The Relationship between Heel Fat Pad Thickness and Flexibility and Physical Demographics
Kazuaki Kinoshita1¶*, Takehito Hananouchi2¶, Masayuki Fukuda3, Mai Kitagawa3, Mika Hirata3
1Department of Physical Therapy, Faculty of Rehabilitation, Shijonawate Gakuen University, Osaka 574-0011, Japan
2Medical Engineering Laboratory, Department of Mechanical Engineering, Faculty of Engineering, Osaka Sangyo University, Osaka, 574-8530, Japan
3Center of Rehabilitation, Kobe Kaisei Hospital, Hyogo 657-0068, Japan
*Corresponding author: Kazuaki Kinoshita, Department of Physical Therapy, Faculty of Rehabilitation, Shijonawate Gakuen University, Osaka 574-0011, Japan. Takehito Hananouchi, Department of Mechanical Engineering, Faculty of Engineering, Osaka Sangyo University, Osaka, 574-8530, Japan
¶These authors contributed equally to this work
Received: 01 December 2022; Accepted: 07 December 2022; Published: 15 December 2022
Article Information
Citation: Kazuaki Kinoshita, Takehito Hananouchi, Masayuki Fukuda, Mai Kitagawa, Mika Hirata. The Relationship between Heel Fat Pad Thickness and Flexibility and Physical Demographics. Fortune Journal of Health Sciences 5 (2022): 588-595.
View / Download Pdf Share at FacebookAbstract
To clarify the factors that contribute to the development of heel pain in young individuals, this study investigated the relationship of heel thickness and flexibility with physical demographics among Japanese elementary and junior high school students. A total of 69 heels were included as participants in this study. The measurement items were age, height, weight, rohrer index, heel fat pad thickness and flexibility. The heel fat pad thickness and flexibility was measured using an ultrasound probe and softgram (Shinko Denshi co.,ltd.). The heel fat pad thickness measurements were distance from the skin to the calcaneus was measured by applying the ultrasound probe both without (hereafter referred to as "non-pressure thickness") and with manually applied pressure to the measurement point (hereafter referred to as “pressure thickness”) . The heel fat pad flexibility evaluation consisted of subtracting non-pressure thickness by pressure thickness (thickness of change difference), and computing for the value when the thickness of change difference is divided by the non-pressure thickness and multiplied by 100 (thickness of change rate). In addition to this, heel fat pad flexibility evaluation consisted of measuring using the softness sensor Softgram. Multiple regression analysis showed that weight and age were predictors of non-pressure thickness. On the other hand, pressure thickness was weight was a predictor of pressure thickness. In the relationship between heel fat pad flexibility and demographic data, the Softgram measurements showed a significantly fair correlation with age and significantly moderate correlations with height and weight. Multiple regression analysis further showed that body weight was a predictor of Softgram measurements. This results suggest that heel fat pad thickness and flexibility in elementary and junior high school students became thicker and stiffer, respectively, with increasing age, hei
Keywords
<p>Heel Fat Pad, Thickness, Flexibility, Physical Demographics, ultrasound, softness sensor</p>
Article Details
1. Introduction
The heel fat pad, which is made of fibrous adipose tissue, is designed to protect the lower limb by dispersing shock to the lower limb during loading [1,2]. Although its thickness has been reported to be related to heel pain, there has been no uniform consensus on whether heel pain occurs when the heel fat pad becomes thicker or thinner [1, 3-5]. Generally, the thickness of the heel fat pad increases with age until approximately 50 years of age and becomes thinner thereafter [6, 7]. However, few studies have reported detailed age-group data with regard to heel pain and heel pad thickness, especially in younger age groups. One example of heel pain in young individuals is Sever's disease, which afflicts many athletes [8, 9]. Sever's disease is reported to occur most often in individuals aged 5-11 years with calcaneal apophysitis, specifically affecting those who play sports or move their heels a lot. Moreover, this disease is directly related to calcaneal impact and muscle tendon overuse [10]. Therefore, examining the relationship between heel fat pad thickness and heel pain in young people may be useful in the prevention and treatment of heel pain.
Aside from its thickness, heel fat pad flexibility is also related to heel pain. According to a report by researchers. [4, 11-13], patients with heel pain had less flexible heels. However, it should be noted that reports on heel flexibility were only based on older patients. In other words, the flexibility of the heel fat pad has not be known in young subjects. To clarify the factors that cause heel pain in young individuals, we believe that it is important to investigate how heel thickness and flexibility affect heel pain. Therefore, this study was designed to investigate the relationship between heel thickness and flexibility and physical demographics (e.g., age, body size, height, and weight) of young individuals in Japanese elementary and junior high schools. Specifically, thickness was measured using an ultrasound device, and flexibility was assessed by evaluating the difference between normal measurement and constant pressure application using Softgram (Shinko Denshi Co., Ltd., Tokyo, Japan), a compression test measurement device [14-16].
