FDA-Approved Excipient N, N-Dimethylacetamide Attenuates Inflammatory Bowel Disease in In Vitro and In Vivo Models
Jagadish B Koya1, Tong Shen2, Geming Lu3, Alex Gauthier1, Lin Mantell1, Charles R. Ashby Jr1, Sandra E. Reznik1, 4,*
1Department of Pharmaceutical Sciences, St. John’s University, Queens, NY
2Department of Structural and Chemical Biology, Mount Sinai Medical Center, New York, NY
3Department of Immunology, Mount Sinai Medical Center, New York, NY
4Departments of Pathology and Obstetrics and Gynecology and Women’s Health, Albert Einstein College of Medicine, Bronx, NY
*Corresponding author: Sandra E. Reznik, Department of Pharmaceutical Sciences, St. John’s University, Queens, NY and Departments of Pathology and Obstetrics and Gynecology and Women’s Health, Albert Einstein College of Medicine, Bronx, NY.
Received: 17 June 2022; Accepted: 24 June 2022; Published: 26 August 2022
Article Information
Citation: Jagadish B Koya, Tong Shen, Geming Lu, Alex Gauthier, Lin Mantell, Charles R Ashby Jr, Sandra E. Reznik. Development of a Web-Based Student Internship Portal for Students of Health Colleges. Fortune Journal of Health Sciences 5 (2022): 499-509.
View / Download Pdf Share at FacebookAbstract
Inflammatory bowel disease (IBD) affects almost 7 million people worldwide and is increasing in incidence. While the precise pathogenesis of IBD remains unknown, the production of inflammatory cytokines and chemokines play a central role. We have previously found that N, N-dimethylacetamide (DMA), a widely used non-toxic drug excipient, suppresses cytokine and chemokine secretion in vitro and prevents inflammation-induced preterm birth in vivo. Using sandwich enzyme-linked immunosorbent assays (ELISAs), we tested whether DMA attenuates cytokine and chemokine secretion from LPS- or TNFα-stimulated human intestinal epithelial cells and human monocytes and HMGB1 release from RAW 264.7 cells. To test our hypothesis that the mechanism of DMA’s effects in in vitro and in vivo models of IBD is inhibition of the NF-kB pathway, we used western blotting to track levels of the nuclear factor kappa B (NF-kB) inhibitory molecule I kappa B alpha (IkBα) in THP-1 human monocytes in the absence or presence of DMA. Finally, we induced colitis in C57Bl/6 mice with dextran sodium sulfate (DSS) and then tested whether i.p injections of DMA at 2.1 g/kg/day attenuates clinical and histopathologic signs of colitis. DMA attenuated cytokine and chemokine release from human intestinal epithelial cells and human monocytes and HMGB1 release from RAW 264.7 cells. Importantly, DMA prevented degradation of IkBα in THP-1 cells, thereby suggesting one mechanism for DMA’s effects. Finally, we show here, for the first time, that DMA attenuates clinical and histologic features of DSS-induced colitis. Based on these data, DMA should be further explored in preclinical and clinical trials for its potential as novel drug therapy for IBD.
Keywords
<p>N, N-dimethylacetamide, inflammatory bowel disease, nuclear factor kappa B, inflammation, excipient</p>
Article Details
1. Introduction
Inflammatory bowel disease (IBD) is a group of chronic inflammatory conditions of the gastrointestinal tract, which includes Crohn’s disease and ulcerative colitis [1]. Patients with IBD suffer from concomitant chronic diseases and infectious conditions, including cardiovascular disease, respiratory disease, digestive disease, malignancy and osteoporosis [2, 3]. An estimated 6.8 million people worldwide currently suffer from IBD [4] and its prevalence more than doubled in the United States from 2009-2016 [5].
While the precise etiology of IBD remains unknown, the production of pro-inflammatory cytokines, such as TNFa, Interleukin (IL)-1b and IL-6 [6, 7] and chemokines such as IL-8, which recruits neutrophils to sites of tissue injury [8-11], are central to the pathogenesis of this disorder. These cytokines are secreted from both intestinal epithelial cells and intestinal macrophages [9, 12-14]. One driver of these pro-inflammatory responses is high mobility group box 1 (HMGB1), a ubiquitous nuclear protein that activates innate immune responses when released from cells [15]. HMGB1 has been recently recognized as a therapeutic target in various inflammatory diseases [16-19], including IBD [20].
We have previously reported that N,N-dimethylacetamide (DMA), a widely used drug excipient, formerly believed to be inert, attenuates inflammation-induced preterm birth in mice by preventing nuclear factor kappa B (NF-kB) activation [21]. We have also shown that DMA suppresses cytokine secretion in lipopolysaccharide (LPS)-challenged RAW 264.7 cells, tumor necrosis factor alpha (TNFa)-induced human placental JEG-3 cells and LPS-stimulated human placental explants [22] specifically by acting on the NF-kB pathway. Ghayor et al. have shown that DMA prevents osteoporosis in rats via the inhibition of osteoclast mediated bone resorption [23] and enhances bone regeneration impaired by excess inflammation [24]. In other re-purposing studies, DMA has been shown to prevent high-fat diet induced weight gain [25] and has been investigated as a potential reversible contraceptive [26]. Ghayor et al.’s observation that DMA acts by inhibiting NF-kB is consistent with our findings [21-23]. Given DMA’s mechanism of action as an NF-kB inhibitor and given the well-known role of NF-kB in inducing intestinal mucosal inflammation and IBD [27, 28], we hypothesized that DMA would attenuate DSS-induced colitis by inhibiting NF-kB.
