First Observations of Plasmodium malariae on the Long-Term Malaria Vector Control Program on Villages of the Balombo Region of Angola
Sylvie Manguin*, 1, Vincent Foumane2, Jean-Claude Toto2, Franck Martineaud3, Maria Adelaide Dos Santos3, Filomeno Fortes4, Pierre Carnevale5
1HSM, University of Montpellier, CNRS, IRD, Montpellier, France.
2Organization of Cooperation and Coordination for Endemic Diseases Control in Central Africa (OCEAC), PO 288 Yaoundé Cameroon.
3Clinic and Malaria Control Programme, Sonamet, Lobito, Angola.
4Institute of Hygiene and Tropical Medicine, Lisbon, Portugal.
5Le Majoral Building, 34420 Portiragnes Plage, France.
*Corresponding author: Sylvie Manguin, HSM, University of Montpellier, CNRS, IRD, Montpellier, France.
Received: 24 January 2024; Accepted: 30 January 2024; Published: 13 February 2024
Article Information
Citation: Sylvie Manguin, Vincent Foumane, Jean-Claude Toto, Franck Martineaud, Maria Adelaide Dos Santos, Filomeno Fortes, Pierre Carnevale. First Observations of Plasmodium malariae on the Long-Term Malaria Vector Control Program on Villages of the Balombo Region of Angola. Fortune Journal of Health Sciences. 7 (2024): 44-55.
View / Download Pdf Share at FacebookAbstract
At the request of the National Malaria Control Program (NMCP), a long-term vector control program was implemented in 2007 in eight villages around the Balombo town (Angola) to compare the efficacy of four methods of indoor vector control. These methods included (1) Long-Lasting deltamethrin Insecticide treated Nets (LLIN PermaNet© 2.0 model or P.2.0); (2) association of P.2.0 and deltamethrin Insecticide Treated Plastic Sheeting (delta-ITPS)-Zero Fly© model; (3) delta-ITPS alone; and (4) 2 rounds of lambdacyhalothrin Inside Residual Spraying (IRS) followed by installation of delta-ITPS. Cross-sectional parasitological surveys (CSS) were done every two months. Plasmodium species determination, parasitemia and gametocytes presence, and evolution in time were analyzed. A total of 190 CSS was done between 2007 and 2011, Plasmodium spp. were observed in 5,431 of the 21,804 TBF done (24.9%). Plasmodium malariae alone was observed in 22 TBF (0.4%) and mixed infections P. falciparum and P. malariae in 44 TBF (0.8%). Our study confirms the presence of P. malariae in Angola, which must be known due to its special clinical impact, quartan fever, kidney failure, chronicity, symptomless carriers, persistence for several years with long term recrudescence and reported cases of resistance to classical ACTs. The prevalence of P. malariae decreased after implementation of vector control methods. The burden of P. malariae needs to be studied to reach the goal of malaria elimination by 2030.
Keywords
<p>Cross-sectional malaria surveys, vector control, Plasmodium malariae, mixed infections</p>
Article Details
1. Introduction
A great amount of work has been devoted to Plasmodium malariae since its first description by Laveran as Oscillaria malariae in 1881, then by Grassi and Feletti in 1890 under the name Haemamoeba malariae [1-10], until recent studies included genome sequencing [11- 19]. P. malariae is a particular species, especially at the clinical and parasitological levels, with a 72-hr erythrocyte cycle inducing a quartan fever with a slow development (15 days in both liver and Anopheles mosquito) [20, 21]. The parasite density is always low compared to Plasmodium falciparum and it produces clinical relapses, considered now as recrudescence, due to the asexual forms that persist in the host’s blood [20]. Considered benign, this Plasmodium species was used in the 40’s in experimental infections for the treatment of neurosyphilis [10]. Though, symptoms include nephropathies [22-26], long asymptomatic persistence of several years before access to recrudescence [27] that may induce transfusional malaria [28]. Cases of glomerulonephropathy associated with P. malariae infections were also reported with a disease that may persist and evolve despite the apparent elimination of parasites and with detectable chronic renal disease observed even 3-5 years after the so-called primary infection [29].
This species presents genetic polymorphism [30-34], it can be transmitted by palearctic or tropical vectors [35, 36], and due to its possibility to infect human and simian subjects [37, 38], it was considered as an anthropozoonotic species. This plasmodial species has a scattered world-wide distribution in malarious regions of Africa [39, 40], South America [41], Asia and Pacific [42] with a variable frequency in time and space according to the technique of investigations and biological analysis. With the recent acceleration of malaria control operations, the frequency of P. falciparum is decreasing [17, 43, 44] and a special interest has to be given to other plasmodial species, including P. malariae, which could persist for many years [45], or even the whole life of infected persons [19]. The genome of P. malariae has recently been decrypted [18, 46], which explains a case of resistance with a clinical recrudescence after treatment with the combination of artemether-lumefantrine (a full course of 6 doses over 3 days, equivalent to a total dosage of 6.2 mg/kg of artemether and 37.4 mg/kg of lumefantrine). The explanation given was that the primary infection would have been polyclonal, but the recrudescence was due to a resistant clone [46]. In 1937, the death of a 27-years old man in India was reported following what appeared to be the third malaria crisis access, with no particularly high fever, but parasitological examination revealed a high density of P. malariae. Despite treatment with quinine (injection and oral), resulting in the disappearance of microscopically observed Plasmodium, the subject died possibly from heart problems [47]. Overall, P. malariae is still a neglected human malaria parasite, not well understood that still contributes up to 10% of malaria infections in sub-Saharan Africa [34]. The objective of the study was to evaluate the prevalence of both P. malariae and P. falciparum in a mesoendemic malaria region of Angola where our study has been ongoing since 2007 [48-50]. In addition, it seemed important to pay a particular attention to these Plasmodium species in the framework of the study aiming at comparing the efficacy of four vector control methods, including long- asting deltamethrin-impregnated mosquito nets (LLIN), deltamethrin insecticide-treated plastic sheeting (ITPS), association LLIN and ITPS, and lambdacyhalothrin indoor residual spraying (IRS), in the Balombo region of Angola and to monitor its possible evolution with the implementation of different vector control operations.