2. Materials and Methods
2.1 Participants
A total of 38 healthy junior gymnasts with 76 heels were included as participants in this study (Table 1). The exclusion criteria were as follows: history of foot surgery, foot injury at the start of the study, and congenital malformations of the foot. This study adhered to the ethical standards of the Declaration of Helsinki and was approved by the Ethics Committee of Shijonawate-gakuen University (No. 20-9). All participants and their guardians gave their consent before participating.
Table 1: Characteristics of the Study Subjects
|
Sex |
N |
Male: 10 Female: 28 |
|||
|
Age |
years |
11.3 |
± |
2 |
(7-14) |
|
Height |
cm |
137.3 |
± |
11.2 |
(118.5-156.5) |
|
Weight |
kg |
32 |
± |
7.6 |
(19.8-49.9) |
|
Rohrer index |
kg/m3 |
121.8 |
± |
9.6 |
(104.5-145.3) |
2.2 Measurement of heel fat pad thickness
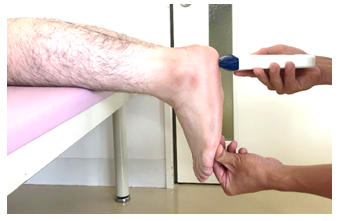
Thickness was measured using a Cprobe-5L ultrasound probe (Sonostar Technologies Co., Limited, China), and data were recorded in millimeters on an iPad (Apple 2017). One examiner performed the measurement. Measurements were taken using a modified version of Daniel et al.'s measurement method [1], with a measured intraclass correlation if 0.95 (0.90-0.97) in the seated with their foot at 90° to the tibia position. In this study, the seated position was modified to the prone position, , and the position of the ankle joint is the same as in previous studies. Then, the examiner checked for maximum heel bulge on the plantar surface of the foot and placed the ultrasound probe just perpendicular to the plantar surface (Fig. 1).
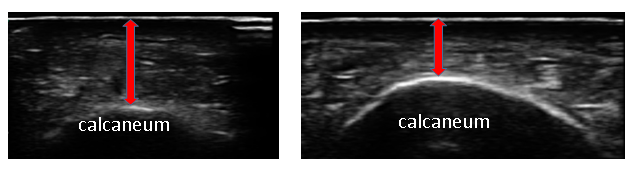
The distance from the skin to the calcaneus was measured by applying the ultrasound probe both without (hereafter referred to as "non-pressure thickness") (Fig. 2) and with manually applied pressure to the measurement point (hereafter referred to as “pressure thickness”) (Fig. 3). Measurements were taken three times each at random on the left and right sides, and the average value was recorded. Using the study data, the relationship of non-pressure and pressure thickness with demographic data (age, height, weight, and Laurel index) was investigated. Multiple regression analysis was also performed with non-pressure and pressure thickness as dependent variables and demographic data as independent variables.
Figure 1: The Position of the Limb When Using the Ultrasound System to Measure Heel Fat Pad Thickness. The examiner checks the area of maximum heel bulge on the plantar surface of the foot and places the ultrasound probe just perpendicular to the plantar surface. (A) Non-pressure thickness. (B) Pressure thickness.
Figure 2: Measurement for Non-pressure and Pressure Thickness. (A) The distance from the skin to the calcaneus is measured by applying the ultrasound probe without applying pressure to the measurement point. (B) The distance from the skin to the calcaneus is measured by applying the ultrasound probe with maximum pressure at the measurement point.
