Anti-Inflammatory and Neuroprotective Activity of Viphyllin A Standardized Extract of β-Caryophyllene from Piper Nigrum L (Piperaceae) and its Associated Mechanisms in Mouse Macrophage Cells and Human Neuroblastoma SH-SY5Y Cells
Kuluvar Gouthamchandra*, 1, Heggar Venkataramana Sudeep 1, Anusha Sathish1, Amritharaj1, Lingaraju HB2, Naveen Puttaswamy3, Shyam Prasad Kodimule2, 3
1Department of Biomedicinal Research, R&D Centre for Excellence, Vidya Herbs Pvt. Ltd, #14A, Jigani I Phase, Bangalore-560105, Karnataka, India
2Department of Phytochemistry, R&D Centre for Excellence, Vidya Herbs Pvt. Ltd, #14A, Jigani, I Phase, Bangalore-560105, Karnataka, India
3Department of Analytical Development Research Lab. R&D Centre for Excellence, Vidya Herbs Pvt. Ltd, #14A, Jigani I Phase, Bangalore-560105, Karnataka, India
*Corresponding author: Dr. Kuluvar Gouthamchandra, Department of Biomedicinal Research, R&D Centre for Excellence, Vidya Herbs Pvt. Ltd, #14A, Jigani I Phase, Bangalore-560105, Karnataka, India
Received: 28 December 2023; Accepted: 06 January 2024; Published: 22 January 2024
Article Information
Citation: Kuluvar Gouthamchandra, Sudeep Heggar Venkataramana, Anusha Sathish, Amritharaj, Lingaraju HB, Naveen Puttaswamy, Shyam Prasad Kodimule. Anti-Inflammatory and Neuroprotective Activity of Viphyllin A Standardized Extract of β-caryophyllene from Piper Nigrum L (Piperaceae) and its Associated Mechanisms in Mouse Macrophage Cells and Human Neuroblastoma SH-SY5Y Cells. Fortune Journal of Health Sciences. 7 (2024): 25-36.
View / Download Pdf Share at FacebookAbstract
Oxidative stress breeds various chronic lifestyle ailments including inflammatory conditions and neurodegenerative diseases. β-caryophyllene natural bicyclic sesquiterpene, obtained from various plants sources found to be effective against inflammation and neuroprotection. In this study, we have evaluated the protective effect of Viphyllin, a standardized extract of β-caryophyllene from black pepper against inflammation induced by lipopolysaccharide in RAW264.7 macrophage cells and mechanisms involved in hydrogen peroxide (H2O2)- challenged oxidative stress in human neuroblastoma SH-SY5Y cells. Viphyllin demonstrated the anti-inflammatory activity by subsiding the release of the pro-inflammatory intermediaries like NO, cytokines, interleukins, and protein expression levels of cyclooxygenase (COX-2) and inducible nitric oxide synthase (iNOS). In addition, Viphyllin suppressed the extracellular signal-regulated kinase (ERK). c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) phosphorylation. On the other hand, Viphyllin showed neuroprotective effect against neuronal oxidative damage caused by H2O2. Viphyllin lessened the expression B-cell lymphoma-2 (Bcl-2), B-cell lymphoma-2-associated X protein (BAX), cleaved caspase-9, and PARP-1 proteins associated with apoptosis. Our findings indicate that Viphyllin ameliorated LPS-mediated inflammation in macrophages by regulating inflammation and Viphyllin exerted remarkable anti apoptotic effect against neuronal damage challenged by H2O2. Altogether, Viphyllin could be potential functional food ingredient for inflammation and neurodegenerative diseases.
Keywords
<p>Inflammation, cytokines, oxidative stress, apoptosis, COX-2, MTT</p>
Article Details
1. Introduction
Pharmacological propertiesof medicinal plants are primarily due to the presence of secondary metabolites like polyphenols, steroids, flavonoids, alkaloids andterpenoids [1, 2]. Terpenoids have been studied as an alternative to treat different disease conditions. Certain terpenoids for instance Taxol and Artemisinin has been using as anticancer and antimalarial drug, respectively [3, 4]. Terpenoids play diverse role in the field of food, drugs, and cosmetics, studies show the relationship of terpenoid consumption with the reduction of inflammation for example Citronellol, geraniol, d-limonene and Ursolic acid (UA), a pentacyclic triterpenoid carboxylic acid, is the major component of many plants. β-caryophyllene is a “natural bicyclic sesquiterpene hydrocarbon” it is most abundant constituent exist in many plant-originated essential oils, like as lemon balm, cloves, Cannabis sativa and black pepper [5, 6]. Recent studies are reported on significant biological activities of β-caryophyllene against inflammation, cancer and chronic pain and chemo sensitizing properties for chemotherapies like oxaliplatin, doxorubicin sorafenib, and 5-fluorouracil. On the other hand, β-caryophyllene is a plant (or food) derived cannabinoid ligand, and it is also reported that it functions as a selective agonist of cannabinoid receptor 2 (CB2). It is thought that CB2 receptor activation is suitable medicinal approach for the treatment of inflammation related disorders. United States Food and Drug Administration considers β-caryophyllene is a dietary phyto cannabinoids and has been included in the list of “generally regarded as safe” ingredients for dietary use [7, 8]. In the current investigation, we have enriched and systematized the extract in a manner that is not less than 30% β-caryophyllene from black pepper and termed it as Viphyllin, subsequently investigated for anti-inflammatory activity in LPS induced mouse macrophage cells and neuroprotective property against oxidative stress induced by H2O2.
