Metadichol; An Agonist that Expresses the Anti-aging gene Klotho in Various Cell Lines
Palayakotai R. Raghavan
Nanorx Inc., PO Box 131, Chappaqua, NY 10514, USA
*Corresponding author: Palayakotai R. Raghavan, Nanorx Inc., PO Box 131, Chappaqua, NY 10514, USA.
Received: 16 September 2023; Accepted: 22 September 2023; Published: 29 September 2023
Article Information
Citation: Palayakotai R. Raghavan. Metadichol; An Agonist that Expresses the Anti-aging gene Klotho in Various Cell Lines. Fortune Journal of Health Sciences. 6 (2023): 357-362.
View / Download Pdf Share at FacebookAbstract
Klotho is a well-known tumor suppressor hormone that exhibits anti-cancer and anti-aging properties. Klotho levels are low or non-existent in cancer patients. Klotho protein levels decrease with aging; maintaining consistent levels may prevent disease and promote healthier aging. Metadichol is a nanoemulsion of long-chain alcohols C26, C28, and C30, of which C-28 constitutes over 85%. Any small molecule that can elevate Klotho can, in principle, help reverse many diseases in which Klotho levels are low. Previously, we showed that treatment of the pancreatic cancer cell lines PANC1, MIA-PACA, and COLO-205, combined with Metadichol, a lipid emulsion consisting of long-chain alcohols at 1-100 pg/mL concentrations, resulted in a 4- to 10-fold increase in Klotho expression as determined by qRT-PCR, This study aimed to demonstrate that Metadichol promotes Klotho expression in a wide variety of cell lines, such as primary cancer, stem, and somatic cell lines. Cells were treated with various concentrations of Metadichol ranging from 1 pg (picogram) to 1µg 9microgram). Three-to-fifteen-fold increase in Klotho expression was observed compared with baseline, as measured by qRT-PCR and qualified by western blot analysis. Metadichol is a natural agonist of Klotho expression and is non-toxic at levels up to 5000 mg/kg in rats. and has a potential therapeutic role in cancer and reversing aging.
Keywords
<p>Klotho, Metadichol, anti-aging, anti-tumor, primary cancer cells, fibroblasts</p>
Article Details
1. Introduction
In Greek methodology, Klotho was known as the goddess of lifespan regulation [1]. Following its initial discovery in the kidneys in 1997 [2], Klotho has been shown to block tumor growth and metastasis, modulate chemotherapeutic drug resistance, and improve overall survival [3]. The injection of Klotho into animals decreased breast cancer growth regarding tumor size, weight, and visual appearance and resulted in no significant side effects. Low levels of Klotho are associated with cancer and many other diseases [4-6], as shown in Table 1. An increasing number of studies [4] have focused on the use of Klotho in extending longevity and counteracting the effects of aging on physical function. Recently, significant progress has been made in identifying factor [7] contributing to the aging process, which has contributed to developing therapeutic strategies to prevent, delay, or reverse age-related decline. Many studies have attempted to induce Klotho gene expression, emphasizing its potential benefits when injected into animals. Clinical and preclinical studies have demonstrated that Klotho is an essential anti-aging molecule [8] that affects lifespan, health, and cognitive function [9]. Laboratory animals that do not express Klotho exhibit a shorter lifespan and cognitive impairment.