2.3 Measurement of heel fat pad flexibility
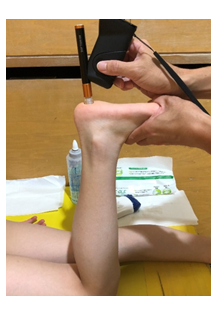
Flexibility evaluation consisted of measuring using the softness sensor Softgram, subtracting non-pressure thickness by pressure thickness (thickness of change difference), and computing for the value when the thickness of change difference is divided by the non-pressure thickness and multiplied by 100 (thickness of change rate). The Softgram is a device that measures flexibility by measuring through spherical indentation and damped oscillation when the tip of the sensor is pressed against the measurement site [14-16]. Sawai et al. reported less than 5% variation in soft gram measurements. [17]. Subjects in this study underwent an examination in the prone position with the knee flexed at 90° and the ankle joint in the mid-range (Fig. 3). The skin surface of the heel was wiped thoroughly with alcohol cotton, and the Softgram was applied perpendicularly to the maximum bulge of the heel for measurement, which was performed three times each at random on the left and right. The average value was used as the study data. For the thickness of change rate, higher numerical values were interpreted to be softer. Using the study data, the relationships of measured Softgram values, thickness of change difference, and thickness of change rate with demographic data were investigated. Furthermore, variables that showed a strong relationship with the demographic data were further investigated as dependent variables.
2.4 Statistical analyses
The Shapiro-Wilk test was first performed to assess normality. Based on these results, the unpaired t-test or Mann-Whitney test was used for between-group comparisons. Pearson's correlation coefficient or Spearman's rank correlation coefficient was used to examine correlations. Multiple regression analysis was also performed using the stepwise method. The sample size was calculated using the G*power 3.1.9 software (Heine University, Dusseldorf, Germany), resulting in a minimum sample size of 34 heels with an alpha level of 0.05, a power analysis of 0.80, and an estimated effect size of 0.50. Correlation analysis results were then classified as follows: 0.00-0.20 (poor correlation), 0.21-0.40 (fair correlation), 0.41-0.60 (moderate correlation), 0.61-0.80 (strong correlation), and 0.81-1.00 (very strong correlation). Statistical analysis was performed using the IBM SPSS Statistics for Windows, version 20.0 software (IBM Corp., NY, USA), and statistical significance was set at p < 0.05.
3. Results
From the 76 heels included in the study, seven heels developed pain, leading to the analysis of only 69 heels (Table 2).
In the relationship between heel fat pad thickness and demographic data, non-pressure thickness showed a very strongly significant correlation (r = 0.826) with age and a strongly significant correlation (r = 0.781 and r = 0.800, respectively) with height and weight. Meanwhile, pressure thickness showed a strongly significant correlation (r = 0.631, r = 0.619, and r = 0.661, respectively) with age, height, and weight (Table 3). Multiple regression analysis showed that the predictive equation for non-pressure thickness was 2.422 + 0.177 body weight + 0.324 age, indicating that weight and age were predictors of non-pressure thickness (F: 73.523, R2: 0.681, p < 0.01) (Table 4). On the other hand, the predictive equation for pressure thickness was 1.040 + 0.125 body weight, indicating that weight was a predictor of pressure thickness (F: 51.115, R2: 0.424, p < 0.01) (Table 5). The Laurel index was not significantly correlated with any of the items (Table 3).
Table 2: Demographic Data of the Study Participants
|
Sex |
heel |
Male: 20 Female: 49 |
||
|
Age |
years |
11.1 |
± |
2 |
|
Height |
cm |
136.2 |
± |
11 |
|
Weight |
kg |
31.5 |
± |
7.6 |
|
Rohrer index |
kg/m3 |
122.6 |
± |
9.6 |
|
Non-pressure thickness |
mm |
11.6 |
± |
2.3 |
|
Pressure thickness |
mm |
5 |
± |
1.4 |
|
Softgram |
kPa |
124.8 |
± |
49.6 |
|
Thickness of change difference |
mm |
6.6 |
± |
1.6 |
|
Thickness of change rate |
% |
57.3 |
± |
7.9 |
Table 3: Correlation Coefficients of the Measurement Items
( ) : p Value *p < 0.05. **p < 0.01.
Table 4: Results of Multiple Regression Analysis for Non-pressure Thickness
|
Unstandardized coefficients |
Standardized coefficients (β) |
p |
95% confidence interval |
|
||
|
(β) |
Lower |
Upper |
VIF |
|||
|
Constant |
2.422 |
0.01 |
0.598 |
4.247 |
||
|
Weight |
0.177 |
0.581 |
0 |
0.097 |
0.256 |
3.635 |
|
Age |
0.324 |
0.279 |
0.037 |
0.021 |
0.627 |
3.635 |
VIF: Variance Inflation Factor
Table 5: Results of multiple regression analysis for pressure thickness
|
|
Unstandardized coefficients |
Standardized coefficients (β) |
p |
95% confidence interval |
|
|
|
(β) |
Lower |
Upper |
VIF |
|||
|
Constant |
1.04 |
0.07 |
-0.089 |
2.169 |
||
|
Weight |
0.125 |
0.658 |
0 |
0.09 |
0.16 |
1 |
VIF: Variance Inflation Factor
In the relationship between heel fat pad flexibility and demographic data, the Softgram measurements showed a fair significantly correlation with age (r = 0.397) and a moderate significantly correlations with height and weight (r = 0.442 and r = 0.412, respectively) (Table 3). Multiple regression analysis further showed that the predictive equation for Softgram measurements was 27.003 + 3.104 body weight, indicating that body weight was a predictor of Softgram measurements (F: 19.698, R2: 0.216, p < 0.01) (Table 6).