2. Materials and Methods
2.1 Chemical and Reagents
Lipopolysaccharide (LPS, Escherichia coli 0128: B12) (Catalog No. L2630), Hydrogen peroxide 30% (Catalog No: 7722-84-1) was purchased from Sigma-Aldrich. The Mouse Prostaglandin E2, PG-E2 (Cat No: KLM2578). Mouse TNF-α (Catalog No: KB2145). Mouse IL-6 (Catalog No KB2068). Mouse IL-1β (Catalog No: KB2063) ELISA kits were purchased from KRISHGEN BioSystems. Antibodies for cyclooxygenase 2 (COX-2) (Catalog No. sc-1999), iNOS (Catalog No. sc-7271), phosphorylated Erk (Catalog No. sc-81492), phosphorylated JNK (Catalog No. sc-6254), phosphorylated p38 (Catalog No. sc-7973), PARP1 (Catalog No. sc-8007), Caspase-9 (Catalog No.sc-73548), BCL2 (Catalog No. sc-7382) and BAX (Catalog No. sc-20067) were purchased from Santa Cruz. Relative peroxidase-conjugated secondary antibody (Catalog No. A4416), β-actin (Catalog No. A3854) were purchased from the Sigma Aldrich, Bradford reagent (Catalog No. 5000006) from Bio-Rad and Pierce™ ECL Western Blotting Substrate (Catalog No. 32106) were purchased from ThermoFisherScientific.
2.2 Sample preparation
Viphyllin a standardized extract of β-caryophyllene from black pepper (Piper Nigrum L) was collected from the Department of Phytochemistry, Vidya Herbs Pvt. Ltd. (Bangalore, Karnataka, India), and dissolved in cell culture media (DMEM) at the appropriate concentrations. (Supplementary figure S1).
2.3 Cell culture
RAW 264.7 Murine macrophage and Human neuroblastoma SH-SY5Y cells were obtained through NCCS (Pune, India), RAW264.7 cells were cultured in DMEM media containing 10% foetal bovine serum and antibiotic solution in a humidified 5%CO2 incubator (Thermo Fisher Scientific). The cell culture medium was replaced with fresh DMEM media every 2 to 3 days, once cells reached 80% confluence, the cells were subcultured. SH-SY5Y cells were grown in DMEM and F12 nutrient mix subsidized with FBS (10%) and antibiotic solution respectively at 37 °C in the CO2 incubator.
2.4 Cell viability assay
MTT assay was adopted to check the influence of Viphyllin on the viability of RAW 264.7 cells. Briefly, cell suspensions were seeded at density of 1×104cells/well in 96-well plates. After 18 h of incubation, the cells were treated with the different concentrations(5-50μg/mL) of Viphyllin, further incubated for 24 h. The supernatant was discarded and 100 μL of MTT solution (0.5 mg/mL) was replaced and cells were incubated for an extra 2-4hrs, subsequently 100 μL of MTT stop solution (DMSO) was added to wells to dissolve the formazan crystal. The absorbance was read at 550 nm using a microplate reader (Tecan Infinite M200 Pro). Likewise, cell viability assay was conducted on SHSY5Y cells. The preventive effect of Viphyllin versus H2O2- elicited neurotoxicity was investigated by incubating cells with 12 and 24µg/mL of Viphyllin for 6 h and then treated them with 500μM H2O2 for 24 h. Cell viability of cells were determined using the MTT assay as described above.
2.5 Determination of NO
NO production was determined by by Griess reagent. Briefly, RAW macrophages were set at 4×104 cells/well in 96-well plates for 18 h. Then the cells were incubated with different concentrations (5-50μg/mL) of Viphyllin before they were stimulated with 1μg/ml lipopolysaccharide. After completion of 24h incubation, using the cell culture supernatant NO content was measured by Griess reagent.
2.6 Evaluation of cytokine secretion level in macrophages
The secretion level of PGE-2, TNF-α, IL-6 and IL-1β, inflammatory cytokines levels were measured by the ELISA using in the cell culture supernatant of RAW 264.7 macrophages according to manufacturer’s protocol (KrishgenBiosystems, Mumbai,India).
2.7 Lactate dehydrogenase (LDH) assay
The assay was performed by employing an LDH cytotoxicity detection kit (Sigma aldrich). Briefly, SH-SY5Y cells were added at the density of 2.5×104 cells/ mL in 96-well. Then the cells were treated with Viphyllin at different concentrations (5-50μg/mL) for 24h. with and without H2O2. The amount of LDH release was determined by using a microplate reader (Tecan Infinite M200 Pro) at 490 nm.
2.8 Western Blot Analysis
RAW macrophages were incubated overnight and subjected to two different concentrations of Viphyllin 12 and 24µg/mL prior to LPS (1µg/mL) treatment. Cells were collected by trypsinization after 24 h incubation and were lysed with 100µL of radioimmunoprecipitation (RIPA) lysis buffer. Total protein concentration was measured using the Bradford reagent (Bio-Rad). Equivalent amounts of protein (30µg) were separated by sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the protein bands were transferred onto a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% non-fat milk solution (and incubated with 1:1000 diluted primary antibodies (cox2. iNOS, p-ERK, p-JNK, p-p38 and β-actin) at 4οC for overnight, and subsequently probed with specific secondary antibodies coupled to horseradish peroxidase (Santa Cruz Biotech, Dallas, TX, USA) and bands were visualizedusingECLreagents, and quantified using the ImageJ software (National Institutes of Health, Bethesda, MD, USA). Similarly, western blot experiment was conducted in H2O2 induced oxidative damage in SH-SY5Y cells using PARP1, caspase 9, Bcl-2 and BAX primary antibodies.