2. Methodology
2.1 Study area
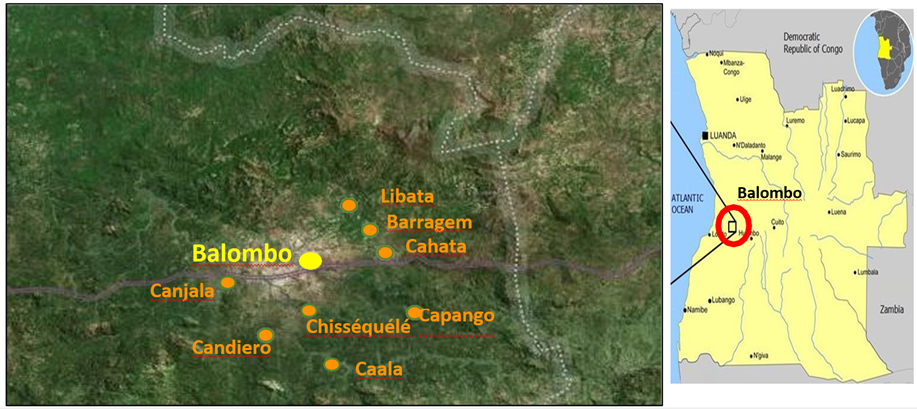
The Balombo study area in the Benguela Province of Angola was already described [48]. This study was made between February 2007 and December 2011, with sampling collections made every 2 months, in eight villages around the town of Balombo (12°21'S, 14°46’E) located at an altitude of 1,220 meters in a mountainous area of ancient volcanoes with a thermal spring at the west entrance to the city (Fig. 1). The vegetation is a tropical savannah with shrub prairie, the original forest has been severely degraded for crop development and domestic use. There is a well-marked dry and cold season in June to August and abundant rains from November to April. Temperatures vary between a minimum of 10°-15°C in June-August and a maximum of 30-35°C in March and April.
2.2 Parasitological surveys
Parasitological surveys were carried out as part of a multidisciplinary evaluation of a vector control operation aiming at comparing four vector control methods in paired villages. The complete protocol of vector control method implementation is detailed in Brosseau et al. [48] and it included the following steps: (1) PermaNet 2.0 Long lasting deltamethrin-impregnated mosquito nets (LLIN) provided in Cahata and Caala; (2) association of LLIN with Zero Fly®, deltamethrin insecticide-treated plastic sheeting (ITPS) that were pinned on the hut walls in Capango and Canjala; (3) deltamethrin-treated ITPS Wall Lining (WL) model alone and also pinned on the walls in Chissequele and Barragem; and (4) conventional lambdacyhalothrin indoor Residual Spraying (IRS) with two rounds/year (every six months), followed by the installation of ITPS in Libata and Candiero in order to increase the long lasting efficacy of the treatment. Cross-sectional parasitological surveys were conducted on a regular basis every two months focusing on random samples of the population under 15 years of age. At the time of the field investigations, part of the population present in the village usually came to the team to have a blood test and, for obvious ethical reasons, a thick blood film (TBF) was prepared from each one of these volunteers and these TBF were coloured in the field with Giemsa. Then, microscopic observations were done at the medical department of the Angolese Sonamet Company located in Lobito, which supported the study through their Malaria Control Program (MCP).
To compare the efficacy of each vector control method, a subsample of children ≤ 15 years old was extracted from the original list to standardize the samples to be compared, but in the present study dealing with P. malariae the whole samples were considered with no age limits. Then, the number of blood smears done per village varied between 1,856 TBF in Capango to 3,387 TBF in Caala during the five years of the study (Table 1) and were constituted of people from all age groups, from 2 to 75 years old. Young children were accompanied by their volunteer mother. During the surveys a supplementary drop of blood was also obtained on filter paper for further immunological analysis at IRD in Montpellier, France for estimating the Anopheles biting pressure on the population before and after implementation of the four vector control methods [48]. Ethical approval was obtained from the National Malaria Control Program of the Ministry of Health of Angola, the Ethical authority in charge of approving studies on malaria research in Angola. In addition, written consent signed by the head of each household was obtained for all individuals enrolled in the study by Malaria Control Program (MCP) of the Sonamet Company. Microscopic parasitological examinations were done by the same team throughout the study with a regular double-checking by V. Foumane, parasitologist at OCEAC (Organisation de coordination pour la lutte contre les endémies en Afrique Centrale), Yaoundé, Cameroon, of 10% random sample slides. Results of these analyses were provided to the village health worker for further action to be taken by the National Malaria Control Program (NMCP) of Angola.
2.3 Statistical analysis
Data were analyzed and graphs constructed with GraphPad Prism6® software (San Diego, CA, USA). Distribution of parasitemia were analyzed with the non-parametric Mann-Whitney statistical test.
3. Results
3.1 Prevalence of Plasmodium malariae
From February 2007 to December 2011, 190 field surveys were done and 21,804 thick blood smears were prepared from which 5,431 were positive for Plasmodium (24.9%), 5,365 with P. falciparum (98.8%), 22 (0.4%) with P. malariae, and 44 (0.8%) with a mix of P. falciparum and P. malariae (Table 1).
Table 1: Parasitological surveys done from 2007 to 2011 in 8 villages of the Balombo region
|
Village |
No |
No TBF |
No TBF + |
No P.f. |
No P.m. |
No P.f. + P.m. |
|
surveys |
done |
|||||
|
Caala |
25 |
3,387 |
1,004 |
1,000 |
1 |
3 |
|
Cahata |
25 |
2,873 |
833 |
819 |
7 |
7 |
|
Capango |
24 |
1,856 |
314 |
312 |
1 |
1 |
|
Canjala |
24 |
2,952 |
931 |
915 |
4 |
12 |
|
Chisséquélé |
23 |
2,551 |
463 |
460 |
2 |
1 |
|
Barragem |
23 |
2,525 |
548 |
534 |
3 |
11 |
|
Candiero |
23 |
2,761 |
524 |
523 |
0 |
1 |
|
Libata |
23 |
2,899 |
814 |
802 |
4 |
8 |
|
Total |
190 |
21,804 |
5,431 |
5,365 |
22 |
44 |
|
-100% |
-24.90% |
-98.80% |
-0.40% |
-0.80% |
No, number; TBF, thick blood film; TBF+, positive thick blood film; P.f., P. falciparum; P.m., P. malariae
3.2 Parasitemia of P. malariae alone or coinfections P. malariae and P. falciparum
- malariae parasitemia, alone or associated with P. falciparum, were significantly not different with p-value = 0.1127. For single or mixed infections, the geometric means were of 466 and 787, and the medians of 450 and 746 respectively (Table 2).