Table 6: Results of Multiple Regression Analysis for Softgram Measurements
|
|
Unstandardized coefficients |
Standardized coefficients (β) |
p |
95% confidence interval |
|
|
|
(β) |
Lower |
Upper |
VIF |
|||
|
Constant |
27.003 |
. 238 |
-18.233 |
72.24 |
||
|
Weight |
3.104 |
. 477 |
0 |
1.708 |
4.5 |
1 |
VIF: Variance Inflation Factor
Lastly, thickness of change difference showed a moderate significantly correlations (r = 0.602, r = 0.530, and r = 0.528, respectively) with age, height, and weight and a strongly significant correlation (r = 0.778) with non-pressure thickness. Multiple regression analysis further showed that the predictive equation for thickness of change difference was 2.693 + 0.124 body weight, indicating that weight was a predictor of thickness of change difference (F: 39.267, R2: 0.360, p < 0.01) (Table 7). In contrast, no significant correlations were observed for thickness of change rate.
Table 7: Results of Multiple Regression Analysis for Thickness of Change Difference
|
|
Unstandardized coefficients |
Standardized coefficients (β) |
p |
95% confidence interval |
|
|
|
(β) |
Lower |
Upper |
VIF |
|||
|
Constant |
2.693 |
0 |
1.408 |
3.977 |
||
|
Weight |
0.124 |
0.608 |
0 |
0.085 |
0.164 |
1 |
VIF: Variance Inflation Factor
4. Discussion
This study investigated the relationship of heel thickness and flexibility with physical demographics (e.g., age, body size, height, and weight) among Japanese elementary and junior high school students. Results showed that heel fat pad thickness became thicker with increasing body weight and age and that flexibility became stiffer with increasing body weight.
The heel fat pad thickness of healthy adults is reported to be in the range of 12-28 mm [18-23], and the mean values in Japanese individuals aged 6-14 years are reported to be 12.7 mm for male individuals and 12.1 mm for female individuals [7]. In this study, the mean values measured were 12.1 mm for males individuals and 11.6 mm for female individuals, which were similar to the values reported in previous reports [7, 18-23]. Heel fat pad thickness in young individuals has been shown to increase with increasing weight and age in this studies. Maemichi et al. [7] reported that heel fat pad thickness increased from 1-5 to 30-44 years of age, whereas it decreased from 30-44 to 80-96 years of age. Furthermore, they reported that weight and height were well-correlated with the heel fat pads in male individuals. A study by Tas et al. [24] including subjects aged 19-50 years of age showed a good correlation (r = 0.68, p < .001) with body weight and a moderate correlation (r = 0.42, p = -.001) with height, suggesting that the heel fat pad becomes thicker with increasing age, height, and weight up to a certain point in middle age.
Considering the results of this study, heel fat pad thickness in young individuals was dependent on age, height, and weight, since it adapts to protect impact from the heel, especially with increased age and weight. However, no correlation was found between heel fat pad thickness and the Laurel index. Tas et al. [24] also reported a moderate correlation between heel fat pad thickness and body mass index (BMI) in 87 participants. On the other hand, a comparison of men and women with similar BMI showed that men had significantly thicker heel fat pads than women [25]. As conflicting findings suggested that a consistent opinion on BMI has not been reached, multiple regression analysis was conducted with age, height, weight, and BMI as explanatory variables. As a result, weight and age were significant for non-pressure thickness, and weight was significant for pressure thickness. Therefore, weight was considered to be the main factor for heel fat pad thickening.
