinking water or through the food chain, accumulates in the human body and especially in the kidney and liver [26,27]. Cadmium intake through water as vehicle enters the body through the gastrointestinal tract. It first accumulates in the liver after it is transported into the blood, and induces the synthesis of metallothionein in this location [28,29]. Metallothionein is a low molecular weight, thiol-rich, metal-binding protein, whose function is maintaining a balance of zinc and copper in-vivo [29]. However, it also plays a role in guiding against heavy metal toxicity in the body, including cadmium via the formation of metallothionein complexes [28]. It has been reported that the heart does not exhibit any metallothionein-inducing capacity [30]. This observation is of significance since it suggests that heart may be more prone to cadmium toxicity because it lacks metallothionein that binds to and detoxifies Cd.
As a non-essential element, it is unlikely that Cd enters the organs via a Cd-specific transport mechanism [31]. It rather crosses various membranes utilizing the transport mechanism of other elements, including Ca [32]. It is evident in studies that cadmium uptake increases significantly when given to rats in conjunction with alcohol [33]. It is now generally accepted that alcohol can induce in vivo changes in membrane lipid composition and fluidity [34], which may affect cellular functions. Because alcohol is known to increase the fluidity of membranes, making them more permeable to cadmium [35], alcoholics might become more susceptible to the hazards of cadmium as compared to their non-alcoholic counterparts even in the absence of direct occupational exposure. This increase in uptake from the environment or food and drinking water is likely due to the increased permeability of membrane in the presence of ethanol. The observations made in the present study indicate that ethanol administered at high concentration even for a short period of time with a simultaneous exposure to cadmium influences the turnover of this heavy metal.
It has also been reported that administration of ethanol and in conjunction with cadmium/magnesium decreased magnesium concentration in the liver and kidney in an increasing alcohol concentration manner [33]. Progressive decrease in Mg levels in organs and especially the heart results in increased concentration of alcohol intake due to of increase excretion of the minerals. It has been reported that chronic alcoholics have altered Mg homeostasis [36]. Marked hypomagnesemia associated with hypermagneseuria has also been observed in alcoholics [37]. Magnesium plays a role in the active transport of calcium and potassium ions across cell membranes, a process that is important to nerve impulse conduction, muscle contraction, and normal heart rhythm [37]. This native metal is de-mobilized and excreted in the presence of alcohol with subsequent substitution by Cd. This event results in the delivery of the metal to multiple extra- pulmonary sites where they form reactive centers that continually catalyze the generation of reactive oxygen species and induce oxidative stress [38]. Consequently, this will invariably lead to the induction of hypertension [39], with apparent direct toxic impact on gene transcription in the vascular endothelium [40]. Then comes peripheral arterial disease [41], increased vascular intima media thickness, Cd accumulating in the wall of the aorta [42], increasing aortic resistance [43] and myocardial infarction [44]. Cd-linked sudden cardiac death may per ultimately follow [44].
Conflict of Interests
The authors declare that there are no conflicts of interest related to this study.
Authors’ Contribution
CO conceived, researched and funded the study; CO also drafted the manuscript; JD designed, supervised and provided significant input in all sections of the work to improve the quality of the manuscript; KJ Co-supervised and was involved in literature search. All authors read and approved the final manuscript.
Conclusion
The results of this study show that the higher the concentration of alcohol administered in a subject, with a constant concentration of Cd and Mg, the higher the concentrations of cardiac injury biomarkers in the serum of the subject. This might be due to the demobilization and excretion of magnesium from the cardiac cells by ethanol, and subsequent substitution by cadmium. This means that alcoholics have higher risk of cardiac Cd toxicities in areas where the water source is polluted with cadmium, as is the case with our study area.
References
- Ali H & Khan E. Environmental chemistry in the twenty-first century. Environmental Chemistry Letters 15 (2016): 329-346.
- Hashem, MA, Nur-A-Tomal, MS, Mondal, NR & Rahman, MA. Hair burning and liming in tanneries is a source of pollution by arsenic, lead, zinc, manganese and iron. Environmental Chemistry Letters 15 (2017): 501-506.