Table 2: Parasitemia of P. malariae alone or associated to P. falciparum
|
Indicator/ infections |
P. malariae |
P. malariae + P. falciparum |
|
Arithmetic mean (parasites/µl) |
1,065 ±1,840 |
1,955 ±4,008 |
|
Geometric mean |
466 |
787 |
|
Median (sample size) |
450 (n=22) |
746 (n=44) |
|
Minimum value (parasites/µl) |
32 |
133 |
|
Maximum value (parasites/µl) |
8,301 |
24,000 |
3.3 Influence of vector control methods
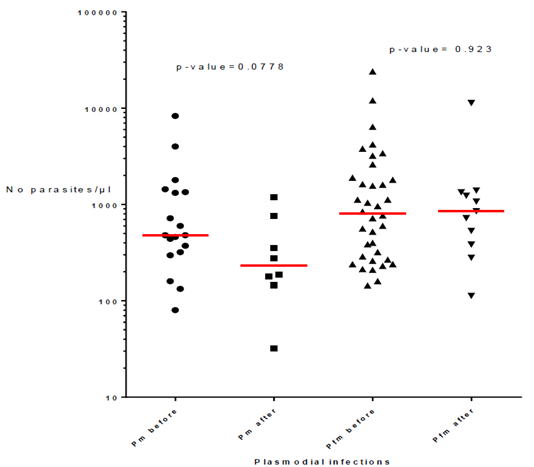
The presence of P. malariae, alone or associated with P. falciparum, according to the vector control method is reported in Table 3. Similar percentages of infections were found when considering P. malariae and mixed P. malariae + P. falciparum with values ranging from 1% in villages with LLIN alone and IRS, 1.4% in villages with LLIN and Zero Fly, and 1.7% in villages with ITPS, while P. falciparum percentages vary between 98.3% and 99%. Besides, the number of infections with P. malariae alone or mixed with P. falciparum decreased drastically, by a factor of 4.5 after vector control operations with values of 54 out of 66 infections (82%) before to 12 (18%) after vector control implementation (Table 4). However, the parasitemia of P. malariae infections alone or in coinfection with P. falciparum did not change with vector control implementation as shown by p-values of 0.0778 for P. malariae infections alone (median = 480 before and 233 after VC) and p-value= 0.923 for coinfections (median = 804 before and 853 after VC) (Fig. 2).
Table 3: Presence of Plasmodium malariae according to vector control method implemented (TBF= thick blood film; LLIN= Long lasting insecticide treated net; ITPS= insecticide treated plastic sheeting; ZF= Zero Fly model of ITPS; IRS= Inside residual spraying)
|
Method of vector control |
No |
No TBF |
No TBF + |
No P.f. |
No P.m. |
No of mixed P.f. + P.m. |
|
surveys |
done |
|
||||
|
LLIN alone |
50 |
6,260 |
1,837 |
1,819 |
8 |
10 |
|
-100% |
-29.30% |
-99.00% |
-0.40% |
-0.50% |
||
|
LLIN+ZF |
48 |
4,808 |
1,245 |
1,227 |
5 |
13 |
|
-100% |
-25.90% |
-98.60% |
-0.40% |
-1.00% |
||
|
ITPS alone |
46 |
5,076 |
1,011 |
994 |
5 |
12 |
|
-100% |
-19.90% |
98.3%) |
-0.50% |
-1.20% |
||
|
IRS then ITPS |
46 |
5,660 |
1,338 |
1,325 |
4 |
9 |
|
-100% |
-23.60% |
-99.00% |
-0.30% |
-0.70% |
Table 4: Prevalence and number of cases in parentheses of P. malariae alone (P.m.) or associated to P. falciparum (P.m. + P.f.) in surveys done before and after vector control (VC) implementation
|
Vector control methods |
Before VC |
After VC |
||
|
P.m. |
P.m.+P.f. |
P.m. |
P.m.+P.f. |
|
|
LLIN alone |
57% (8) |
43% (6) |
0 |
100% (4) |
|
LLIN + ZF |
14% (2) |
86% (12) |
75% (3) |
25% (1) |
|
ITPS alone |
27% (4) |
73% (11) |
50% (1) |
50% (1) |
|
IRS then ITPS |
36% (4) |
63% (7) |
0 |
100% (2) |
|
Sub-Total |
82% (18/22) |
82% (36/44) |
18% (4/22) |
18% (8/44) |
|
Total |
82% (54/66) |
18% (12/66) |
||
LLIN= Long lasting insecticide treated net; ITPS= insecticide treated plastic sheeting; ZF= Zero Fly model of ITPS; IRS= Inside residual spraying.
3.4 Influence of the season
The analysis of P. malariae infections observed during the dry and rainy season surveys shows a similar overall number with respectively 30 and 36 infections, with twice less P. malariae alone infections in the rainy (32%) than the dry season (68%) (Table 5).
Table 5: Number of positive slides and prevalence of P. malariae (P.m.) alone or associated to P. falciparum (P.f.+P.m.) according to season
|
Rainy season |
Dry season |
|||
|
Plasmodium species |
P.m. |
P.f. + P.m. |
P.m. |
P.f. + P.m. |
|
No positive slides |
Jul-22 |
23/44 |
15/22 |
21/44 |
|
Prevalence |
32% |
52% |
68% |
48% |
|
Total |
30/66 (45%) |
36/66 (55%) |
||
3.5 Influence of gender
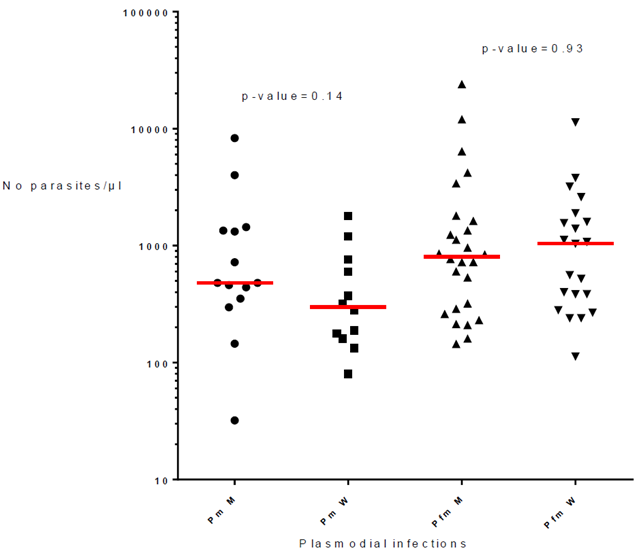
The 66 infections of P. malariae alone or associated with P. falciparum included 28 men and 38 women. The parasite load of P. malariae were similar in men and women (p-value= 0.14; respective median 480 and 299) and the parasitemia of mixed infections P. malariae + P. falciparum were also similar in men and women (p-value= 0.93; respective median = 804 and 1,040) (Fig. 3).