Table 1: List of diseases associated with low levels of Klotho
|
Acetylcholine and Nitric Oxide Dysregulation |
Hypertension |
|
Aging (highly accelerated) |
Impaired Cognition (such as Alzheimer's Disease) |
|
All-Cause Mortality |
Hyperphosphatemia |
|
Anemia |
Lung Damage |
|
Anorexia |
Multiple System Atrophy |
|
Atherosclerosis (as well as calcification of the arteries) |
Pseudo exfoliation Syndrome |
|
Bone Loss (such as osteoporosis and low bone mass) |
Rheumatoid Arthritis |
|
Cancers · Bone · Brain · Breast · Colon · Stomach · Kidney · Liver |
Sarcopenia |
|
Skin Atrophy (such as scleroderma) |
|
|
Stroke |
|
|
Vascular Disease (such as coronary artery disease) |
|
|
Hyperparathyroidism |
|
|
Inflammatory Bowel Disease |
In contrast, mice that overexpress Klotho have a longer lifespan and enhanced cognition and memory [10]. Klotho significantly inhibited the growth of lung cancer [11], pancreatic carcinoma [12], colorectal carcinoma [13], breast cancer [14], hepatocellular carcinoma [15], ovarian carcinoma [16], melanoma [17], diffuse large B cell lymphoma [18]. Several molecules, such as PPARγ agonists [19], testosterone [20], and resveratrol [21], either directly promote Klotho expression in vitro or inhibit Klotho regulation in vivo. Jung et al reported a novel molecular mechanism by which a small molecule (N-(2-chlorophenyl)-1H-indole-3-caboxamid) induces Klotho expression [22].
Other studies [23] have used CRISPR methodology to upregulate Klotho transcription and production in two different cell lines, one of which was a neuron-like cell line. Pharmaceutical companies have been developing Klotho agonists that up-regulate Klotho expression and are of significant interest in treating diseases. For example, Klotho Therapeutics has developed a patent-pending treatment based on Klotho that affects aging [24]. Kleenex has been working on a two-pronged approach to target endogenous Klotho's natural production and regulation and deliver Klotho genetic material directly to patient cells, thus enabling them to produce the protein [25].
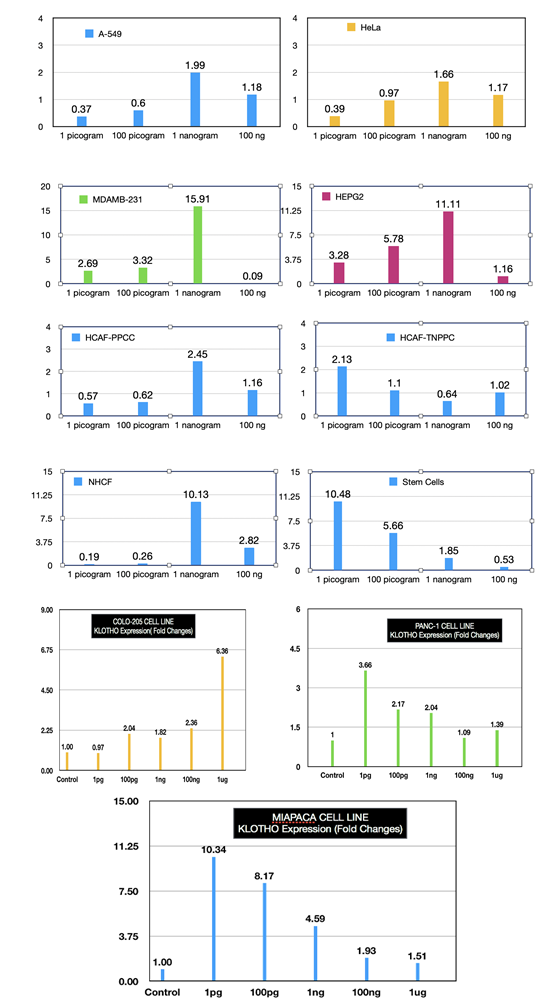
Previously, we showed that treatment of the pancreatic cancer cell lines PANC1, MIA-PACA, and COLO-205, combined with Metadichol, a lipid emulsion consisting of long-chain alcohols at 1-100 pg/mL concentrations, resulted in a 4- to 15-fold increase in Klotho expression as determined by qRT-PCR [26]. In the present study, we have extended our original work to determine the effects of metadichol in many other cell lines, including primary cancer cell lines, stem cell lines, and fibroblasts (Figure1).
2. Methods
2.1 Experimental procedures
This work was carried out by a service provider: Skanda Life Sciences, Bangalore, India.