- Khan FU, Rahman AU, Jan A & Riaz M. Toxic and trace metals (Pb, Cd, Zn, Cu, Mn, Ni, Co and Cr) in dust, dustfall/soil. Journal of the Chemical Society of Pakistan (Pakistan), 26 (2004).
- Chen Y, Hu Z, Bai H, Shen W. Variation in Road Dust Heavy Metal Concentration, Pollution, and Health Risk with Distance from the Factories in a City-Industry Integration Area, China. Int J Environ Res Public Health 19 (2022): 14562.
- Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 9 (2021): 42.
- Spiegel H. Trace element accumulation in selected bioindicators exposed to emissions along the industrial facilities of Danube Lowland. Turkish Journal of Chemistry 26 (2002): 815-824.
- Wieczorek-Dabrowska M, Tomza-Marciniak A, Pilarczyk B & Balicka-Ramisz A. Roe and red deer as bioindicators of heavy metals contamination in north-western Poland. Chemistry and Ecology 29 (2013): 100-110.
- Ahmed F & Ishiga H. Trace metal concentrations in street dusts of Dhaka city, Bangladesh. AtmosphericEnvironment 40 (2006): 3835-3844.
- Dabak JD, Gazuwa SY and Ubom GA. Effect of grade concentrations of Ca on nephrotic cells of Cd and Pb co-intoxicated rats. Journal of Environmental Toxicology and public Health 3 (2018): 9-17.
- Koboldt DC, Fulton RF, McLellan M, Schmidt H, Kalicki-Veizer J, McMichael J, ... &. 2012. Comprehensive molecular portraits of human breast tumours. Nature 490 (2012): 61-70.
- Omar AMS. Histopathological and physiological effects of liver and kidney in rats exposed to cadmium and ethanol. Global Advanced Research Journal of Environmental 2 (2013): 93-106.
- Dabak JD, Gazuwa SY and Ubom GA. Nephroprotective effect of graded concentrations of Mg on cd and Pb co-intoxicated rats. Journal of Basic and Applied Research International 23 (2017): 41-50.
- Brzóska MM, Galazyn-Sidorczuk M & Dzwilewska I. 2013. Ethanol consumption modifies the body turnover of cadmium: a study in a rat model of human exposure. Journal of Applied Toxicology 33 (2013): 784-798.
- Prozialeck WC & Edwards JR. 2012. Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. Journal of Pharmacology and Experimental Therapeutics 343 (2012): 2-12.
- Gobe G & Crane D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicology letters 198 (2010): 49-55.
- Prozialeck WC & Edwards JR. Early biomarkers of cadmium exposure and nephrotoxicity. Biometals, 23 (2010): 793-809.
- Teschke R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines 6 (2018): 106.
- Nordberg GF, Jin T, Nordberg M. Subcellular targets of cadmium nephrotoxicity: cadmium binding to renal membrane proteins in animals with or without protective metallothionein synthesis. Environ Health Perspect 102 (1994): 191-4.
- Eman MA, Gordon AF. Heavy Metal Poisoning and Cardiovascular Disease. Journal of Toxicology (2011).
- García-Niño WR, & Pedraza-Chaverrí J. Protective effect of curcumin against heavy metals-induced liver damage. Food and Chemical Toxicology 69 (2014): 182-201.
- Jacob R, Khan M. Cardiac Biomarkers: What Is and What Can Be. Indian J Cardiovasc Dis Women WINCARS 3 (2018): 240-244.
- Savonitto S, Granger CB, Ardissino D, Gardner L, Cavallini C, Galvani M, et al. GUSTO-IIb Investigators. The prognostic value of creatine kinase elevations extends across the whole spectrum of acute coronary syndromes. J Am Coll Cardiol 39 (2002): 22-9.
- Sharma S, Jackson PG, Makan J. 2004. Cardiac troponins. J Clin Pathol 57 (10): 1025-6.
- Katus HA, Remppis A, Neumann FJ, Scheffold T, Diederich KW et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation 83 (1991): 902-912.