3.6 Influence of age
Our sample was not prepared to be representative of the village populations since it was intended to mainly evaluate the impact of vector control operations on P. falciparum infections in children (≤ 15 years). However, for P. malariae evaluation, 2 age groups were considered such as <5 years old, considered as at-risk group, and > 5 years old. The results showed a similar number of infections in both groups with 11 carriers <5 years old and 11 carriers > 5 years old for infections with P. malariae alone, and 20 carriers <5 years old and 24 carriers > 5 years old for mixed infections P. malariae + P. falciparum.
4. Discussion
According to the last WHO malaria report [44], Angola is the fifth country with the highest number of malaria cases and deaths in the world, accounting respectively for 3.4% and 3.2% of the global cases and deaths in 2022, ranking 5th and 7th among the African countries. For the past 10 years, malaria figures in Angola have been steadily increasing with an incidence that jumped from 3.5 million confirmed cases in 2011 to 9.2 million in 2022, and a mortality that nearly doubled from 6,909 to 12,474 deaths respectively out of a total population of 35.6 million, all living in malaria-risk regions. In the Balombo region of Angola (Benguela Province), malaria is mesoendemic and all reported cases are due to P. falciparum with no report of the occurrence of P. malariae or P. ovale, due to their rarity, although records have shown the presence of these Plasmodium species in other parts of Angola [51- 55]. For instance in northern Angola, on a total of 3,316 blood samples collected, 541 (16.3%) were Plasmodium infected, out of them 477 (88.2%) were due to P. falciparum alone and 35 (6.5%) were coinfections P. falciparum and P. malariae [53]. In this study by Fancony et al. [53], P. malariae infections were reported by molecular methods and not observed with usual optical microscopy such as our study.
The five years longitudinal surveys in the eight villages around Balombo (Angola) confirmed the presence of P. malariae in the Balombo region of west-central Angola and its very low prevalence, with 22 cases out of 21,804 thick blood smears observed (0.1%), and this could explain why this species is rarely reported in single sectional surveys, deserving continuous training of microscopic technicians [56]. For example, during continuous training operations for microscopists from health centers in Bengo, Benguela and Luanda Provinces in Angola, there was an increase in the sensitivity and specificity of examinations after training, while noting false positive P. vivax case in Bengo and P. malariae infection on a slide considered as negative [57]. Using combined techniques such as microscopy and PCR assays, the prevalence of P. malariae mono-infections at 1% was found in the Democratic Republic of Congo, country neighbouring Angola [58], while it was at 2.5% in Cameroon and coinfections P. falciparum/P. malariae at 17% using the same two techniques and RDTs [59].
In Angola, a cross-sectional survey conducted in 733 Chinese and Southeast Asian migrants showed that most infections were due to P. falciparum, although infection and/or exposure to P. vivax and P. malariae was also detected [54]. Based on these very few reports, more attention should be given to the detection of non-falciparum infections in order to know what and where plasmodial species are circulating in Angola, and more widely in the south of the Saharan region for a more precise diagnosis of patients in order to provide them an appropriate malaria prophylaxis [60]. In addition, it is important to know where this plasmodial species is circulating for the diagnosis of patients being examined in non-endemic areas, to avoid kidney failure or any other complications due to P. malariae. Plasmodium infections with non-falciparum species, also referred to Non-Falciparum Malaria (NFM), are responsible for 25% of cases imported into Europe [55] and a retrospective analysis of the records from January 2006 to August 2016, recorded in a hospital in Portugal, revealed 19 cases of NFM among the 225 malaria cases including 12 cases from Angola. Out of these 19 NFM cases, 7 (37%) were due to P. malariae. The most frequent symptom was fever and the biological analysis showed thrombocytopenia. Treatments provided to these 19 NFM cases were quinine-doxycycline (11 patients), chloroquine (six patients) or artemether-lumefanthrin (two patients) with cure in all cases. In Henan Province of China, three patients, including two returning from Angola and one from Equatorial Guinea, had symptoms such as irregular fever, headache, chills. Two cases had elevated total bilirubin and splenomegaly and were confirmed as P. malariae infection by microscopic examination. Both were cured with artemisinin-based combination therapy (ACT) [52], while the usual drug can have crucial side effect of G6PD deficiency and must be given only with strict medical care in main hospital. In Jiangsu Province of China, an observational study of imported malaria cases was carried out for the period of 2011-2014 [51]. Out of 1,268 imported malaria cases, P. falciparum cases accounted for 83.4% (n = 1,058), and it was noticed that the proportions of P. ovale and P. malariae increased during these four year-period. Therefore, this increase in rare Plasmodium species originating from south-Saharan Africa and Southeast Asia needs to be better monitored at all levels of health providers focusing on diagnosis and treatment of malaria. In addition, due to long latency periods and misdiagnosis of P. malariae and P. ovale, there is an increased risk of re-introduction of malaria in China, declared malaria-free by WHO in 2021 [51, 61, 62]. A recent meta-analysis of the occurrence of P. malariae and P. ovale in the world revealed that 51% of these species were reported from the African region and each species was accounting for 3.16% and 1.69% respectively on this continent [17]. This prevalence of P. malariae is 10 times higher than the one found in this study (0.3% including mixed infections), which may be due to the fact that this species is often misidentified as P. falciparum or totally missed under microscopic examination due to the low parasite density compared to P. falciparum [63]. Then, the use of optical microscopy to search for Plasmodium parasites must be, in future studied, accompanied by PCR assays to confirm, and eventually increase the number of positive samples. This is particularly important as P. malariae shows a lower parasitemia compared to P. falciparum, therefore more difficult to detect under a microscope [21, 64].