For heel fat pad flexibility, Softgram measurements showed that the heel fat pad became stiffer with increasing age, height, and weight. More importantly, weight was extracted as a significant factor for heel fat pad flexibility. Ozdemir et al. [4] examined 67 heels of individuals aged 23-73 years and reported that an increase in the heel fat thickness pad due to aging and weight gain decreased heel fat pad flexibility. Similarly, Belhan et al. [5] reported a decrease in elasticity with the increasing thickness of the heel fat pad. In addition, this study showed that this relationship between flexibility and thickness remained true in younger individuals, wherein the heel fat pad was estimated to absorb 20-25% of the contact force applied to the heel during walking [26]. Furthermore, Jørgensen [27] reported that running doubles the pressure on the heel fat pad. Thus, when heel fat pad flexibility is reduced due to degeneration or inflammation in older adults, heel pain is often accompanied with greater impact [4,11-13]. In growing subjects such as those included in this study, the phenomenon of decreasing flexibility with thickening heel fat pad may be attributed to the process of growth. However, this study did not investigate this relationship with pain. In the future, it might be beneficial to examine the relationship between decreased flexibility and heel pain in young adults.
Thickness of change difference and thickness of change rate were measured as indirect indices to evaluate the quality of the heel fat pad. Normally, if the thickness of change difference is large, a high flexibility is inferred. However, this only holds true when the heel fat pad thickness is approximately the same, and one can assume that a thicker heel fat pad is associated with a larger thickness of change difference. In this study, the correlation coefficient between thickness of change difference and non-pressure thickness was 0.778, which indicated a good correlation. Therefore, it was considered appropriate to evaluate the heel fat pad flexibility by the thickness of change rate. In previous studies, a similar evaluation of flexibility was performed using the heel pad compressibility index [28, 29].
This index was calculated with the help of radiography by dividing the thickness of the heel fat pad during loading by the thickness of the heel fat pad during non-loading. In this study, there was no significant correlation between the thickness of change rate and values measured by Softgram. This is because the thickness of change rate is the compression rate of the entire heel fat pad, whereas Softgram measures the flexibility of the depth up to 5 mm. It is therefore possible that measurements were evaluated from different perspectives. For example, the heel fat pad consists of two layers—the microchamber and macrochamber layers. The thickness of the microchamber layer increases from 15-29 years of age and does not change thereafter, whereas the macrochamber layer increases from 30-44 years of age [7]. In this study, the average thickness of the microchamber layer from the surface layer was 2.2 mm in subjects aged 6-14 years, and the average thickness of the macrochamber layer was reported to be 9.9-10.5 mm. Given this, the structure of the heel fat pad can be considered to be different inside, which was not well captured due to the tissue changes within 5 mm. Thus, it is possible that the values shown by Softgram did not correlate with the thickness of change rate. In the future, it is important to verify these findings by examining how the Softgram values and thickness of change rate are related.
Despite these findings, there are certain limitations in this study. First, the results may vary with different ages due to the limited age range of the subjects. Moreover, the possibility of errors in Softgram measurements due to various reasons (e.g., skin thickening) has not been investigated. This error is also true for ultrasound measurements. Finally, the pooling of left and right limbs is problematic, and it is unclear if or how the regression models.
5. Conclusion
To clarify the factors that cause heel pain in young individuals, we investigated the relationship of heel fat pad thickness and flexibility with the physical demographics (e.g., age, body size, height, and weight) of young individuals in Japanese elementary and junior high schools. Results showed that heel fat pad thickness and flexibility in elementary and junior high school students became thicker and stiffer, respectively, with increasing age, height and weight. Notably, both thickness and flexibility were particularly related to body weight.
Acknowledgments
This work was supported by JSPS KAKENHI grant Number 18KK0104. We would like to express our sincere gratitude to the study participants and the past and present members of my laboratory.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Lopez-Lopez D, Becerro-de-Bengoa-Vallejo R, Losa-Iglesias ME, Soriano-Medrano A, Palomo-López P, Morales-Ponce A, et al. Relationship between decreased subcalcaneal fat pad thickness and plantar heel pain. A case control study. Pain Physician (2019): 109-116.
- Wearing SC, Hooper SL, Dubois P, Smeathers JE, Dietze A. Force-deformation properties of the human heel pad during barefoot walking. Medicine & Science in Sports & Exercise (2014): 1588-1594.
- Rome K, Campbell R, Flint A, Haslock I. Heel pad thickness—a contributing factor associated with plantar heel pain in young adults. Foot & ankle international (2002): 142-147.
- Ozdemir H, Söyüncü Y, Ozgörgen M, Dabak K. Effects of changes in heel fat pad thickness and elasticity on heel pain. Journal of the American Podiatric Medical Association (2004): 47-52.
- Belhan O, Kaya M, Gurger M. The thickness of heel fat-pad in patients with plantar fasciitis. Acta orthopaedica et traumatologica turcica (2019): 463-467.