2.9 Statistical analysis
One -way ANOVA and Dunnett’s multiple comparisons test has been used to determine the statistical significance of differences among control and treated groups. Experiments were carried out in triplicate and all the values that were expressed as a mean ± standard error. GraphPad Prism 9.1 was used for statistical analysis.
3. Results
3.1 Cell viability
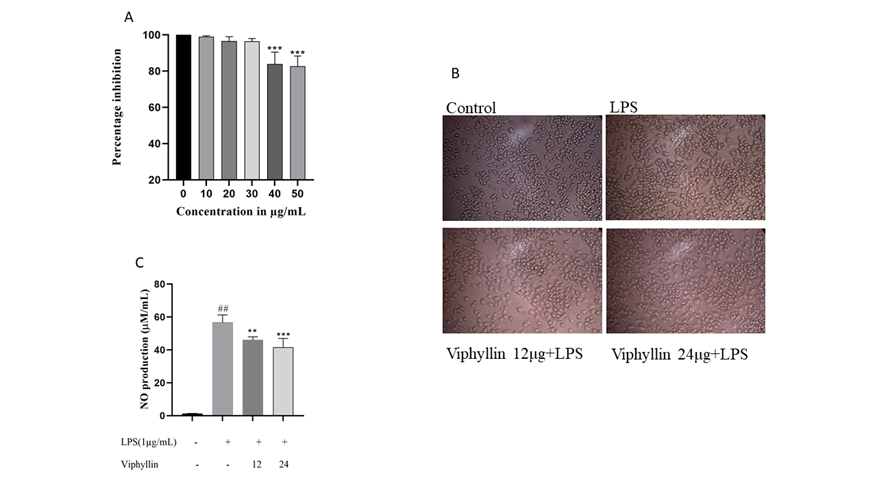
We investigated the cytotoxic effect of Viphyllin in RAW264.7 murine macrophages. As shown in Figure 1A. Viphyllin had no cytotoxic effect on macrophages up to the dosage of 30 µg, whereas higher dose of Viphyllin showed significant cytotoxicity in RAW264.7 cells. Hence, we concluded that concentrations up to 30 μg/ml of the Viphyllin could be safe. Thus, in the subsequent experiments, RAW274.7 cells were treated with nontoxic concentrations of Viphyllin. The morphology of LPS elicited cells had an uneven shape and spreading compared to untreated cells, whereas Viphyllin treated cells offer protection against LPS treatment by maintaining normal shape of cells (Figure 1B).
3.2 Effect of Viphyllin on Nitric Oxide (NO) production
NO is thought to be as a pro-inflammatory mediator that causes inflammation due to over production in abnormal conditions. In our study we observed substantial changes in the production of NO in the cell culture supernatant of LPS treated RAW 264.4 murine macrophages cells. Interestingly, Viphyllin treatment dose dependently reduced NO production in cell culture supernatants of RAW 264.4 murine macrophages treated with LPS compared to LPS alone treated RAW 264.4 murine macrophages. (Figure 1C).
Figure 1: Effect of Viphyllin on cell viability, morphological changes, and production of Nitric oxide (NO) in LPS induced RAW 264.7 cells. (A) RAW 264.7 cells were treated with various concentration of Viphyllin (10-60µg/mL) for 24 h and cell viability was determined. Values are expressed as a percentage of control. Each bar is the mean ± SEM (n = 3). ***p≤0.001 indicates a significant difference compared with untreated controls. (B) Morphological changes. (C) RAW 264.7 cells were pretreated with 12 and 24/µg/mL of Viphyllin followed by LPS (1µg/mL) treatment for 24 h. NO production was determined. Data were expressed as mean ± SEM. ## p<0.0001 indicates a significant difference compared with untreated control ***(p<0.001). indicates a significant difference compared with LPS.
3.3 Influence of Viphyllin on secretion of PGE2, TNF-α. IL-6 and IL-1β in LPS challenged RAW264.7 macrophages.
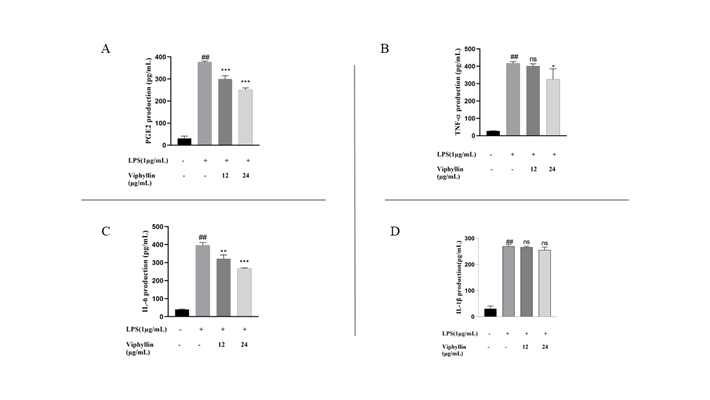
The effect of Viphyllin treatment on the secretion of inflammatory mediators PGE2 and cytokines (TNF-α IL-6 and IL-1β) in the supernatant of RAW264.7 macrophages challenged with LPS were examined. As shown in Figure 2A-D, LPS treatment significantly elevated the level of cytokines in the cell culture supernatant of RAW264.7 macrophages (P-value<0.001) respectively, compared untreated normal cells. Interestingly, Viphyllin treatment significantly (P-value<0.001) subsided the release of proinflammatory cytokines IL-6, PGE-2, and TNF-α in a dose-dependent way. However, the expression of IL-1β remained the same.