2.2 Chemicals and reagents
The A549, Colo-205, PANC1, MDAMB31, Hela, HepG2, and human cardiac fibroblast cells were purchased from the ATCC (USA). Primary breast cancer cells were obtained from BIOIVT (Detroit, Michigan, USA). Primary antibodies were purchased from AB clonal (Woburn, Massachusetts, USA) and E-lab Science (Maryland, USA). Primers were obtained from Saha Gene, Hyderabad, India (Table 2). All other molecular biology reagents were purchased from Sigma Aldrich.
Table 2: Primers used for qPCR
|
Gene |
Primer pair |
Sequence |
Tm |
Product size (base pairs) |
|
β–Actin |
FP |
TCCTCCTGAGCGCAAGTACTCT |
62.1 |
153 |
|
RP |
GCTCAGTAACAGTCCGCCTAGAA |
62.4 |
||
|
KLOTHO |
FP |
GGGAGGTCAGGTGTCCATTG |
55.88 |
152 |
|
RP |
TGCTCTCGGGATAGTCACCA |
53.83 |
2.3 Cell line maintenance and seeding
The cells were cultured in a suitable medium with or without supplements in the presence of 1% antibiotics in a humidified atmosphere of 5% CO2 at 37°C. The medium was changed periodically until the cells reached confluency. Cell survival was determined using a hemocytometer. When the cells reached 70–80% confluency, single-cell suspensions were seeded into 6-well plates at a density of 106 cells per well and incubated for 24 h at 37°C in 5% CO2. Afterward, we rinsed the cell monolayer with a serum-free medium and added metadichol at predefined concentrations.
2.4 Cell treatment
Metadichol was prepared at various concentrations (1 pg/mL, 100 pg/mL, one ng/mL, and 100 ng/mL) in serum-free media, and the mixture was added to predesignated wells. The control cells received media without drugs. The cells were incubated, then gently rinsed with sterile phosphate-buffered saline (PBS) solution. Quantitative RT-PCR (qRT-PCR) and western blot analysis were done as described below.
2.5 RNA isolation
RNA was isolated from each treatment group using TRIzol reagent (Invitrogen). Cells (106) were collected into 1.5-mL microcentrifuge tubes and centrifuged at 5,000 rpm for 5 min at 4°C. Then, 650 µL of TRIzol was added to the pellet, mixed, and incubated on ice for 20 min. Subsequently, we added 300 µL of chloroform, mixed the samples with gentle inversion for 1–2 min, and set them on ice for 10 min. The samples were centrifuged at 12,000 rpm for 15 min at 4°C. The upper aqueous layer was transferred to a new sterile 1.5 mL centrifuge tube, and an equal amount of prechilled isopropanol was added. The samples were incubated at −20°C for 60 min, then centrifuged at 12,000 rpm for 15 min at 4°C. The supernatant was carefully removed, and the RNA pellet was washed with 1.0 mL of 100% ethanol and 700 µL of 70% ethanol by centrifugation as previously described. The RNA pellet was air-dried at room temperature for approximately 15–20 min and resuspended in 30 µL of DEPC-treated water. RNA concentration was measured using a Spectra drop (Spectramax i3x, USA) spectrophotometer (Molecular Devices).
2.6 cDNA synthesis
Complementary DNA (cDNA) was synthesized from 2 µg of total RNA using the Prime Script cDNA synthesis kit (Takara) with oligo dT primers following the manufacturer’s instructions. The reaction volume was 20 μL, and cDNA synthesis was performed at 50°C for 30 min, then incubated at 85°C for 5 min using an Applied Biosystems instrument (Veritii). The resulting cDNA was used as a template for qPCR.