2. Results
2.1 Cell viability
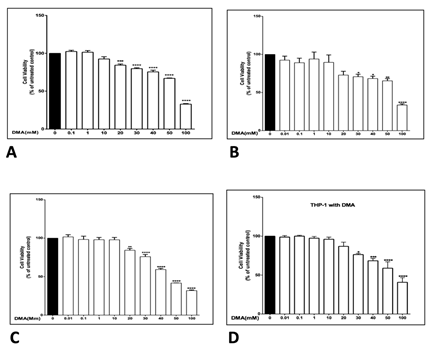
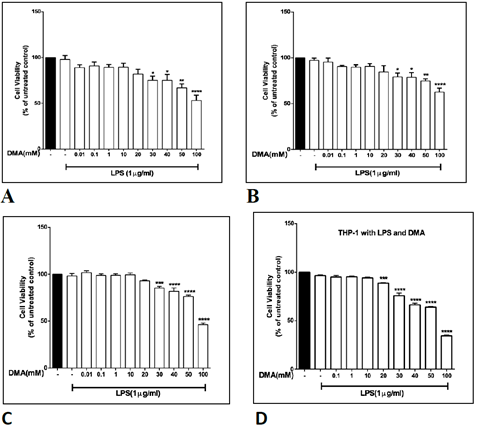
The effect of various concentrations of DMA on the viability of HT-29, HCT-116, SW620 and THP-1 cells was determined in order to identify a non-toxic range of concentrations that could be used in further experiments. DMA was found to produce no significant change in cell viability at concentrations up to at least 10 mM in all four cell lines in unstimulated cells (Supplemental Figure 1), in all four cell lines with stimulation by 1 mg mL-1 LPS (Supplemental Figure 2) and in the three colonic epithelial cell lines with stimulation by TNFa (Supplemental Figure 3). Therefore, in vitro assays were carried out with no more than 10 mM DMA.
2.2 DMA attenuates IL-8 secretion from HT-29, HCT-116 and SW620 cells
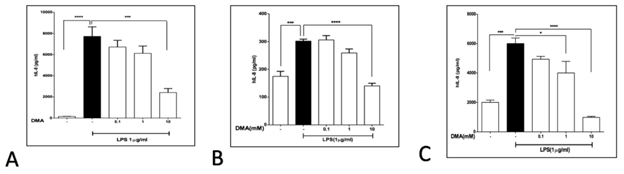
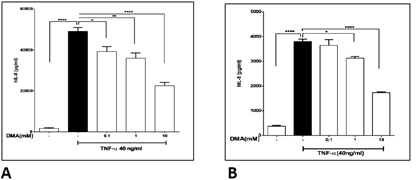
DMA significantly attenuated IL-8 secretion in HT-29 cells (Figure 1A) and HCT-116 cells (Figure 1B) stimulated with 1mg mL-1 LPS at a concentration of 10 mM and significantly decreased IL-8 secretion in LPS stimulated SW620 cells at both 1 and 10 mM (Figure 1C). Concentrations as low as 0.1 mM DMA attenuated IL-8 secretion in HT-29 cells (Figure 2A) and concentrations of 1 mM and above reduced IL-8 secretion in SW620 cells (Figure 2B) stimulated with 40 ng mL-1 TNFa.
Figure 1: DMA attenuates IL-8 secretion from LPS-stimulated colonic epithelial cells. Levels of IL-8 in cell supernatants after stimulation with 1mg ml-1 LPS in the absence or presence of increasing concentrations of DMA as shown. A, HT-29 cells; B, HCT-116 cells; C, SW620 cells. **P<.01; ***P<.001; ****P<.0001.
Levels of IL-8 in cell supernatants after stimulation with 40 ng mL-1 TNFa in the absence or presence of increasing concentrations of DMA as shown. A, HT-29 cells; B, SW620 cells. **P<.01; ***P<.001.
2.3 DMA attenuates pro-inflammatory cytokine secretion from THP-1 cells
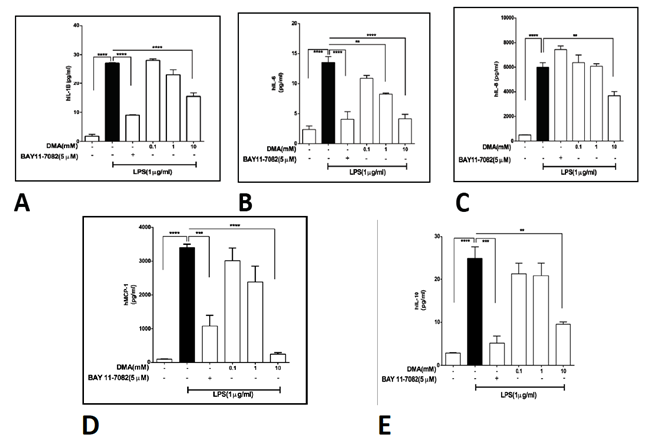
While colonic epithelial cells are likely a relatively minor source of inflammatory cytokines in vivo, monocytes and macrophages contribute significantly to the cytokine response in IBD. Therefore, we tested the ability of DMA to suppress cytokine secretion in THP-1 cells, a human monocyte line. In THP-1 cells stimulated with 1 mg mL-1 LPS. DMA significantly reduced IL-1b secretion at 10 mM (Figure 3A), IL-6 secretion at 1 and 10 mM (Figure 3B), IL-8 secretion at 10 mM (Figure 3C), MCP-1 secretion at 10 mM (Figure 3D) and IL-10 secretion at 10 mM (Figure 3E). BAY 11-7082, an NF-kB inhibitor was used at 5 mM as a positive control.