Figure 2: Effects of Viphyllin on the production of inflammatory mediator PGE2 and inflammatory cytokines TNF- α, IL-6 and IL-1β in LPS-induced RAW 264.7 cells. Cells were pre-treated with Viphyllin for 4 h and then elicited with 1μg/mL of LPS for 24 h.(A) The productions of PGE2 (B)TNF– α. (C) IL-6 and (D)IL-1β in the cell culture supernatant were measured using an ELISA Kit. The data represent the mean ± SEM (n = 3). ## p < 0.0001 vs. control group. *p < 0.05, **p < 0.001 *** p < 0.0001 and ns: not significant vs. LPS group.up.
3.4 Effect of Viphyllin on the expression amount of COX-2, and iNOS protein in LPS elicited RAW 264.7 macrophages.
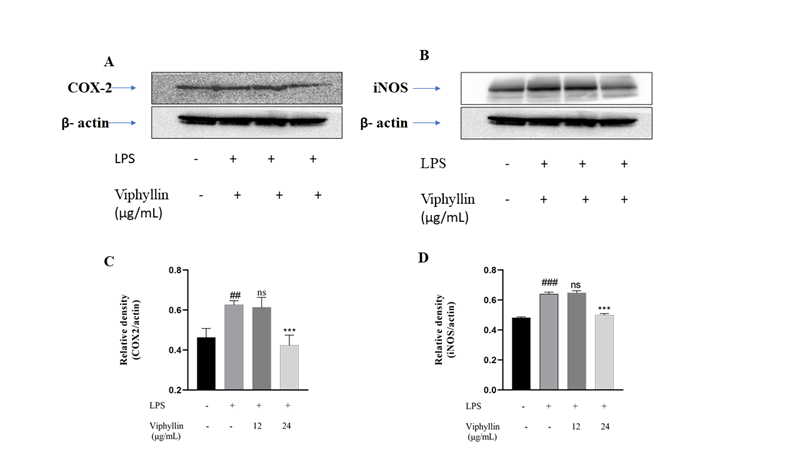
To examine the influence of Viphyllin as anti-inflammatory agent we have carried out western blotting experiments to check the expression amounts of COX-2, and iNOS protein in murine macrophages challenged by LPS(1µg/ml). 24h later, the presence of LPS (1µg/ml) in RAW 264.7 cells caused the dramatic upregulation of COX-2 and INOS protein levels when compared untreated normal cells. However, treatment with Viphyllin, in presence of LPS significantly subsided the expression levels of COX-2 and iNOS proteins at concentration at 24µg/mL compared to LPS alone treated RAW 264.7 macrophages (Figure 3).
Figure 3: Inhibitory effect of Viphyllin in LPS induced protein expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in RAW 264.7 macrophage cells. Quantifications of COX-2 (A) and iNOS (B) immunoblots were normalized to that of β-actin followed by statistical analysis of relative image density in comparison to control. ## p < 0.0001 significantly different from the control group. ** p < 0.001, *** p < 0.0001 significantly different from the LPS-induced group.
3.5 Effect of Viphyllin on MAPK signalling pathway in LPS-Induced RAW 264.7 Cells
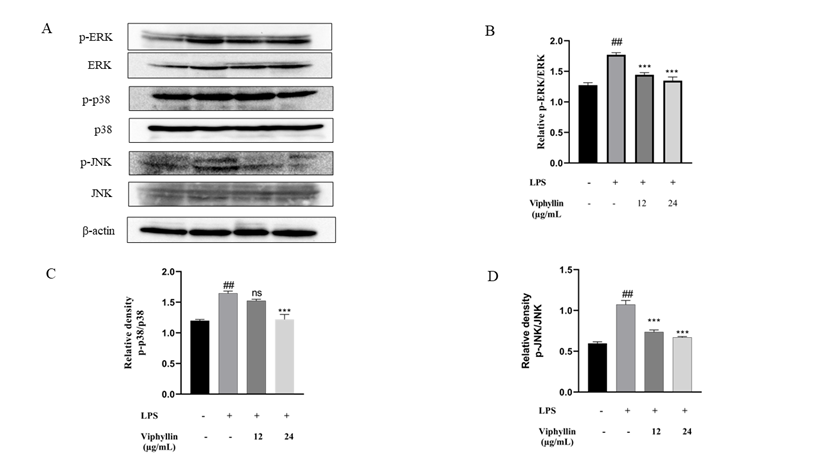
In many diseases including cancer, viral infection and neurological disorders, extreme inflammation is well established as a crucial factor. Enhanced activity of MAPK, specifically p38 and their involvement in the management of the synthesis of inflammation intermediaries make them targets for anti-inflammatory drugs. To explore if these signalling pathways are affected by Viphyllin, we conducted western blot experiments. Western blot results showed that LPS stimulation significantly elicited phosphorylation of p-ERK, p-JNK and p-p38 MAPK compared to control untreated cells. In contrast, we noticed Viphyllin pre-treatment with 12 and 24μg/mL mitigated phosphorylation of JNK and p38 (Fig.4A-D). However, remarkably we observed Viphyllin at the concentration of 12µg/mL had significant effect on suppression of p-ERK. These results suggest that Viphyllin could inhibit inflammatory response through regulation of MAPKs signalling in LPS-activated murine macrophages.