2.7 Primers and qPCR
A final reaction volume of 20 µL of PCR mixture was prepared, consisting of 1 µL of cDNA, 10 µL of SYBR green Master Mix, and one µM complementary forward and reverse gene-specific primers. The samples were run under the following conditions: initial denaturation at 95°C for 5 min, followed by 30 cycles of secondary denaturation at 95°C for 30 seconds, annealing at the optimized temperature for 30 seconds, and extension at 72°C for 1 min. We defined the number of cycles that allowed amplification in the exponential range without reaching a plateau as the optimal number of cycles. The results were obtained using CFX Maestro software. We calculated fold-change using the comparative CT method and determined the relative expression of each target gene relative to a housekeeping gene (β-actin) and untreated control cells. ΔCT was calculated for each treatment using the following formulas: ΔCt = Ct (target gene)–Ct (reference gene), ΔΔ, Ct = ΔCt (treatment group)–ΔCt (control group). The fold change was calculated for target gene expression for each treatment using the formula: Fold change = 2^ (−ΔΔCt).
2.8 Protein isolation and western blot analysis
Total protein was isolated from 106 cells using radioimmuno precipitation assay buffer supplemented with the protease inhibitor phenylmethyl sulfonyl fluoride. The cells were lysed for 30 min at four °C with gentle inversion, centrifuged at 10,000 rpm for 15 min, and the supernatant was transferred to a new sterile tube. The Bradford method (BIO-RAD USA) was used to measure the protein concentration. Protein (25 µg) was mixed with 1X sample loading dye containing SDS and loaded onto a polyacrylamide gel. The proteins were separated under denaturing conditions using a Tris-glycine running buffer. The proteins were transferred to PVDF membranes (Invitrogen) using a Turbo transblot system (Bio-Rad, USA), blocked with 5% BSA for one h, incubated with the respective primary antibody overnight at four °C, followed by a species-specific secondary antibody for one h at RT. After washing, the membranes were incubated with ECL substrate (Merck) for 1 min in the dark. The images at suitable exposure settings were captured using the ChemiDoc XRS system (Bio–Rad, USA).
4. Discussion
The highest Klotho expression was observed in MDAMB-231 (Triple-Negative Breast Cancer cells developed at MD Anderson Institute Houston), followed by stem cells, then cardiac fibroblasts (NHCFs) (Figure 1). Although the expression of Klotho induced by small molecules is elevated in kidney cells [27], we found that Metadichol increased Klotho expression in many different cell types and thus may represent a universal Klotho agonist. Because Klotho exhibits anticancer activity, its level of expression in cancer cells is significant. Down regulation of Klotho has been observed in several cancers, such as pancreatic cancer, HCC, and others [28, 29]. Epigenetic modulation, such as promoter methylation and histone deacetylation, also contributes to the dysregulation of Klotho in cancer. Forester et al (30, 31). Suggested that the liganded vitamin D receptor (VDR) upregulates Klotho via vitamin D response elements (VDRE). Metadichol is a VDR inverse/protein agonist [32, 33]. Among the consequences of the enhanced expression of Klotho is an increase in telomerase activity. Similarly, Metadichol can upregulate telomerase [34] and thus potentially prevent stem cell aging [35]. In addition, the nuclear receptor PPAR gamma regulated Klotho expression. [35]. We have recently shown that Metadichol increases PPAR gamma expression 2-3-fold, increase in Stem and human dermal Fibroblast cells [36].
Consequently, the downregulation of Klotho enhances proliferation and reduces apoptosis in cancer cells. Conversely, the over expression of Klotho results in cancer cell inhibition [37-40]. Similarly, our results suggest that metadichol could be useful in inducing apoptosis in cancer cells by increasing Klotho expression, which also has benefits in other diseases. The current study and previously published results [41-44] suggest that the observed results could have been due to increased Klotho expression [45]. The potential therapeutic utility of metadichol in elevating Klotho levels warrants further study in vitro and in vivo.
Abbreviated Cell Lines Description
PANC1: human pancreatic cancer cell line isolated from a pancreatic carcinoma of ductal cell origin
HCAF-PPCC: (Human Cancer-associated Fibroblast primary prostate cancer cell.
MIA-PACA: Human pancreatic cancer cell line
HCAF-TNBCC; Human Cancer-associated Fibroblasts Triple Negative Breast Cancer Panel
COLO-205: The cell line is made up of epithelial cells isolated from ascitic fluid derived from a 70-year-old male with colon cancer.