- Tas S. Effect of gender on mechanical properties of the plantar fascia and heel fat pad. Foot & Ankle Specialis (2018): 403-409.
- Maemichi T, Tsutsui T, Matsumoto M, Iizuka S, Torii S, Kumai T. The relationship of heel fat pad thickness with age and physiques in Japanese. Clinical Biomechanics (2020): 105110.
- Agyekum EK, Ma K. Heel pain: a systematic review. Chinese Journal of Traumatology (2015): 164-169.
- Scharfbillig RW, Jones S, Scutter SD. Sever’s disease: what does the literature really tell us? Journal of the American Podiatric Medical Association (2008): 212-223.
- Cassas KJ, Cassettari-Wayhs A. Childhood and adolescent sports-related overuse injuries. American family physician (2006): 1014-1022.
- Hsu TC, Wang CL, Tsai WC, Kuo JK, Tang FT. Comparison of the mechanical properties of the heel pad between young and elderly adults. Archives of physical medicine and rehabilitation (1998): 1101e1104.
- Wearing SC, Smeathers JE, Yates B, Urry SR, Dubois P. Bulk compressive properties of the heel fat pad during walking: a pilot investigation in plantar Clinical Biomechanics (Bristol, Avon) (2009) : 397-402.
- Falsetti P, Frediani B, Acciai C, Baldi F, Filippou G, Marcolongo R. Heel fat pad involvement in rheumatoid arthritis and in spondyloarthropathies: an ultrasonographic study. Scandinavian journal of rheumatology (2004): 327-331.
- Hananouchi T, Suzuki T, Dorthe EW, Du J, D’Lima DD. The resistance force of the anterior cruciate ligament during pull probing is related to the mechanical property. Bioengineering (2021): 4.
- Sawai A, Mitsuhashi R, Zaboronok A, Warashina Y., Mathis BJ. Serum creatine kinase increases after acute strength training in college. Women (2021): 71-79.
- Hihara H, Tagaino R, Washio J, Laosuwan K, Wicaksono DP, Izumita K., et al. Effectiveness and safety of a new dental plaque removal device utilizing micro mist spray for removing oral biofilm in vitro. BMC Oral Health (2021): 286.
- Akemi Sawai, Risa Mitsuhashi, Alexander Zaboronok, Yuki Warashina, Bryan J. Mathis. Serum Creatine Kinase Increases after Acute Strength Training in College Athletes with Menstrual Irregularities. Women (2021): 71–79.
- Ker RF. The time-dependent mechanical properties of the human heel pad in the context of locomotion. The Journal of experimental biology (1996): 1501-1508.
- Prichasuk S, Mulpruek P, Siriwongpairat P. The heel-pad compressibility. Clinical orthopaedics and related research (1994): 197-200.
- Jahss MH, Michelson JD, Desai P. Investigations into the fat pads of the sole of the foot: anatomy and histology. Foot & Ankle (1992): 233-242.
- Kerr PS, Silver DA, Telford K, Andrews HS, Atkins RM. Heel-pad compressibility after calcaneal fractures: ultrasound assessment. The Journal of Bone and Joint Surgery. British volume (1995): 504-505.
- Perry J. Anatomy and biomechanics of the hindfoot. Clinical orthopaedics and related research (1983): 9-15.
- Hassab H.K, el-Sherif AS. Drilling of the os-calcis for painful heel with calcanean spur. Acta Orthopaedica Scandinavica (1974): 152-157.
- Tas S, Bek N, Onur MR, Korkusuz F. Effects of body mass index on mechanical properties of the plantar fascia and heel pad in asymptomatic participants. Foot & ankle international (2017): 779-784.
- Tas S. Effect of gender on mechanical properties of the plantar fascia and heel fat pad. Foot & Ankle Specialist (2018): 403-409.
- Jørgensen U, Bojsen-Møller F. Shock absorbency of factors in the shoe/heel interaction--with special focus on the role of the heel pad. Foot & ankle (1989): 294-299.
- Jørgensen U. Achillodynia and loss of heel pad shock absorbency. The American journal of sports medicine (1985): 128-132.
- Uzel M, Cetinus E, Bilgic E, Ekerbicer H, Karaoguz A. Comparison of ultrasonography and radiography in the assessment of the heel pad compressibility. Measurement of the fat pad in plantar heel pain syndrome. Joint Bone Spine (2006): 196-199.
- Prichasuk S, Mulpruek P, Siriwongpairat P. The heel-pad compressibility. Clinical orthopaedics and related research (1994): 197-200.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 