Levels of various cytokines in cell supernatants after stimulation with 1 mg ml-1 LPS in the absence or presence of increasing concentrations of DMA. BAY 11-7082 (5 mM), an NF-kB inhibitor [42] is included as a positive control. A, IL-1?; B, IL-6; C, IL-8; D, MCP-1; E, IL-10. **P<.01; *** P<.001; ****P<.0001.
2.4 DMA prevents IkBa degradation in THP-1 cells
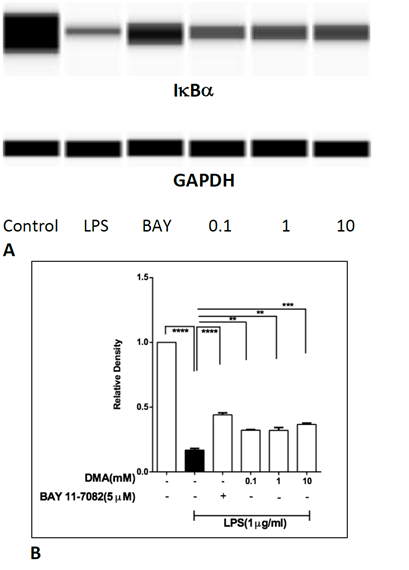
To test whether DMA affects levels of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IkBa) in THP-1 cells, the time course of IkBa degradation in THP-1 cells stimulated with 1mg mL-1 LPS was examined, using immunoblotting. Lowest levels of IkBa were found in cell lysates at 30 min (Supplemental Figure 4). Therefore, DMA’s effect on IkBa degradation was evaluated in THP-1 cells stimulated with LPS for 30 min. DMA protected IkBa from LPS-induced degradation at 0.1, 1 and 10 mM (Figure 4).
Levels of IkBa in THP-1 cell lysates after stimulation with LPS in the absence or presence of increasing concentrations of DMA. BAY 11-7082 (5 mM), an NF-kB inhibitor, is included as a positive control.
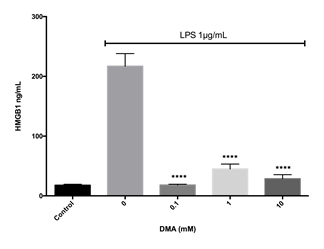
2.5 DMA attenuates HMGB1 secretion from RAW 264.7 cells at micromolar concentrations
HMGB1 secretion reflects oxidative stress, a key component of the pathogenesis of IBD. In addition, HMGB-1 activates the NF-kB pathway. Interestingly, DMA prevented HMGB1 secretion from LPS stimulated RAW 264.7 cells, a well characterized mouse macrophage cell line known to secrete HMGB1, at all concentrations tested (Figure 5).
Levels of HMGB1 in cell supernatants after stimulation with 1 mg ml-1 LPS in the absence or presence of increasing concentrations of DMA. ****P<.0001.
2.6 DMA attenuates DSS-induced colitis
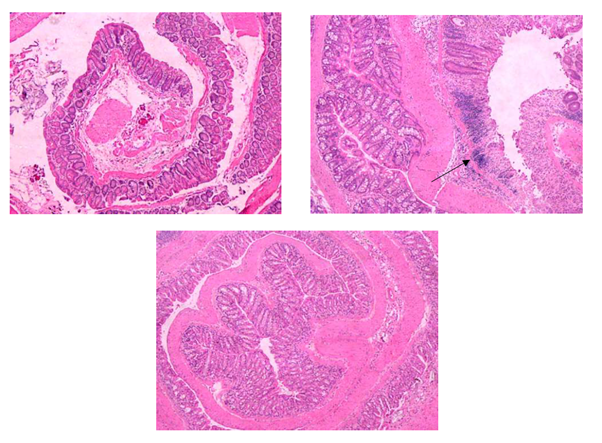
Control DSS challenged mice (n=5) all developed bloody diarrhea by the eighth day of the experiment, whereas all the DMA rescued DSS challenged mice (n=5) developed bloody diarrhea on the ninth day of the study. In addition, histologic analysis of intestinal sections from the DSS control mice showed inflammation, crypt injury and ulceration, but these effects were attenuated by DMA with significant reduction in crypt injury (P<.05) and total histologic scores (P<.05, Figure 6). Mean histologic scores for inflammation in DSS challenged mice with and without DMA rescue were 2.4 + 0.9 and 3.0, respectively. Mean scores for crypt injury for the two groups were 3.8 + 0.8 and 5.0, respectively (P<.05). Mean scores for ulceration for these two groups were 2.2 + 0.8 and 3, respectively. Finally, mean total histologic scores were 8.4 + 2.1 and 11.0, respectively (P<.05). Raw histologic scoring data are provided in Supplemental Table 1.