To improve the diagnosis of certain fevers, anaemia [65] and nephropathies, it is important to be aware of the presence of P. malariae and thus avoiding misdiagnosis [66] in endemic and non-endemic areas [64, 67, 68] with the critical issues of mistreatment [60, 69-74]. The persistence of P. malariae even after ACT treatment is of great concern [75, 76], as well as its well-known long chronicity [3] with an amazing persistence for several years, such as a P. malariae case reported 32 years after the initial infection of stay in malaria-endemic areas [77]. It was reported that infections have been transmitted by blood transfusion twelve years, twenty years, and thirty years after infection of the donors [3]. In addition, it is important to highlight the fact P. malariae is a parasite of both man and African apes (chimpanzee and gorilla) [38, 78] and with current and foreseen environmental changes and forest destruction, the risk of human infections could greatly increase as it was recently seen with P. knowlesi in Indonesia and even more Malaysia [79-89].
5. Conclusion
Angola belongs to the Malaria Elimination Eight Regional Initiative (E8), as one of the 8 countries of southern Africa aiming to eliminate malaria by 2030 [90]. The expansion of malaria interventions in Angola in the 20 past years aimed to consolidate malaria control and move towards pre-elimination [91]. However, the case of residual malaria is of great concern and may jeopardize malaria elimination [92-94], especially if all efforts are concentrated on P. falciparum only. Non-falciparum species, such as P. malariae, are largely forgotten, although this species showed a prevalence of 3.16% in Africa, based on 50 studies, and 17% of coinfections with P. falciparum were recently reported from Cameroon [17, 59]. Better knowledge on the prevalence and burden of non-falciparum species is crucial in the aim of achieving malaria elimination in Angola and in the concerned countries of the E8 initiative. To accomplish this task, well-trained microscopists in the identification of non-falciparum species are needed; molecular techniques for the diagnosis of Plasmodium species must be popularized; and less expensive diagnostic techniques (such as hypersensitive RTDs) need to be developed.
Résumé
A la demande du Programme National de Lutte contre le Paludisme (PNLP), un programme de lutte antivectorielle a été mis en œuvre dès 2007 dans huit villages autour de Balombo (Angola) pour comparer l’efficacité de quatre méthodes de lutte antivectorielle dans les habitations. Ces méthodes comprenaient (1) des moustiquaires à imprégnation durable (MID) traitée à la deltaméthrine (PermaNet© 2.0 ou P.2.0); (2) l’association de MID P.2.0 et de bâches en plastique traitées avec un insecticide (BPTI), la deltaméthrine (delta-BPTI) - Zero Fly©; (3) delta-BPTI seul; et (4) 2 cycles de pulvérisation de lambdacyhalothrine à effet rémanent suivis de l’installation de delta-BPTI. Des enquêtes parasitologiques transversales (EPT) ont été effectuées tous les deux mois. L’identification des espèces de Plasmodium, la parasitémie, la présence de gamétocytes, et l’évolution dans le temps de ces paramètres ont été analysées. Un total de 190 EPT a été fait entre 2007 et 2011, Plasmodium spp. ont été observés dans 5.431 des 21.804 gouttes épaisses (GE) effectuées (24.9%). Plasmodium malariae seul a été observé dans 22 GE (0,4%) et des infections mixtes P. falciparum et P. malariae dans 44 GE (0,8%). Notre étude confirme la présence de P. malariae en Angola, qui doit être suivie en raison de son impact clinique, fièvre quarte, insuffisance rénale, chronicité, porteurs asymptomatiques, persistance pendant plusieurs années avec recrudescence à long terme et cas signalés de résistance aux ACT classiques. La prévalence de P. malariae a diminué après la mise en œuvre des méthodes de lutte antivectorielle. Le fardeau que représente P. malariae doit être étudié afin d’atteindre l’objectif d’élimination du paludisme d’ici 2030.
Acknowledgments
We are grateful to the Angolese Sonamet Company based at Lobito and its Medical Department in the framework of the Malaria Control Program (MCP), as well as the Subsea7 Company, which supported this study. We thank National and Provincial Public Health authorities, which allowed and supported the surveys. All the data were collected by the intense work of the MCP, Sonamet. We gratefully acknowledge Dr F. Fortes, chief of the National Malaria Control Programme of Angola during the study and now Director of the Institute of Hygiene & Tropical Medicine of the University Nova in Portugal, for his important and permanent support before and during this study.
Author Contributions
PC designed the study protocol; participated to field surveys, analyzed data and drafted the manuscript. FF supported the study and participated to field surveys. MADS, VF, JCT carried out the field surveys. MADS read all blood slides. VF did initial and ongoing training, participated in laboratory analysis and double-checked part of the blood slides. SM did data analysis and was fully involved in drafting and writing the manuscript. FM participated in data analysis and revised the manuscript. All authors read and approved the final manuscript.
Funding
The Angolese Sonamet Company based at Lobito and the Subsea7 Company funded this study.
Availability of data and materials
All data are fully available without restriction.
Declarations
Ethical Approval and consent to participate
This study was conducted in accordance with the Edinburgh revision of the Helsinki Declaration and was approved by the National Malaria Control Program of the Ministry of Health of Angola, the Ethical authority in charge of approving studies on malaria research in Angola. Written consent (signed by the head of each household) was obtained for all individuals enrolled in the study by the SONAMET Company - Malaria Control Program (MCP).
Conflicts of Interest
The authors declare that they have no conflicts of interest in relation to this article.
References
- Boyd M. Observations on naturally and artificially induced quartan malaria. Am J Trop Med 20 (1940): 749.
- Boyd M. A Note on the Chronicity of a Quartan Infection. Bruxelles, Belgium: Goemaere 1947.
- Brumpt E. The human parasites of the genus Plasmodium. In Malariology. Edited by Boyd MF. Philadelphia, USA: Saunders WB (1949): 65-121
- Chwatt LJ. Infection of reticulocytes by Plasmodium falciparum and Plasmodium malariae in hyperendemic indigenous malaria. Ann Trop Med Parasitol 42 (1948): 101-112.
- Einhorn NH. Tertian, quartan and mixed malarial infections; a survey of three hundred and thirty-four cases of infection with Plasmodium vivax, ten cases of infection with Plasmodium malariae and eight cases of mixed malarial infections in children. Am J Dis Child 73 (1947): 55-86.
- Geiger JC, Kelly FL. Plasmodium malariae (Quartan)-A type new to California: Report of two cases. Cal State J Med 14 (1916): 198.
- Kitchen S. The Infection of Mature and Immature Erythrocytes by falciparum and P. malariae. Am J Trop Med 19 (1939): 47.