Figure 3: Inhibitory effect of Viphyllin in LPS induced protein expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in RAW 264.7 macrophage cells. Quantifications of COX-2 (A) and iNOS (B) immunoblots were normalized to that of β-actin followed by statistical analysis of relative image density in comparison to control. ## p < 0.0001 significantly different from the control group. ** p < 0.001, *** p < 0.0001 significantly different from the LPS-induced group.
Figure 4: Inhibition of the phosphorylation of MAPKs signalling by Viphyllin during RAW 264.7 macrophage cells activation by LPS. Western blot analysis by using antibodies specific for phosphorylated forms of, ERK1/2, JNK and p38 MAPK as described in the methods. The relative protein levels were quantified normalized to β-actin, The results were expressed as means ± SE (n = 3), ## p < 0.0001: significantly different from the control group. * p < 0.05, ** p < 0.001 significantly different from the LPS-induced group.
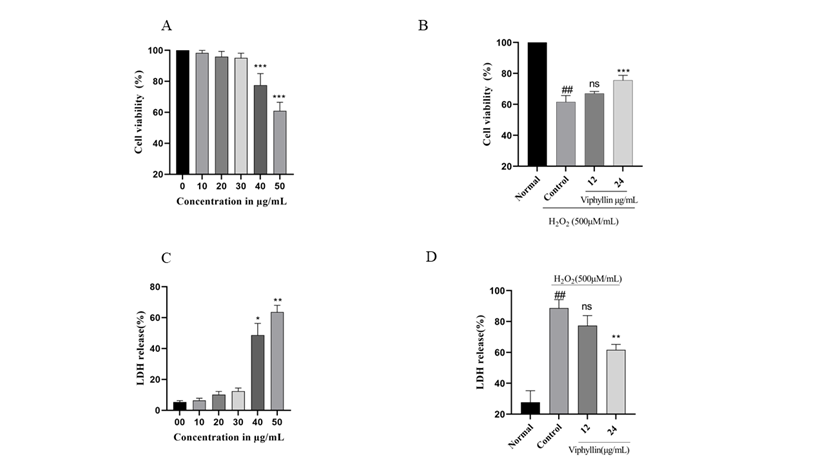
3.6 Neuroprotective Effect of Viphyllin on H2O2-challenged Human Neuroblastoma SH-SY5Y cells.
Investigate the effect of Viphyllin presence on cell viability, SH-SY5Y cells were treated with Viphyllin at various concentrations ranging from 5–50µg/mL for 24 hr (Figure 5A). It has been observed that Viphyllin had no significant effect on SH-SY5Y cells up to the dosage of 30μg/ml where its viability ranges from (98.3, 95.97% and 95.12%) respectively, whereas higher concentration of Viphyllin subsided the cell viability. Therefore, we have selected the nontoxic dose (12 and 24μg/ml) of Viphyllin for further experiments. H2O2 is well established neurotoxin, which generates highly reactive oxygen species like hydroxyl, superoxide anion radicals, resulting in cellular damage. Determine protective effect of Viphyllin against H2O2 induced oxidative stress in SH-SY5Y cells, cells were pre-incubated with two different concentration (12 and 24μg/mL) of Viphyllin for 4 h and then subjected to 500μM of H2O2 for 24 h. cell viability assay demonstrated that presence of H2O2 decreased viability of SH-SY5Y cells, while interestingly 24μg/mL of Viphyllin pre-treatment showed considerable protection against H2O2-generated loss of cells (Figure 5B).
3.7 LDH assay
LDH is an intracellular enzyme that exists in the cells, elevated levels of LDH enzyme in the cell culture medium implies cellular membrane burst and it is considered as label of cell death. Impact of various concentration of Viphyllin on LDH release was examined, as shown in Figure 5C. Viphyllin up to the concentration of 30μg/mL did not affect the LDH release, on higher concentration significant release of LDH was observed, consequently nontoxic dose of Viphyllin was evaluated for its protective effect against H2O2 induced toxicity. LDH secretion in the H2O2-treated control group was significantly elevated compared to untreated control cells with the release of 88.66%. However, pre-treatment of Viphyllin significantly decreased H2O2-induced LDH release suggesting suppression of neuronal toxicity stimulated by H2O2 in SH-SY5Y cells (Figure 5D).
Figure 5: Effects of Viphyllin on the cell viability (MTT assay) and LDH release of SHSY-5Y cells. SHSY-5Y cells pre-treated with 10-50μg/mL of Viphyllin for 24 h followed by subsequent exposure to 500 μM H2O2 for 24h. (A) Viphyllyn alone; (B) Viphyllin+500μM H2O2; (C) Viphyllyn alone on LDH release; (D) Viphyllin+500μM H2O2 on LDH release. Results are expressed as mean ± SEM for three independent experiments. ##P < 0.0001vs control group, ***P < 0.0001 vs. H2O2 group.