Figure 6: DMA prevents DSS-induced colitis. Hematoxylin and eosin stained tissue sections of colon from C57Bl/6 mice treated with 2.5% DSS in the absence or presence of rescue by daily treatments of 2.1 g/kg DMA administered ip. A, sham; B, DSS with no DMA treatment; C, DSS plus DMA rescue. Arrow indicates crypt abscess. Original magnification 200X.
3. Discussion
While the precise etiology of IBD remains elusive, it is clear that NF-kB driven pro-inflammatory responses play a central role. Current pharmacotherapeutic approaches to IBD such as sulfasalazine and biologics work by preventing activation of the NF-kB pathway either directly or indirectly [29, 30]. However, sulfasalazine, while it attenuates IBD symptoms and is readily accessible, does not show efficacy in some patients and, like all sulfonamides, is associated with hepatotoxicity [31]. On the other hand, biologics, such as monoclonal antibodies, may have greater efficacy, but remain extremely expensive [32]. DMA is an inexpensive, non-toxic widely used drug excipient, which has been previously shown by us and others to inhibit NF-kB [21, 23] and which has been proven to be non-toxic in pediatric cancer patients found to have plasma concentrations in the mM range [33]. Therefore, we hypothesized that DMA would attenuate DSS-induced colitis.
After establishing the highest non-toxic concentration of DMA in cell viability assays, we proceeded to investigate the effect of DMA on inflammatory mediators (cytokines and chemokines) in human IECs and in human monocytes. Initially, we screened for the presence of cytokines and chemokines including TNFα, IL-6, IL-1β, IL-8, IL-10, MCP-1, and GM-CSF in the three IEC cell lines and the monocytes. The stimulated IECs, which are not immune in origin, only upregulated the production of IL-8. Chemokine IL-8 recruits neutrophils to sites of tissue injury [34] and triggers the formation of crypt abscesses, a predominant feature in UC. Moreover, levels of neutrophils in colonic biopsies from UC patients are proportional to levels of disease severity [10, 11]. DMA, at 10 mM, attenuated IL-8 secretion from the IECs stimulated with either LPS or TNFa, which is consistent with our previous finding that DMA decreases neutrophil counts in placentas harvested from LPS-stimulated pregnant C57Bl/6 mice [21].
In addition to investigating DMA’s effect on IL-8 secretion from IECs, we tested DMA’s effect on the secretion of various cytokines from LPS-stimulated THP-1 monocytes. Monocytes reside under the lamina propria in the intestine, express toll-like receptor (TLR)-4 on their surface and play an active part in producing chronic gut inflammation [35, 36]. Cytokines IL-6, IL-1β and IL-10 and chemokines IL-8 and MCP-1 were significantly reduced with treatment of DMA at 10 mM in THP-1 cells. Consistent with these results, levels of IL-6 and IL-1β are positively correlated with disease severity in both Crohn’s disease and UC [37, 38]. While IL-10 is classically considered an anti-inflammatory cytokine, it has also been reported to show immune stimulatory effects by upregulating major histocompatibility class II expression in B-lymphocytes, inducing cytotoxic T-cell differentiation [39]. Moreover, IL-10 deficient mice develop colitis [40]. It is likely that IL-10 is acting as a pro-inflammatory cytokine in our cultured THP-1 cells.
Importantly, we show here that DMA inhibits degradation of the NF-kB inhibitory molecule IkBa in THP-1 cells. This finding is consistent with our previously reported result that DMA prevents IkBa degradation in RAW 264.7 cells [22] and suggests one mechanism whereby DMA prevents NF-kB driven up-regulation of cytokines and chemokines. In addition, we demonstrate that DMA decreases HMGB1 secretion from RAW 264.7 cells. HMGB1, when secreted, acts as a pro-inflammatory mediator. DMA’s ability to prevent HMGB1 release from cells, therefore, reinforces its effect on NF-kB signaling. Interestingly, DMA prevents HMGB1 secretion at 0.1 mM, whereas much higher concentrations of DMA are required to produce its effects on cytokine levels. This disparity suggests that while decreased HMGB1 release may contribute to DMA’s anti-inflammatory effects, reduced HMGB1 secretion alone does not fully account for DMA’s mechanism of action. Ghayor et al. have also shown that DMA is a bromodomain ligand [23], a result that we have confirmed in our laboratory. Taken together, the data suggest that DMA, a small, highly soluble molecule, may act via multiple mechanisms. Finally, we show here, for the first time, that DMA attenuates DSS-induced colitis in a murine model. In particular, DMA prevents the formation of crypt abscesses, a hallmark feature of UC in human patients. The data reported here indicate that DMA should be further investigated as potential drug therapy for IBD.
4. Methods
4.1 Cell culture and reagents
The human monocyte THP-1 (ATCC TIB-202) cell line; the human colon epithelial cell lines HT-29, HCT-116 and SW 620; and the mouse macrophage RAW 264.7 cell line were purchased from the American Type Culture Collection (Manassas, VA). HT-29, HCT-116, SW 620 and RAW 264.7 cells were cultured in complete growth media (CGM) using Dulbecco’s Modified Eagle’s Medium (DMEM) with 4.5 g/L glucose, L-glutamine and sodium pyruvate (Cellgro, Corning, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Atlanta Biologics, Lawrenceville, GA) and 1% penicillin-streptomycin (Cellgro, Corning, NY). THP-1 cells were grown in Roswell Park Memorial Institute (RPMI) medium with L-glutamine (Cellgro, Corning, NY) supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin. All cells were maintained in an incubator set at 37°C and 5% CO2 and allowed to grow to 80-90% confluency before being sub-cultured or used in experiments. Purity of DMA was confirmed by gas chromatography-mass spectroscopy. All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) or VWR (Bridgeport, NJ).