- Mackerras MJ, Ercole QN. Observations on the life-cycle of Plasmodium malariae. Aust J Exp Biol Med Sci 26 (1948): 515-519.
- McDaniel GE, Hemphill FM. Plasmodium malariae in school surveys in South Carolina. J Natl Malar Soc 7 (1948): 65-75.
- Kitchen S. Quartan malaria. In Malariology A comprehensive survey of all aspects of this group of diseases from a global standpoint. Edited by Boyd MF. Philadelphia USA. Saunders Company (1949): 1643
- Aznar ML, Zarzuela F, Sulleiro E, Trevino B, Serre N, Pou D, et al: Plasmodium malariae in a Spanish traveller returning from Colombia. J Travel Med 26 (2019).
- Patrocinio-Jesus R, Cunha J, Trigo D, Flor-de-Lima B, Pacheco P. Artemether/Lumefantrine for the treatment of malariae in a patient on hemodialysis. Case Rep Med (2019): 1326171.
- Subissi L, Kanoi BN, Balikagala B, Egwang TG, Oguike M, Verra F, et al. Plasmodium malariae and Plasmodium ovale infections and their association with common red blood cell polymorphisms in a highly endemic area of Uganda. Trans R Soc Trop Med Hyg 113 (2019): 370- 378.
- Sutherland CJ. Persistent Parasitism: The Adaptive Biology of Malariae and Ovale Malaria. Trends Parasitol 32 (2016): 808-819.
- Sutherland CJ. A New Window on Plasmodium malariae J Infect Dis 221 (2020): 864-866.
- Woodford J, Collins KA, Odedra A, Wang C, Jang IK, Domingo GJ, et al. An experimental human blood- stage model for studying Plasmodium malariae J Infect Dis 221 (2020): 948-955.
- Hawadak J, Dongang Nana RR, Singh V. Global trend of Plasmodium malariae and Plasmodium ovale malaria infections in the last two decades (2000-2020): a systematic review and meta-analysis. Parasit Vectors 14 (2021): 297.
- Ansari HR, Templeton TJ, Subudhi AK, Ramaprasad A, Tang J, Lu F, et al. Genome-scale comparison of expanded gene families in Plasmodium ovale wallikeri and Plasmodium ovale curtisi with Plasmodium malariae and with other Plasmodium Int J Parasitol 46 (2016): 685-696.
- Rutledge GG, Bohme U, Sanders M, Reid AJ, Cotton JA, Maiga-Ascofare O, et al. Plasmodium malariae and ovale genomes provide insights into malaria parasite evolution. Nature 542 (2017): 101-104.
- Garnham PCC. Malaria parasites of man: life cycles and morphology (excluding ultrastructure). London, UK Churchill Livingstone (1988).
- Manguin S, Carnevale P, Mouchet J, Coosemans M, Julvez J, Richard-Lenoble D, et al. Biodiversity of malaria in the world. Paris, France: John Libbey Eurotext (2008).
- Badiane AS, Diongue K, Diallo S, Ndongo AA, Diedhiou CK, Deme AB, et al. Acute kidney injury associated with Plasmodium malariae Malar J 13 (2014): 226.
- Barsoum RS. Malarial acute renal failure. J Am Soc Nephrol 11 (2000): 2147-2154.
- Duvic C, Nedelec G, Debord T, Herody M, Didelot F. [Important parasitic nephropathies: update from the recent literature]. Nephrologie 20 (1999): 65-74.
- Halleux D, Moerman F, Gavage P, Carpentier M, Van Esbroeck M, Craenen S, et al. A nephrotic syndrome of tropical origin: case report and short review of the aetiology. Acta Clin Belg 69 (2014): 379-381.
- Silva GBDJ, Pinto JR, Barros EJG, Farias GMN, Daher EF. Kidney involvement in malaria: an update. Rev Inst Med Trop Sao Paulo 59 (2017): e53.
- Vinetz JM, Li J, McCutchan TF, Kaslow DC. Plasmodium malariae infection in an asymptomatic 74-year-old Greek woman with splenomegaly. N Engl J Med 338 (1998): 367-371.
- Brouwer EE, van Hellemond JJ, van Genderen PJ, Slot E, van Lieshout L, Visser LG, et al. A case report of transfusion-transmitted Plasmodium malariae from an asymptomatic non-immune traveller. Malar J 12 (2013): 439.
- Elsheikha HM, Sheashaa HA. Epidemiology, pathophysiology, management and outcome of renal dysfunction associated with plasmodia infection. Parasitol Res 101 (2007): 1183-1190.
- Lo E, Nguyen K, Nguyen J, Hemming-Schroeder E, Xu J, Etemesi H, et al. Plasmodium malariae prevalence and csp gene diversity, Kenya, 2014 and 2015. Emerg Infect Dis 23 (2017): 601-610.
- Saralamba N, Mayxay M, Newton PN, Smithuis F, Nosten F, Archasuksan L, et al. Genetic polymorphisms in the circumsporozoite protein of Plasmodium malariae show a geographical bias. Malar J 17 (2018): 269.
- Srisutham S, Saralamba N, Sriprawat K, Mayxay M, Smithuis F, Nosten F, et al. Genetic diversity of three surface protein genes in Plasmodium malariae from three Asian countries. Malar J 17 (2018): 24.
- Tahar R, Ringwald P, Basco LK. Heterogeneity in the circumsporozoite protein gene of Plasmodium malariae isolates from sub-Saharan Africa. Mol Biochem Parasitol 92 (1998): 71-78.
- Oriero EC, Demba MA, Diop MF, Ishengoma DS, Amenga-Etego LN, Ghansah A, et al. Plasmodium malariae structure and genetic diversity in sub-Saharan Africa determined from microsatellite variants and linked SNPs in orthologues of antimalarial resistance genes. Sci Rep 12 (2022): 21881.
- Collins WE, McClure H, Strobert E, Skinner JC, Richardson BB, Roberts JM, et al. Experimental infection of Anopheles gambiaes., Anopheles freeborni and Anopheles stephensi with Plasmodium malariae and Plasmodium brasilianum. J Am Mosq Control Assoc 9 (1993): 68-71.
- Girod R, Gaborit P, Carinci R, Issaly J, Fouque F. Anopheles darlingi bionomics and transmission of Plasmodium falciparum, Plasmodium vivax and Plasmodium malariae in Amerindian villages of the Upper-Maroni Amazonian forest, French Guiana. Mem Inst Oswaldo Cruz 103 (2008): 702-710.