A-549: Adenocarcinomic human alveolar basal epithelial cell
HEPG2: human liver cancer cell line
HELA: Immortalized cell line used in scientific research. It is the oldest and most commonly used human cell line. The line is derived from cervical cancer cells
hESC BGO1V: cells are pluripotent and can differentiate to representatives of the three primary germ layers.
MDAMB 231: isolated at M D Anderson Institute, Houston, USA, from a pleural effusion of a patient with invasive ductal carcinoma) is commonly used to model late-stage breast cancer
Author Contributions
All work was planned and supervised by the author (PPR), who is solely responsible for its content.
Conflicts of Interest
None
Funding:
Nanorx, Inc. R&D Budget provided funding.
Competing Interests:
None
Availability of data and materials
All data are in the manuscript and the supplementary material provided.
Supplementary material:
Western Blot data and Klotho Diseases NetworkThe paper was published as Preprint; Metadichol, an agonist that expresses the anti-aging gene Klotho in various cell lines," has been posted to Research Square. DOI; 10.21203/rs.3.rs-2635049/v1
References
- Michael Lichtenauer, et al. Uncoupling fate: Klotho—Goddess of fate and regulator of life and ageing; Australasian Journal of aging 39 (2020): 161-163.
- Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling aging. Nature 390 (1997): 45–51.
- Ligumsky H, et al 2022. The role of α-klotho in human cancer: molecular and clinical aspects. Oncogene 41 (2022): 4487–97.
- Kuro-o M. The Klotho proteins in health and disease. Nature Reviews Nephrology 15 (2019): 27-44.
- Buchanan S, et al. Klotho, aging, and the failing kidney. Frontiers in endocrinology 11 (2020): 560.
- Chen K, et al. Klotho deficiency causes heart aging via impairing the Nrf2-GR pathway. Circulation research, 128 (2021): 492-507.
- Feder A, et al. The biology of human resilience: opportunities for enhancing resilience across the life span. Biol psychiatry 86 (2019): 443-53.
- Prud’homme GJ, et al. Pathobiology of the klotho anti-aging protein and therapeutic considerations. Front Aging 3 (2022): 931331.
- Abraham CR, et al. Klotho is a neuroprotective and cognition-enhancing protein. Vitam Horm. 2016; 101 (2016): 215–38.
- Kurosu H, Kuro-o M et al. Suppression of aging in mice by the hormone klotho. Science. 2005 309 (2005): 1829–33.
- Doi S, Kuro-O M et al. Klotho inhibits transforming growth factor-β1 (TGF-1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 2011 286 (2011): 8655–65.
- Abramovitz L, teal. KL1 internal repeat mediates klotho tumor suppressor activities and inhibits bFGF and IGF-I signaling in pancreatic cancer. Clin Cancer Res 17 (2011): 4254–66.
- Li XX, et al. Klotho suppresses the growth and invasion of colon cancer cells through inhibition of the IGF1R-mediated PI3K/AKT pathway. Int J Oncol 45 (2014): 611–8.
- Ligumsky et al. Tumor suppressor activity of klotho in breast cancer is revealed by structure-function analysis. Mol Cancer Res13 (2015): 1398–1407.
- Tang X, et al. Klotho: a tumor suppressor and modulator of the Wnt/ β-catenin pathway in human hepatocellular carcinoma. Lab Invest 96 (2016): 197–205.
- Yan Y, et al. Reduced Klotho expression contributes to poor survival rates in human patients with ovarian cancer, and over expression of Klotho inhibits the progression of ovarian cancer partly via the inhibition of systemic inflammation in nude mice. Mol Med Rep 15 (2017): 1777–85.
- Behera R, et al. Inhibition of age-related therapy resistance in melanoma by rosiglitazone-mediated induction of klotho. Clin Cancer Res 23 (2017): 3181–90.
- Zhou X, et al. Klotho, an anti-aging gene, is a tumor suppressor and inhibitor of IGF-1R signaling in diffuse large B cell lymphoma. J Hematol Oncol 10 (2017): 37.