4.2 MTT assay for cell viability
Cell viability assays were performed as previously described [22]. A solution of 3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT) was prepared in phosphate buffered saline (PBS) at a concentration of 5 mg mL-1. HCT-116, SW620, and HT-29 cells were seeded at 18,000 cells/well whereas THP-1 cells were seeded at 30,000 cells/well in 96-well plates and incubated at 37°C and 5% CO2 overnight. The next day, cells were washed with phosphate buffered saline (PBS), pre-treated with different concentrations of DMA (0.01, 0.1, 1, 10, 20, 30, 40, 50 and 100 mM) in CGM for 2 h and then treated with LPS (Escherichia coli 026:B6) (Sigma, St. Louis, MO) at 1 µg mL-l and human TNFa (R and D systems, Minneapolis, MN) at 40 ng mL-1 for 24 h. At the end of 24 h of treatment, 20 µl of MTT solution (5 mg mL-1) (Alfa Aesar, Ward Hill, MA) was added to each well at a final concentration of 0.5 mg mL-1. After incubation for 2 h at 37°C and 5% CO2, media was aspirated and dimethyl sulfoxide (DMSO) (100 µl/well) (BDH, Randor, PA) was added to dissolve formed purple formazan crystals. The plates were shaken on an orbital microplate shaker for 10 min to ensure complete solubilization of the crystals and the absorbance of the resulting purple solution was measured at 570 nm using an Opsys MR microplate reader (Dynex Technologies, Chantilly, VA).
4.3 Enzyme-linked immunosorbent assay (ELISA)
Sandwich ELISA was used to determine the levels of various cytokines and chemokines such as TNFa, Interleukin IL-6, IL-1b, IL-8, MCP, GM-CSF-1 and IL-10 and HMGB1 in cell culture supernatants. Ready-SET-Go! sandwich ELISA kits (eBioscience, San Diego, CA) for various human cytokines and chemokines were used as per manufacturers’ protocols. First, the optimal sample dilution for each target cytokine was determined to ensure that the sample absorbance readings fall within the range of their respective standard curves. Standard curves were produced by serially diluting lyophilized or recombinant standards, provided with the kits, according to the manufacturers’ instructions.
As per manufacturers’ protocols, clear flat-bottom Maxisorp 96-well plates (Thermo Fisher Scientific, Waltham, MA) were coated and incubated overnight at 4°C with capture antibody diluted in coating buffer for each analyte. The following day, each well was washed with washing buffer (1X PBS with 0.05% Tween 20) three times and then blocked for non-specific binding sites with assay buffer for one h. Samples were added to the wells in duplicate undiluted or diluted with assay buffer and incubated for 2 h at room temperature. After the incubation period, each well was washed three times and the detection antibody diluted in assay buffer for the respective analytes was added and samples were incubated for one more h. Following the detection antibody incubation each well was washed thrice and avidin-HRP labeled secondary antibody diluted in assay buffer was added and incubated for another 30 min. Before proceeding to the last step, wells were washed five times and tetramethylbenzidine (TMB) substrate was added and the samples were incubated for 15 min, which resulted in a blue-colored solution. After 15 min, the reaction was concluded by adding a stop solution (1M H3PO4), turning the blue solution to yellow and the sample absorbance was measured at 450 nm using an Opsys MR microplate reader. The concentration of each analyte was interpolated using a second order polynomial equation created from the standard curve, using GraphPad Prism 6 software, and then the value was multiplied by the dilution factor used in the assay to calculate the concentration of the analyte in the original undiluted sample.
4.4 Protein immunoblotting
To study the mechanism of DMA’s effect on cytokine secretion, its effect on IkBa expression in LPS stimulated THP-1 cells was examined. THP-1 cells were seeded at a density of 3 x 106 per T25 cm2 tissue culture flask and incubated overnight at 37°C and 5% CO2 using THP-1 appropriate CGM. The next day, cells were washed with sterile PBS and pre-treated with different concentrations of DMA (0.1, 1 or 10 mM) or plain CGM for the untreated group, for 2 h. Then, LPS was added to all cells, except for the untreated group, at a final concentration of 1 µg mL-1 and cells were incubated for another 30 min. Whole cell lysates were prepared at the end of the respective treatment periods as described below.