- Contacos PG, Collins WE. Plasmodium malariae: transmission from monkey to man by mosquito bite. Science 165 (1969): 918-919.
- Rodhain J, Dellaert R. L'infection à Plasmodium malariae du chimpanzé chez l'homme. Étude d'une première souche isolée de l'anthropoïde Pan Satyrus verus. Ann Soc belge Med Trop 23 (1943): 19.
- Boudin C, Robert V, Verhave JP, Carnevale P, Ambroise-Thomas P. Plasmodium falciparum and malariae epidemiology in a West African village. Bull World Health Organ 69 (1991): 199-205.
- Molineaux L, Storey J, Cohen JE, Thomas A. A longitudinal study of human malaria in the West African Savanna in the absence of control measures: relationships between different Plasmodium species, in particular falciparum and P. malariae. Am J Trop Med Hyg 29 (1980): 725-737.
- Scopel KK, Fontes CJ, Nunes AC, Horta MF, Braga EM. High prevalence of Plasmodium malariae infections in a Brazilian Amazon endemic area (Apiacas-Mato Grosso State) as detected by polymerase chain reaction. Acta Trop 90 (2004): 61-64.
- Kaneko A, Taleo G, Kalkoa M, Yaviong J, Reeve PA, Ganczakowski M, et al. Malaria epidemiology, glucose 6- phosphate dehydrogenase deficiency and human settlement in the Vanuatu Archipelago. Acta Trop 70 (1998): 285-302.
- World malaria report. Geneva, Switzerland: World Health Organization (2020): 299.
- World malaria report. Geneva, Switzerland: World Health Organization (2023): 356.
- Assennato SM, Berzuini A, Foglieni B, Spreafico M, Allain JP, Prati D. Plasmodium genome in blood donors at risk for malaria after several years of residence in Italy. Transfusion 54 (2014): 2419-2424.
- Rutledge GG, Marr I, Huang GKL, Auburn S, Marfurt J, Sanders M, et al. Genomic Characterization of Recrudescent Plasmodium malariae after Treatment with Artemether/Lumefantrine. Emerg Infect Dis 23 (2017): 1300-1307.
- Ghose AK. A Fatal Case of Cerebral Malaria Caused by Plasmodium malariae. Ind Med Gaz 72 (1937): 419.
- Brosseau L, Drame PM, Besnard P, Toto JC, Foumane V, Le Mire J, Mouchet F, Remoue F, Allan R, Fortes F, et al. Human antibody response to Anopheles saliva for comparing the efficacy of three malaria vector control methods in Balombo, Angola. PLoS One 2012, 7:e44189.
- Carnevale P, Dos Santos MA, Alcides MS, Besnard P, Foumane V, Fortes F, et al. Parasitological surveys on malaria in rural Balombo (Angola) in 2007-2008: Base line data for a malaria vector control project. Internat J Trop Med & Health 31 (2018): 1-12.
- Carnevale P, Foumane Ngane V, Toto JC, Dos Santos MA, Fortes F, Manguin S. The Balombo (Benguela Province, Angola) Project: a village scale malaria vector control programme with a long-term comprehensive evaluation. In Annual conference and exhibition strengthening surveillance systems for vector-borne disease elimination in Africa (2019): 23-25.
- Cao Y, Wang W, Liu Y, Cotter C, Zhou H, Zhu G, et al. The increasing importance of Plasmodium ovale and Plasmodium malariae in a malaria elimination setting: an observational study of imported cases in Jiangsu Province, China, 2011-2014. Malar J 15 (2016): 459.
- Deng Y, Zhou RM, Zhang HW, Qian D, Liu Y, Chen WQ, et al. [Diagnosis and treatment for three imported Plasmodium malariae malaria cases in Henan Province]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 32 (2014): 61-63.
- Fancony C, Gamboa D, Sebastiao Y, Hallett R, Sutherland C, Sousa-Figueiredo JC, et al. Various pfcrt and pfmdr1 genotypes of Plasmodium falciparum cocirculate with malariae, P. ovale spp and P. vivax in northern Angola. Antimicrob Agents Chemother 56 (2012): 5271-5277.
- Martins JF, Marques C, Nieto-Andrade B, Kelley J, Patel D, Nace D, et al. Malaria Risk and Prevention in Asian Migrants to Angola. Am J Trop Med Hyg 103 (2020): 1918-1926.
- Ruas R, Pinto A, Nuak J, Sarmento A, Abreu C. Non-falciparum malaria imported mainly from Africa: a review from a Portuguese hospital. Malar J 16 (2017): 298.
- Diallo MA, Diongue K, Seck MC, Ndiaye M, Diallo I, Diedhiou Y, et al. Quality control of malaria microscopy reveals misdiagnosed non-falciparum species and other microscopically detectable pathogens in Senegal. Ann Clin Microbiol Antimicrob 17 (2018): 8.
- Moura S, Fancony C, Mirante C, Neves M, Bernardino L, Fortes F, et al. Impact of a training course on the quality of malaria diagnosis by microscopy in Angola. Malar J 13 (2014): 437.
- Doctor SM, Liu Y, Anderson OG, Whitesell AN, Mwandagalirwa MK, Muwonga J, et al. Low prevalence of Plasmodium malariae and Plasmodium ovale mono-infections among children in the Democratic Republic of the Congo: a population-based, cross-sectional study. Malar J 15 (2016): 350.
- Nguiffo-Nguete D, Nongley Nkemngo F, Ndo C, Agbor JP, Boussougou-Sambe ST, Salako Djogbenou L, et al. Plasmodium malariae contributes to high levels of malaria transmission in a forest-savannah transition area in Cameroon. Parasit Vectors 16 (2023): 31.
- Manguin S, Foumane V, Besnard P, Fortes F, Carnevale P. Malaria overdiagnosis and subsequent overconsumption of antimalarial drugs in Angola: Consequences and effects on human health. Acta Trop 171 (2017): 58-63.
- From 30 million cases to zero: China is certified malaria-free by WHO. WHO report (2021).
- Zhang SS, Feng J, Zhang L, Ren X, Geoffroy E, Manguin S, et al. Imported malaria cases in former endemic and non-malaria endemic areas in China: are there differences in case profile and time to response? Infect Dis Poverty 8 (2019): 61.