- Zhang H, et al. Klotho is a target gene of PPAR–γ. Kidney Int 74 (2008): 732–9.
- Hsu SC, et al. Testosterone increases renal anti-aging klotho gene expression via the androgen receptor-mediated pathway. Biochem J 464 (2014): 221–9.
- Hsu SC, et al. Resveratrol increases anti-aging klotho gene expression via the activating transcription factor 3/c-Jun complex-mediated signaling pathway. Int J Biochem Cell Biol 53 (2014): 361–71.
- Jung D, et al. Induction of anti-aging klotho with a small chemical compound that demethylates CpG islands. Oncotarget 8 (2018): 46745–55.
- Chen CD, et al. Activation of the anti-aging and cognition-enhancing gene klotho by CRISPR-dCas9 transcriptional effector complex. J Mol Neurosci 64 (2018): 175–84.
- klotho.com.
- klogenix.com.
- Raghavan PR. Metadichol® a novel agonist of the anti-aging klotho gene in cancer cell lines. J Cancer Sci Ther10 (2018): 351–7.
- Zhou X, and Wang X. Klotho: a novel biomarker for cancer. J Cancer Res Clin Oncol 141 (2015): 961–9.
- Li Y, et al. Overexpression of klotho suppresses growth and pulmonary metastasis of osteosarcoma in vivo. Genet Mol Biol 43 (2020): e20190229.
- King GD, et al. Identification of novel small molecules that elevate klotho expression. Biochem J 441 (2012): 453–61.
- Forster RE, et al. Vitamin D receptor controls the expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun 414 (2011): 557–62.
- Haussler MR, et al. 1,25-Dihydroxyvitamin D and klotho: a tale of two renal hormones coming of age. Vitam Horm 100 (2016): 165–230.
- Neubig RR. Missing links: mechanisms of protean agonism, Mol Pharmacol 71 (2007): 1200–2.
- PR Raghavan, Metadichol ® Liquid and gel nanopaarticle formulations. US patent 9 (2015): 006.292.
- Raghavan PR. 2019. The quest for immortality: introducing Metadichol®, a novel telomerase activator. Stem Cell Res Ther 9 (2019): 2.
- Zhang H, et al. Klotho is a target gene of PPAR-gamma. Kidney Int. Sep 74 (2008): 732-9.
- PR Raghavan. Metadichol® is a nano lipid emulsion that expresses all 48 nuclear receptors in stem and somatic cells T (2022).
- Ullah M and Sun Z. Klotho deficiency accelerates stem cell aging by impairing telomerase activity. J Gerontol a Biol Sci Med Sci 74 (9):1396–407.
- Zhou X, Wang X. Klotho: a novel biomarker for cancer. J Cancer Res Clin Oncol 141 (2015): 961-9.
- Bo Chen, et al. Inhibition of lung cancer cells growth, motility and induction of apoptosis by Klotho, a novel secreted Wnt antagonist, in a dose-dependent manner, Cancer Biology & Therapy 13 (2012): 1221-1228.
- Ligumsky H, et al. The role of α-klotho in human cancer: Molecular and clinical aspects. Oncogene, 41 (2022): 4487–97.
- Raghavan PR. Metadichol, a novel ROR gamma inverse agonist, and its applications in psoriasis. J Clin Exp Dermatol Res 8 (2017): 433.
- Raghavan PR. Metadichol® and vitamin C increase in vivo, an open-label study. Vitam Miner 6 (2017): 3.
- Raghavan PR. Rheumatoid arthritis and osteoporosis: a case study. J Arthritis 6 (2017): 1000240.
- Raghavan PR. Systolic and diastolic BP control in metabolic syndrome patients with Metadichol®, a novel nanoemulsion lipid. J Cardiol Cardiovasc Ther 5 (2017): 555660.
- Kuro-O M. The Klotho proteins in health and disease. Nat Rev Nephrol 15 (2019): 27-44.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 