4.5 Preparation of whole cell lysates
Whole cell lysates were prepared using a modified protocol as described by Abcam. Cells were scraped off using a cell scraper (Greiner Bio-one, Monroe, NC) and collected in pre-chilled 5 ml snap cap centrifuge tubes and then centrifuged at 14,000 x g for 2 min. The old medium was aspirated from the 5 ml tubes and cells were washed twice with ice-cold PBS. The washed pellets were again collected into 1.5 ml pre-chilled centrifuge tubes and were then centrifuged at 1000 x g for 5 min. The supernatants were removed without disturbing the cell pellets, which were washed with ice-cold PBS once again and centrifuged at the same settings as mentioned above. Again, the supernatants were removed without disturbing the cell pellets and the pellets were resuspended in 65 µl of radio immunoprecipitation assay (RIPA) lysis and extraction buffer (G-Biosciences, St. Louis, MO). The RIPA lysis buffer was freshly supplemented with EDTA-free protease inhibitor cocktail set III (Calbiochem, San Diego, CA) (1:200 dilution) and with phenylmethylsulfonylfluoride (PMSF) (Calbiochem, San Diego, CA) at a concentration of 100 mM in ethanol (1:100 dilution). The cell pellets were thoroughly mixed with the prepared lysis buffer and were kept on ice with vortexing for 5 sec every 10 min, for a total of 30 min. The lysed cells were centrifuged at 14,000 x g for 20 min at 4°C (Eppendorf 5424R, Hauppauge, NY). The whole cell lysates were collected in labelled 0.65 ml microcentrifuge tubes and stored at -80°C until further analysis.
4.6 Automated capillary western blot analysis (WES Simple Western)
WES, an automated capillary-based electrophoresis system (ProteinSimple, San Jose, CA) was used for performing the protein expression analysis. The total protein concentration of each experimental sample was calculated using Pierce bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific, Waltham, MA). The volumes of the lysates used for WES analysis were determined based on total protein concentrations, using a bovine serum albumin (BSA) (Thermo Fisher Scientific, Waltham, MA) standard curve. The final concentration of protein loaded into the WES plate is optimized to be 1µg mL-1. All the reagents required for WES were prepared as per the manufacturer’s instructions. The provided 10X sample buffer was diluted using ultrapure water to 0.1X, which was used to dilute lysates. First, all standard pack reagents (DTT, 5X fluorescent master mix and biotinylated ladder) were prepared. Then the lysates were mixed with prepared 5X fluorescent master mix in a 4:1 ratio to have the final concentrations of the lysates at 1 µg µl-1. Finally, the lysates were heated at 95°C for 5 min.
The prepared lysates, blocking reagent, primary antibodies, HRP-conjugated secondary antibodies, chemiluminescent substrate mix (Luminol:Peroxide mixture in 1:1 ratio) and wash buffer were dispensed into designated wells in an assay plate provided by the manufacturer and as per the manufacturer’s instructions. The assay plate, along with the respective capillary cartridge, was placed into the WES instrument, which carries out all further assay steps automatically using default settings. Anti-I?Ba antibody (Cell Signaling technologies, Danvers, MA), diluted 1/50, was used as the primary antibody and GAPDH antibody (Cell Signaling Technologies, Danvers, MA), diluted 1/10,000, was used for the gel loading control. All primary antibodies were diluted in an antibody diluent provided by the manufacturer and the optimal dilution for each primary antibody was determined using Simple Western antibody database as a reference. In approximately 3 h, molecular weight and quantitative signals for target proteins were automatically reported by the Compass software (Protein Simple, San Jose, CA). Protein expression was analyzed using ImageJ software (NIH, Bethesda, MD) and normalized to that of GAPDH.
4.7 Animals
Nine-week-old male C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All experimental protocols were approved by the Mount Sinai Medical Center Institutional Animal Care and Use Committee (IACUC). All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). Mice were euthanized by carbon dioxide asphyxiation.
4.8 In vivo studies on the effect of DMA on dextran sodium sulphate (DSS)-induced colitis
A total of ten male C57Bl/6 mice weighing between 20 and 28 g were given drinking water containing 2.5% DSS. Mice were then randomly assigned to two groups. Group I mice (n=5) were negative controls and were injected intraperitoneally (ip) with 0.2 mL of PBS once a day for nine days. Group II mice (n=5) were injected ip with 0.2 mL of 33% DMA (2.1 g/kg) once a day for four days. One sham mouse was allowed to drink plain drinking water and injected with 0.2 mL PBS once a day for nine days. Mice were euthanized on the ninth day of the experiment and necropsied. The intestines were removed in their entirety for histologic examination.
4.9 Histological evaluation and grading
Several sections of the distal portion of the colon from each mouse were fixed in formalin, paraffin embedded, sectioned at 4 mM and stained with hematoxylin and eosin. Sections were examined by a practicing anatomic pathologist, who was blinded to the experimental conditions (S.E.R.), and scored, following the method described by Laroui et al. [41]. Each section was scored for severity of inflammation (0 = rare inflammatory cells in the lamina propria, 1 = increased inflammatory cells in the lamina propria, 2 = confluence of inflammatory cells extending into the submucosa, 3 = transmural inflammation); crypt injury (0 = intact crypts, 1 = loss of the basal one third of crypts, 2 = loss of the basal two thirds of crypts, 3 = loss of entire length of crypts, 4 = focal erosion of epithelial surface, 5 = confluent areas of erosion of the epithelium); and ulceration (0 = absence of ulceration, 1 = 1 or 2 foci of ulceration, 2 = 3 or 4 foci of ulceration, 3 = confluent ulceration). Scores were added to yield maximum histologic grade of 11. Sections were examined with a Nikon Eclipse 80i light microscope and images were captured with a Nikon Digital Sight camera (Nikon, Melville, NY).