- Piper HM, Schwartz P, Spahr R, Hütter JF & Spieckermann PG. Early enzyme release from myocardial cells is not due to irreversible cell damage. Journal of molecular and cellular cardiology 16 (1984): 385-388.
- Dabak JD, Gazuwa SY. and Ubom GA. 2011.Comparative hepatotoxicity test of cadmium and lead in rats. Journal of Medicine in the Tropics 14 (2011): 12-18.
- Brzóska MM, Moniuszko-Jakoniuk J, Pilat-Marcinkiewicz B, Sawicki B. Liver and kidney function and histology in rats exposed to cadmium and ethanol. Alcohol and Alcoholism 38 (2003): 2-10.
- Sabolic I, Breljak D, Skarica M, Herak-Kramberger CM. 2010. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 23 (2010): 897-926.
- Satarug S, Vesey DA & Gobe GC. 2017. Health risk assessment of dietary cadmium intake: do current guidelines indicate how much is safe?Environmental health perspectives 125 (2017): 284-288.
- Ravi K. Biochemical Studies on Iso-metallothioneins in Monkeys Exposed to Cadmium, Ph.D. dissertation, Post graduate Institute of Medical Education and Research, Chan digarh, India (1986).
- Roggeman S, de Boeck G, De Cock H, Blust R, & Bervoets L. Accumulation and detoxification of metals and arsenic in tissues of cattle (Bos taurus), and the risks for human consumption. Science of the Total Environment, 466 (2014): 175-184.
- Martelli A, Rousselet E, Dycke C, Bouron A, & Moulis JM. Cadmium toxicity in animal cells by interference with essential metals. Biochimie 88 (2006): 1807-1814.
- Ogham CJ, Dabak JD and Jaryum KH. 2021. Physicochemical and metal assessment of Bar village well water and the effect of co-administration of cadmium, magnesium and alcohol on some organs. Unpublished PhD Thesis, University of Jos, Plateau State Nigeria (2021): 148-149.
- Wang LK, Chen JP, Hung YT, & Shammas NK. (Eds.) Heavy metals in the environment. CRC press (2010).
- Winiarska-Mieczan, A. Protective effect of tannic acid on the brain of adult rats exposed to cadmium and lead. Environmental toxicology and pharmacology 36 (2013): 9-18.
- McClain CJ. &. Su LC. Zinc deficiency in the alcoholic: a review. Alcohol. Clin. Exp. Res 7 (1983): 5-70.
- Rude RK. Magnesium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, Mass: Lippincott Williams & Wilkins (2012): 159-75.
- Agency for Toxic Substances & Disease Registry (ATSDR). 2016. Priority list of hazardous substances. Nikolaos P. Cardiovascular Diseases: Genetic Susceptibility, Environmental Factors and their Interaction. Elsevier, USA (2016).
- Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol 29 (2009): 1392-8.
- Natalia VS, Jonathan DN, Jeffrey SB, George T, Judith SH & Gervasio AL (2014). American Heart Journal 168 (2014): 812-822.
- Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W., ... & et al. Cadmium exposure and incident cardiovascular disease. Epidemiology (Cambridge, Mass.) 24 (2013): 421.
- Horiguchi H, Oguma E, & Kayama F. Cadmium induces anemia through interdependent progress of hemolysis, body iron accumulation, and insufficient erythropoietin production in rats. Toxicological Sciences 122 (2011): 198-210.
- Madkour LH. Biogenic–biosynthesis metallic nanoparticles (MNPs) for pharmacological, biomedical and environmental nanobiotechnological applications. Chron. Pharm. Sci. J 2 (2018): 384-444.
- Abu-Hayyeh S, Sian M, Jones KG, Manuel A, Powell JT. Cadmium accumulation in aortas of smokers. Arterioscler Thromb Vasc Biol 21 (2001): 863-7.
- Genchi G, Sinicropi MS, Carocci A, Lauria G, & Catalano A. Mercury exposure and heart diseases. International journal of environmental research and public health14 (2017): 74.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 