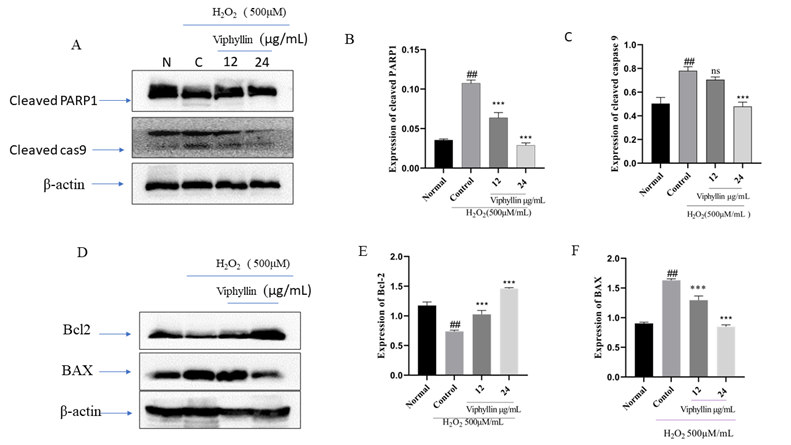
3.8 Effects of Viphyllin on apoptosis-related protein expressions in H2O2-induced SH-SY5Y cells
It has been reported that accumulation of H2O2 causes apoptosis, inflammation and necrosis in the brain is well known in neurodegenerative disease. H2O2 stimulated oxidative stress in SHSY-5Y cells is well established model for studying neurological disorders. Determine the antiapoptotic effect of Viphyllin, we investigated the of pro and antiapoptotic protein expression in SHSY-5Y cells. As presented in Figure 6A-C. we found the increased expressions of cleaved PARP1 and caspase-9 in the H2O2-stimulated SHSY-5Y cells when compared with the untreated normal cells. Interesting we observed Viphyllin treatment at the concentration of 24μg/mL noticeably lessened the -highly expressed cleaved PARP1 and caspase-9. In addition, As represented in Figure 6 D-F. H2O2(500µM) treated group we found significantly elevated expression of the pro-apoptotic gene BAX and subsided expression of Bcl-2 an anti-apoptotic protein in SH-SY5Y cells, whereas Viphyllin treatment dose dependently reversed these effects.
Figure 6: Effect of Viphyllin on apoptosis-related protein expressions in H2O2-treated SH-SY5Y cells(A). western blot analysis and quantification of immunoblot for the cleaved-caspase-9, cleaved-PARP-1and ratio of BAX and Bcl-2 to β-actin respectively. values are expressed as mean ± SEM(n=3). ##P < 0.0001vs control group, ***P < 0.0001 vs. H2O2 group. ns: not significant
4. Discussion
Persistent inflammation has an extensive role in the pathogenesis of various chronic diseases like diabetes, obesity, cardiovascular and neurological disorders [9-11]. Approaches to aim for inflammation has become an attractive approach to defend inflammatory-related ailments [12, 13]. Over the last few years, bioactive constituents or standardized extracts obtained from plant sources still play a significant role in the treatment of many ailments without any adverse effect [14]. Macrophages are crucial participants in the immune response to foreign intruders such as infectious microorganisms. lipopolysaccharide (LPS) is a well-known prototypical endotoxin may directly activate macrophages. Plethora of measures have recommended that activated macrophage produce a greater amount of various proinflammatory intermediaries like NO and PGE2, also proinflammatory cytokines for example TNF-α, and interleukins. All these elements stimulate the development of inflammation and worsen the inflammation over the harmonious interaction with further inflammatory intermediaries and there are reports that in variety of inflammatory ailments including asthma and colitis the expression levels of PGE2 and NO is elevated [15-17]. Our study revealed that Viphyllin efficiently inhibited the secretion of PGE2 and NO, and proinflammatory cytokines in LPS-challenged macrophages at safer dose, our findings are consistent with earlier published studies [18,19]. COX-2. is an important target for the design of drugs against inflammation, particularly NSAIDs. Furthermore, iNOS and COX-2 enzymes play crucial role in the generation of NO and PGE2, Viphyllin significantly subsided the iNOS and COX-2 expression in LPS stimulated macrophages. Our results are consistent with the previous published reports [20, 21].
The MAPK signalling cascade plays a key role in the initiation of response to inflammation. ERK, JNK, and p38 participate in MAPK signalling pathways, which gets activated by TNF-α and interleukins [22]. Earlier studies have shown that LPS or TNF activates MAPKs which are involved in acute lung injury. It has been reported that when MAPK gets activated phosphorylated ERK, p38, and JNK are moved to the nucleus and stimulates response to inflammation by inducing expression of proper gene of interest [23,24]. Pre-treatment of Viphyllin inhibited MAPK signalling by subsiding the phosphorylation of ERK, JNK, and p38 in LPS induced macrophages. It is evident from the study that inhibition of MAPK pathway is a potential mechanism by which Viphyllin wields anti-inflammatory effects. These findings are consistent with those earlier studies [25, 26].