4.10 Statistical analysis
All the data are represented as the mean +/- SEM of at least four independent experiments. The data were analyzed using GraphPad Prism 6 software (San Diego, CA). The statistical significance among and between groups was tested using a one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison post hoc test for the cell viability, cytokine secretion, and immunoblotting protein analyses. The Kruskal Wallis test was used to analyze differences in histologic scores. The a priori significance value was P<.05.
Ethics Approval and Consent to Participate
N/A
Consent for Publication
N/A
Availability of Data and Materials
The data that support the findings in this study are available from the corresponding author upon reasonable request.
Competing Interests
SER and CRA Jr. have a patent pending (US 13/536,946) on the use of N, N-dimethylacetamide for inflammatory disorders.
Funding
This work was supported by funds from the St. John’s University department of pharmaceutical sciences and a contract with the Nassau Health Care Corporation.
Authors’ Contributions
JBK performed most of the experiments and contributed to the writing of the manuscript. TS and GL performed the in vivo study. AG and LM performed the HMGB1 experiment. CRA helped to conceive the project. SER conceived the project, significantly contributed to the writing of the manuscript and provided funding.
Acknowledgments
We are grateful to the animal care facility staff at the Icahn School of Medicine at Mount Sinai, New York, NY for the housing and care of the mice.
References
- Peyrin-Biroulet L, Christopher R, Behan D, Lassen C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev 16 (2017): 495-503.
- Xu F, Dahlhamer JM, Zammitti EP, Wheaton AG, Croft JB. Health-risk behaviors and chronic conditions among adults with inflammatory bowel disease—United States, 2015 and 2016. Morbidity and Mortality Weekly Report 67 (2018): 190.
- Olén O, Askling J, Sachs MC, Neovius M, Smedby KE, Ekbom A, Ludvigsson JF. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964–2014. Gut 69 (2020): 453-61.
- Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, Sadeghi A, Nixon MR, Abdoli A, Abolhassani H. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 5 (2020): 17-30.
- Ye Y, Manne S, Treem WR, Bennett D. Prevalence of inflammatory bowel disease in pediatric and adult populations: recent estimates from large national databases in the United States, 2007–2016. Inflam Bowel Dis 26 (2020): 619-625.
- Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol 14 (2008): 390.
- Torres M, Ríos A. Current view of the immunopathogenesis in inflammatory bowel disease and its implications for therapy. World J Gastroenterol 14 (2008): 1972-1980.
- Cui L, Feng L, Zhang ZH, Jia XB. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int Immunopharmacol 23 (2014): 294-303.
- Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res 14 (2008): 6735- 41.
- Mitsuyama K, Toyonaga A, Sasaki E, Watanabe K, Tateishi H, Nishiyama T, et al. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn's disease. Clin Exper Immunol 96 (1994): 432-36.
- Daig R, Andus T, Aschenbrenner E, Falk W, Scholmerich J, Gross V. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut 38 (1996): 216-22.
- Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev 5 (2002): 79-94.
- Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nature Med 6 (2000): 583-588.
- Roda G, Ng SC, Kotze PG, Argollo M, Panaccione R, Spinelli A, et al. Crohn’s disease. Nature Rev Dis Primers 6 (2020): 1-19.
- Yang H, Wang H, Andersson U. Targeting inflammation driven by HMGB1. Front Immunol (2020).
- Yang H, Tracey KJ. Targeting HMGB1 in inflammation. Biochim Biophys Acta 1799 (2010): 149-56.
- Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Ann Rev Immunol 29 (2011): 139–62.
- Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, et al. HMGB1 in health and disease. Mol Aspects Med 40 (2014): 1–116.
- Andersson U, Yang H, Harris H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin Ther Targets 22 (2018): 263–77.
- Hu Z, Wang X, Gong L, Wu G, Peng X, Tang X. Brit J Role of high-mobility group box protein in inflammatory bowel disease. Brit J Pharm 124 (2015): 131-33
- Sundaram S, Ashby CR, Pekson R, Sampat V, Sitapara R, Mantell L, et al. N, N-Dimethylacetamide regulates the proinflammatory response associated with endotoxin and prevents preterm birth. Am J Pathol 183 (2013): 422-430.
- Pekson R, Poltoratsky V, Gorasiya S, Sundaram S, Ashby CR, Vancurova I, et al. N, N-Dimethylacetamide significantly attenuates LPS- and TNFα-Induced proinflammatory responses via inhibition of the nuclear factor kappa B pathway. Mol Med 22 (2016): 747-758.
- Ghayor C, Gjoksi B, Dong J, Siegenthaler B, Caflisch A, Weber FE. N, N-dimethylacetamide a drug excipient that acts as bromodomain ligand for osteoporosis treatment. Sci Rep 7 (2017): 42108.
- Siegenthaler B, Ghayor C, Ruangsawasdi N, Weber Fe. The release of the bromodomain ligand N,N-dimethylacetamide adds bioactivity to a resorbable guided bone regeneration membrane in rabbit calvarial defect model. Materials (Basel) 13 (2020): 501.
- Bhattacharya I, Ghayor C, Pérez DA, Weber FE. N,N-Dimethylacetamide prevents high-fat diet-induced increase in body weight. Front Pharmacol 10 (2019): 1274.
- Khera N, Ghayor C, Lindholm AK, Pavlova E, Atanassova N, Weber FE. Reversible contraceptive potential of FDA approved excipient N,N-dimethylacetamide in male rats. Front Physiol 10 (2020): 11601084.