- Kotepui M, Kotepui KU, Milanez GD, Masangkay FR. Global prevalence and mortality of severe Plasmodium malariae infection: a systematic review and meta- analysis. Malar J 19 (2020): 274.
- Collins WE, Jeffery GM. Plasmodium malariae: parasite and disease. Clin Microbiol Rev 20 (2007): 579-592.
- Langford S, Douglas NM, Lampah DA, Simpson JA, Kenangalem E, Sugiarto P, et al. Plasmodium malariae Infection Associated with a High Burden of Anemia: A Hospital-Based Surveillance Study. PLoS Negl Trop Dis 9 (2015): e0004195.
- Savargaonkar D, Shah N, Das MK, Srivastava B, Valecha N. Plasmodium malariae infection: a case of missed diagnosis. J Vector Borne Dis 51 (2014): 149-151.
- Danis M, Gentilini M, Paludisme. In Médecine Tropicale. Edited by Lavoisier. Paris, France (2012): 191-231
- Gilles HM, Hendrickse RG. Nephrosis in Nigerian children. Role of Plasmodium malariae, and effect of antimalarial treatment. Br Med J 2 (1963): 27-31.
- Calleri G, Balbiano R, Caramello P. Are artemisinin-based combination therapies effective against Plasmodium malariae? J Antimicrob Chemother 68 (2013): 1447- 1448.
- Dinko B, Oguike MC, Larbi JA, Bousema T, Sutherland CJ. Persistent detection of Plasmodium falciparum, malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian school-children. Int J Parasitol Drugs Drug Resist 3 (2013): 45-50.
- Franken G, Muller-Stover I, Holtfreter MC, Walter S, Mehlhorn H, Labisch A, et al. Why do Plasmodium malariae infections sometimes occur in spite of previous antimalarial medication? Parasitol Res 111 (2012): 943-946.
- Islam S, Hai F. Recrudescing Plasmodium malariae infection despite appropriate treatment in an immigrant toddler. Paediatr Int Child Health 38 (2018): 290-293.
- Kugasia IR, Polara FK, Assallum H. Recrudescence of Plasmodium malariae after Quinine. Case Rep Med (2014): 590265.
- Visser R, de Mast Q, Munnix I, van der Ven A, Dofferhoff T. Failure of atovaquone-proguanil chemoprophylaxis and chloroquine treatment in Plasmodium malariae Travel Med Infect Dis 14 (2016): 644-645.
- Betson M, Sousa-Figueiredo JC, Atuhaire A, Arinaitwe M, Adriko M, Mwesigwa G, et al. Detection of persistent Plasmodium infections in Ugandan children after artemether-lumefantrine treatment. Parasitology 141 (2014): 1880-1890.
- Mombo-Ngoma G, Kleine C, Basra A, Wurbel H, Diop DA, Capan M, et al. Prospective evaluation of artemether-lumefantrine for the treatment of non-falciparum and mixed-species malaria in Gabon. Malar J 11 (2012): 120.
- Macdonald G. The epidemiology and control of malaria. London, UK: Oxford University Press (1957).
- Plenderleith LJ, Liu W, Li Y, Loy DE, Mollison E, Connell J, et al. Zoonotic origin of the human malaria parasite Plasmodium malariae from African apes. Nat Commun 13 (2022): 1868.
- Barber BE, William T, Dhararaj P, Anderios F, Grigg MJ, Yeo TW, et al. Epidemiology of Plasmodium knowlesi malaria in north-east Sabah, Malaysia: family clusters and wide age distribution. Malar J 11 (2012): 401.
- Brock PM, Fornace KM, Parmiter M, Cox J, Drakeley CJ, Ferguson HM, et al. Plasmodium knowlesi transmission: integrating quantitative approaches from epidemiology and ecology to understand malaria as a zoonosis. Parasitology 143 (2016): 389-400.
- Lau YL, Tan LH, Chin LC, Fong MY, Noraishah MA, Rohela M. Plasmodium knowlesi reinfection in human. Emerg Infect Dis 17 (2011): 1314-1315.
- Rajahram GS, Barber BE, William T, Menon J, Anstey NM, Yeo TW. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malar J 11 (2012): 284.
- Singh B, Daneshvar C Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev 26 (2013): 165-184.
- Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363 (2004): 1017-1024.
- Wong ML, Chua TH, Leong CS, Khaw LT, Fornace K, Wan-Sulaiman WY, et al. Seasonal and spatial dynamics of the primary vector of Plasmodium knowlesi within a major transmission focus in Sabah, Malaysia. PLoS Negl Trop Dis 9 (2015): e0004135.
- Hii J, Vythilingam I, Roca-Feltrer A. Human and simian malaria in the Greater Mekong Subregion and challenges for elimination. In Towards malaria elimination - A leap forward. Edited by Manguin S, Dev V. Lodon, UK: IntechOpen (2018): 95- 127
- Chin AZ, Maluda MCM, Jelip J, Jeffree MSB, Culleton R, Ahmed K. Malaria elimination in Malaysia and the rising threat of Plasmodium knowlesi. J Physiol Anthropol 39 (2020): 36.
- Lee WC, Cheong FW, Amir A, Lai MY, Tan JH, Phang WK, et al. Plasmodium knowlesi: the game changer for malaria eradication. Malar J 21 (2022): 140.
- Cooper DJ, Rajahram GS, William T, Jelip J, Mohammad R, Benedict J, et al. Plasmodium knowlesi Malaria in Sabah, Malaysia, 2015-2017: Ongoing Increase in Incidence Despite Near-elimination of the Human-only Plasmodium Species. Clin Infect Dis 70 (2020): 361-367.
- Malaria Elimination Eight Initiative - Reaching the zero-malaria target. In Elimination 8 (2021): 52.
- Tavares W, Morais J, Martins JF, Scalsky RJ, Stabler TC, Medeiros MM, et al. Malaria in Angola: recent progress, challenges and future opportunities using parasite demography studies. Malar J 21 (2022): 396.
- Manguin S, Dev V. Towards malaria elimination - A leap forward. London, UK: IntechOpen (2018).
- Dev V, Manguin S. Defeating malaria in the North-East region: the forerunner for malaria elimination in India. Acta Trop 222 (2021): 106040.
- Carnevale P, Manguin S. Review of Issues on Residual Malaria Transmission. J Infect Dis 223 (2021): S61-S80.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 