Lately, several findings have found neurodegenerative diseases caused by stress and has been extensively associated in neuronal cell death. Previous studies have reported that H2O2 accumulation has been well studied in neurodegenerative disease. Human neuroblastoma SH-SY5Y cells are very well-regarded cellular tool for research on H2O2-stimulated oxidative stress [27-29]. In our recent study we have explored standardized β-caryophyllene extract improves cognitive function in scopolamine-induced amnesia model [30]. In current study, we have studied the protective effect of Viphyllin against H2O2- provoked apoptotic events in SH-SY5Y cells. It has been showed that H2O2 stimulated oxidative damage dramatically declines viability of SH-SY5Y cells [31]. Our study also showed decline of cell viability in the presence of H2O2 in SH-SY5Y cells. Interestingly we have noticed viability of cells enhanced dose dependently by the treatment of Viphyllin. It has been well established that damage to cellular membranes releases LDH into the cell culture supernatant is important marker of cell death [32]. From our data it is noticeably clear that elevated level of LDH was found in the culture medium after H2O2-induction in SH-SY5Y cells. Treatment with the Viphyllin markedly lower the LDH in the SH-SY5Y cell culture medium subjected to H2O2. Hence, we reaffirmed the defensive effects of Viphyllin in H2O2-elicited SH-SY5Y cells. Our outcomes are consistent with previously published study [33]. It has been reported that toxic effect on SHSY-5Y cell is caused by H2O2 is linked with mitochondria-associated apoptotic events and it is considered as one of the valuable tools in neurodegenerative diseases [34]. Cytochrome release occurs when elevated levels of ROS penetrate mitochondrial membrane which leads to the activation of caspase -9 and PARP-1 and gradually induces cell death [35]. Our results demonstrated that markedly elevated proteolytic cleavage of caspase-9 and PARP in H2O2 subjected SHSY-5Y cells. However, Viphyllin protects neuronal cells against H2O2 stimulated oxidative stress by lessening the expression of caspase-9 and PARP1. Furthermore, mitochondrial membrane injury leads to neuronal apoptosis by up-regulation of Bcl-2 and down-regulation of Bax, which leads to inhibition of caspase activation [36]. In current study, Viphyllin treatment lowered Bax level and enhanced Bcl-2 level. Hence, these outcomes demonstrated that Viphyllin along with other active constituents from black pepper offered preventive effects against oxidative damage, our results were consistent with other published studies [37,38].
5. Conclusion
In conclusion, our current unearthing demonstrated that the Viphyllin standardized extract containing β-caryophyllene from black pepper exert an anti-inflammatory function via the downregulation COX-2, iNOS and of the MAPK pathway in LPS challenged macrophages on the other hand it also offers neuroprotective effect. This investigation may suggest Viphyllin is encouraging bioactive food ingredients. However, imminent research is essential relating to the other controlling mechanism of Viphyllin.
Declarations
Authors’ Contributions
GK and SK conceptualized the work. GK, SHV, AS and AR Produced the data and prepared figures 1–6. LH and N P prepared figure S1. GK, SK and SHV designed and analyzed the experiment and results. GK and SK drafted the original manuscript. All authors read and approved the final manuscript.
Competing Interest Statement
The authors declare no conflict of interest.
Funding
The author(s) reveal that financial support was provided by Vidya Herbs Pvt Ltd. Bangalore. India.
Acknowledgments
We would like to thank, Mr. Chandrappa S, Dr. Deepak, and Dr. Vedamuthy B.M, Department of Phytochemistry and Analytical development laboratory, Vidya Herbs Pvt. Ltd, Bangalore, India. Our lab members are also acknowledged for their useful suggestions.
References
- Ota A, Ulrih NP. An Overview of Herbal Products and Secondary Metabolites Used for Management of Type Two Diabetes.Front Pharmacol 8 (2017): 436.
- Kure C, Timmer J, Stough C. The Immunomodulatory Effects of Plant Extracts and Plant Secondary Metabolites on Chronic Neuroinflammation and Cognitive Aging: A Mechanistic and Empirical Review.Front Pharmacol 8 (2017): 117
- Perveen S. Introductory chapter: Terpenes and terpenoids. In Terpenes and Terpenoids; Perveen, S., Ed.; IntechOpen: London UK (2018).
- Kim T, Song B, Cho KS, Lee IS. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases.Int J Mol Sci 21 (2020): 2187.
- Fidyt K, Fiedorowicz A, Strzadala L, Szumny A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties.Cancer Med 5 (2016): 3007-3017
- Singh G, Marimuthu P, de Heluani CS, Catalan CA. Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components.J Agric Food Chem 54 (2006): 174-181
- Gu X, Yao X, Mei J, He H, Gao X, Du Y, et al. β-caryophyllene, a natural bicyclic sesquiterpene, induces apoptosis by inhibiting inflammation-associated proliferation in MOLT-4 leukemia cells. Phcog Mag 17 (2021): 58-66.
- Gertsch J, Leonti M, Raduner S, et al. Beta-caryophyllene is a dietary cannabinoid.Proc Natl Acad Sci U S A 105 (2008): 9099-9104.
- Jayson MI. Vascular damage, fibrosis, and chronic inflammation in mechanical back pain problems.Semin Arthritis Rheum 18 (1989): 73-76
- Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span.Nat Med 25 (2019): 1822-1832
- Jayarathne S, Stull AJ, Park OH, Kim JH, Thompson L, Moustaid-Moussa N. Protective Effects of Anthocyanins in Obesity-Associated Inflammation and Changes in Gut Microbiome.Mol Nutr Food Res 63 (2019).