- Atreya I, Atreya R and Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med 263 (2008): 591-96.
- Ardite E, Panés J, Miranda M, Salas A, Elizalde JI, Sans M, et al. Effects of steroid treatment on activation of nuclear factor kB in patients with inflammatory bowel disease Brit J Pharmacol 124 (1998): 431-33.
- Khanna R, Marshall JK. Sulfasalazine and 5-aminosalicylates for ulcerative colitis. In Crohn’s Disease and Ulcerative Colitis, Baumgart DC (ed). Springer International Publishing (2017): 389-98.
- Hindryckx P, D’Haens Geert. Biological therapy of Crohn’s disease: Natalizumab, Velolizumab, and anti-MadCAM. In Crohn’s Disease and Ulcerative Colitis, Baumgart DC (ed). Springer International Publishing (2017): 375-380.
- European Association for the Study of the Liver. Clinical practice guidelines: Drug-induced liver injury. J Hepatol 70 (2019): 1222-61.
- Chen BK, Yang YT, Bennett CL. Why biologics and biosimilars remain so expensive: despite two wins for biosimilars, the supreme court’s recent rulings do not solve fundamental barriers to competition. Drugs 78 (2018): 1777-81.
- Hempel G, Oechtering D, Lanvers-Kaminsky C, Klingebiel T, Vormoor J, Gruhn B, et al. Cytotoxicity of dimethylacetamide and pharmacokinetics in children receiving intravenous busulfan. J Clin Oncol 25 (2007): 1772-78.
- Wéra O, Lancellotti P, Oury C. The dual role of neutrophils in inflammatory bowel diseases. J Clin Med 5 (2016): 118.
- Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. (1994) Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut 35 (1994): 669-74.
- Matricon J, Barnich N, Ardid D. Immunopathogenesis of inflammatory bowel disease. Self Nonself 1 (2010): 299-309.
- Reinisch W, Gasche C, Tillinger W, Wyatt J, Lichtenberger C, Willheim M, et al. Clinical relevance of serum interleukin-6 in Crohn's disease: single point measurements, therapy monitoring, and prediction of clinical relapse. Am J Gastroenterol 94 (1999): 2156-64.
- Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut 31 (1990): 686-89.
- Li MC and He SH. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J Gastroenterol 10 (2004): 620-25.
- Keubler LM, Buettner M, Häger C, Bleich A. A multi-hit model: colitis lessons from the interleukin-10-deficient mouse. Inflamm Bowel Dis 21 (2015): 1967-75.
- Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV, Merlin D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids. PLoS ONE 7 (2012): e32084.
- Lee J, Rhee MH, Kim E, Cho JY. BAY 11-7082 is a broad spectrum inhibitor with anti-inflammatory activity against multiple targets. Mediators Inflamm (2012).
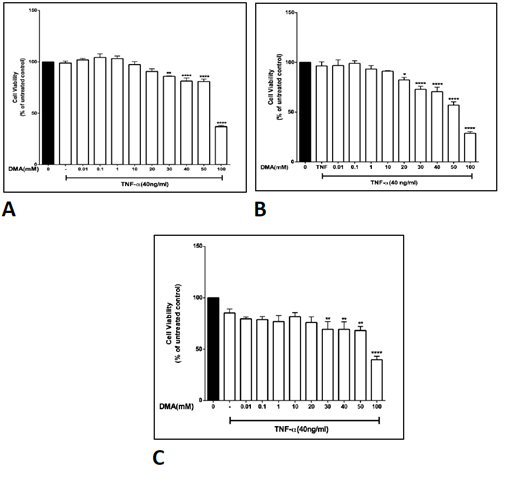
was determined by MTT assay in the absence or presence of increasing concentrations of DMA
after stimulation with 1 μg ml-1 LPS. A, HT-29 cells; B, HCT-116 cells; C, SW620 cells; D, THP-1
cells. *P<.05; **P<.01; ***P<.001; ****P<.0001.
Supplementary Figure 3: The effect of DMA on cell viability in the presence of TNFa. Cell viability was determined by MTT assay in the absence or presence of increasing concentrations of DMA after stimulation with 40 ng mL-1 TNFa. A, HT- 29 cells; B, HCT-116 cells; C, SW620 cells. *P<.05; **P<.01; ****P<.0001.
|
Sample |
Inflammation |
Crypt Abscesses |
Ulceration |
Total |
|
DSS Control 1 |
3 |
5 |
3 |
11 |
|
DSS Control 2 |
3 |
5 |
3 |
11 |
|
DSS Control 3 |
3 |
5 |
3 |
11 |
|
DSS Control 4 |
3 |
5 |
3 |
11 |
|
DSS Control 5 |
3 |
5 |
3 |
11 |
|
DSS + DMA 1 |
3 |
4 |
3 |
10 |
|
DSS + DMA 2 |
2 |
3 |
1 |
6 |
|
DSS + DMA 3 |
3 |
5 |
3 |
11 |
|
DSS + DMA 4 |
3 |
3 |
2 |
8 |
|
DSS + DMA 5 |
1 |
4 |
2 |
7 |
|
No DSS |
1 |
0 |
0 |
1 |
Supplementary Table 1: Effect of DMA on histologic scores in DSS colitis.



 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 