- Kim JH, Kismali G, Gupta SC. Natural Products for the Prevention and Treatment of Chronic Inflammatory Diseases: Integrating Traditional Medicine into Modern Chronic Diseases Care.Evid Based Complement Alternat Med (2018): 9837863
- Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease.Int J Mol Sci 20 (2019): 6008
- Zhang JM, An J. Cytokines, inflammation, and pain.Int Anesthesiol Clin 45 (2007): 27-37
- Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation.Front Immunol 2 (2011): 57
- Mosser DM, & Edwards JP. Exploring the full spectrum of macrophage activation.Nature reviews. Immunology8 (2008): 958–969.
- Silva D, Ferreira MS, Sousa-Lobo JM, Cruz MT, Almeida IF. Anti-Inflammatory Activity of Calendula officinalis L. Flower Extract. Cosmetics 8 (2021): 31.
- Wu X, Xie J, Qiu L, et al. The anti-inflammatory and analgesic activities of the ethyl acetate extract of Viburnum taitoense Hayata.J Ethnopharmacol 269 (2021): 113742
- Xin YJ, Choi S, Roh KB, et al. Anti-Inflammatory Activity and Mechanism of Isookanin, Isolated by Bioassay-Guided Fractionation fromBidens pilosaMolecules 26 (2021): 255.
- Amala R, Sujatha S. Presence of pyrroloquinazoline alkaloid inAdhatoda vasicaattenuates inflammatory response through the downregulation of pro-inflammatory mediators in LPS stimulated RAW 264.7 macrophages.Bioimpacts 11 (2021): 15-22
- Tian Y, Zhou S, Takeda R, Okazaki K, Sekita M, Sakamoto K. Anti-inflammatory activities of amber extract in lipopolysaccharide-induced RAW 264.7 macrophages.Biomed Pharmacother 141 (2021): 111854
- Eo HJ, Jang JH, Park GH. Anti-Inflammatory Effects ofBerchemia floribundain LPS-Stimulated RAW264.7 Cells through Regulation of NF-κB and MAPKs Signaling Pathway.Plants (Basel) 10 (2021): 586.
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases.Biochim Biophys Acta 1802 (2010): 396-405
- Xie X, Sun S, Zhong W, et al. Zingerone attenuates lipopolysaccharide-induced acute lung injury in mice.Int Immunopharmacol 19 (2014): 103-109
- Li L, Chen J, Lin L, et al. Quzhou Fructus Aurantii Extract suppresses inflammation via regulation of MAPK, NF-κB, and AMPK signaling pathway.Sci Rep 10 (2020): 1593.
- Jeong YH, Oh YC, Cho WK, Lee B, Ma JY. Anti-Inflammatory Effects of Melandrii Herba Ethanol Extract via Inhibition of NF-κB and MAPK Signaling Pathways and Induction of HO-1 in RAW 264.7 Cells and Mouse Primary Macrophages.Molecules 21 (2016): 818.
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing.Nature 408 (2000): 239-247.
- Garcimartín A, Merino JJ, González MP, et al. Organic silicon protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide effects.BMC Complement Altern Med 14 (2014): 384.
- Nirmaladevi D, Venkataramana M, Chandranayaka S, Ramesha A, Jameel NM, Srinivas C. Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line.Cell Mol Neurobiol 34 (2014): 973-985.
- Sudeep HV, Venkatakrishna K, Amritharaj, et al. A standardized black pepper seed extract containing β-caryophyllene improves cognitive function in scopolamine-induced amnesia model mice via regulation of brain-derived neurotrophic factor and MAPK proteins.J Food Biochem 45 (2021).
- Li J, Li W, Su J, Liu W, Altura BT, Altura BM. Hydrogen peroxide induces apoptosis in cerebral vascular smooth muscle cells: possible relation to neurodegenerative diseases and strokes.Brain Res Bull 62 (2003): 101-106.
- Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill.J Immunol Methods 119 (1989): 203-210.
- Huang B, Liu J, Fu S, et al. α-Cyperone Attenuates H2O2-Induced Oxidative Stress and Apoptosis in SH-SY5Y CellsviaActivation of Nrf2.Front Pharmacol 11 (2020): 281.
- Hu XL, Niu YX, Zhang Q, et al. Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells.Environ Toxicol Pharmacol 40 (2015): 230-240.
- Wang X, Ohnishi K, Takahashi A, Ohnishi T. Poly (ADP-ribosyl) ation is required for p53-dependent signal transduction induced by radiation.Oncogene 17 (1998): 2819-2825.
- Kim Y, Cho E, Lee Ah Y, Seo W. Apigenin Ameliorates Oxidative Stress-induced Neuronal Apoptosis in SH-SY5Y Cells. Microbiology and Biotechnology Letters 49 (2021): 138-147.
- Tian W, Heo S, Kim DW, et al. Ethanol Extract ofMaclura tricuspidataFruit Protects SH-SY5Y Neuroblastoma Cells against H2O2-Induced Oxidative Damage via Inhibiting MAPK and NF-κB Signaling.Int J Mol Sci 22 (2021): 6946.
- Kumar MR, Yeap SK, Lee HC, et al. Selected Kefir Water from Malaysia Attenuates Hydrogen Peroxide-Induced Oxidative Stress by Upregulating Endogenous Antioxidant Levels in SH-SY5Y Neuroblastoma Cells.Antioxidants (Basel) 10 (2021): 940.



 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 