Data Driven vigiPoint Identification Study of Adverse Event Reporting Patterns for Zimbabwe Reports in VigiBase WHO Global Database of Individual Case Safety Reports for Medicines and Vaccines
Priscilla P.M. Nyambayo1, *, Michael S. Gold2, Ushma C. Mehta3
1Pharmacovigilance and Clinical Trials Division, Medicines Control Authority of Zimbabwe, Harare, Zimbabwe.
2University of Adelaide, Discipline of Paediatric, Women’s and Children’s Health Network, Adelaide, Australia.
3Centre for Infectious Disease Epidemiology and Research, School of Public Health, University of Cape Town, South Africa.
*Corresponding author: Priscilla P.M. Nyambayo. Pharmacovigilance and Clinical Trials Division, Medicines Control Authority of Zimbabwe, Harare, Zimbabwe.
Received: 25 May 2023; Accepted: 02 June 2023; Published: 19 June 2023
Article Information
Citation: Priscilla P.M. Nyambayo, Michael S. Gold, Ushma C. Mehta. Data-Driven vigiPoint identification study of Zimbabwe ICSRs compared with RoW VigiBase data with and without USA reports. Fortune Journal of Health Sciences. 6 (2023): 237-245.
View / Download Pdf Share at FacebookAbstract
vigiPoint: Data driven analytic tool was developed by the Uppsala Monitoring Centre(UMC) to identify key features of VigiBase Individual Case Safety Reports (ICSRs) data subsets. Zimbabwe contributed ICSRs into VigiBase since 1998 hence the importance to understand the reporting patterns of Zimbabwe ICSRs compared to the rest of the world’s (RoW) data with and without the USA reports, which contributes 48% ICSRs to VigiBase.
Objective: The study explored vigiPoint differences in the Zimbabwe medicines and vaccines ICSRs reporting patterns compared to the RoW with and without the USA reports.
Methods and Materials: The study used vigiPoint analysis for VigiBase ICSRs reports analysis to outline data subsets of interest, pinpointing outstanding key features, using odds ratios subjected to statistical shrinkage distinguishing one data subset from another. The vigiPoint methodology compared 5213 Zimbabwe ICSRs reports in VigiBase from 1998-2022 with RoW with and without the USA unduplicated reports. To highlight features that deviate from the expected only, the threshold for the credibility interval of the log odds ratio was set at 0.5 and −0.5, respectively. The shrinkage was set to the vigiPoint default corresponding at 40% of the size of the Zimbabwe unduplicated ICSRs data subset.
Results: A total of 5213 ICSRs (20% vaccines AEFIs, and 80% medicines AEs) were analysed using VigiPoint method. Zimbabwe ICSRs compared with RoW and without USA ICSRs reports had most reports submitted from nurses, AEs for people age ranges 18-44 years (43.1 vs 30.7%), infants and children 1-23 months (13.8 vs 3.0%) and children 2-11 years (12.1 vs 4.0%). Zimbabwe ICSRs were serious 71.6% vs 35.8% RoW mostly cosuspected antiretrovirals, antituberculosis medicines, or vaccines.
Conclusion: Study findings are characteristic of limited healthcare settings, like other studies that found low physician-patient ratio, higher rates of HIV, TB, and comorbid diseases. Further studies of Zimbabwe ICSRs causality assessment outcomes including use of mHealth to enhance consumers/HCWs reporting are required.
Keywords
<p>vigiPoint analysis method, vigiPoint score (shrinkage log-odds ratios) (SLORs), individual case safety reports (ICSRs)</p>
Article Details
1. Introduction and Background
Since 1998 Zimbabwe has participated in the WHO international drug monitoring program which is co-ordinated by the Upsala Monitoring Centre (UMC), by uploading individual case safety reports (ICSRs) to the global database known as VigiBase that currently has over thirty-two million ICSRs [1-5]. The United States of America (USA) is the highest reporter with about 48% (15.3 million) reports [6]. Africa only contributed a total 0.9% ICSRs to VigiBase which excluded most SADC countries hence it was not possible to conduct regional comparative VigiPoint analysis with Zimbabwe ICSRs [2, 7]. Zimbabwe contributed 0.018% (5231) reports from January 1998 to July 2022. Adverse events (AEs)/ICSRs reporting patterns tend to vary between countries, reflecting differences in medicine and vaccine profiles, reporting method cultures, clinical practice, comorbid conditions, and pharmacogenetics [8]. Understanding these reports in the global context can be helpful for signal hypothesis generation and risk minimisation of patient vulnerabilities due to AEs. They can also provide an overview of the coverage of the national spontaneous reporting system, identifying opportunities for strengthening and reprogramming. VigiBase ICSRs data originates from various reporters and different countries with diverse quality information of the ICSRs known as VigiGrade completeness score, and limited causality assessment information that classifies the likelihood that a medicine and/or vaccine caused the AE [8, 9]. The reported AEs are coded with the Medical Dictionary for Regulatory Activities (MedDRA®), medicines and vaccines are encoded with the WHO Drug Global dictionary for medicinal information facilitating interpretation and evaluation of safety signals [6, 10]. The Council for International Organizations of Medical Sciences (CIOMS) defined a signal as information that arises from one or multiple sources (including observations or experiments), which suggests a new potentially causal association or a new aspect of a known association between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action [11].
On a conceptual level, VigiBase has inbuilt signal detection statistical data mining analysis tools known as disproportionate analysis. These include the information component (IC), proportionate reporting ratio (PRR), and relative odds ratio (ROR). These tools are similar since they are all observed versus (vs) expected ratios. They mostly differ on how the scale is presented (logarithm for IC vs no logarithm for PRR and ROR) and what is included in the background (in IC reports the medicine of interest is included in the background, while excluded from the background for PRR). Thus, they always point in the same direction, so if the IC is elevated, then PRR and ROR are also elevated.
vigiPoint is also a disproportionality analysis, but not in the same way as the IC, PRR or ROR measures. The IC, PRR or ROR measures always compare the number of reports from a combination (e.g., paracetamol – rash) to the expected number of reports in the background (which usually includes the entire VigiBase database). In vigiPoint, the comparisons done are also disproportionality, however, the foreground is selected (e.g., all reports from Zimbabwe) and compared with the background (e.g., RoW VigiBase) and then vigiPoint analyses all structured fields in the reports to determine if any of those fields are disproportional to the selected background. It is important to note that none of these comparisons are exposure and event combinations; instead, vigiPoint® compares the fraction of reports with a certain feature in the foreground (e.g., reports with paracetamol, serious reports, reports with rash, reports with female patients, etc.) with the same features for the background. It is important to note that vigiPoint® aims to highlight features where the fraction deviates by at least ~40% between the foreground and the background. vigiPoint aims to answer the very general question: What are the main differences between the foreground dataset (e.g., reports from Zimbabwe) compared to the background dataset (e.g., reports from the rest of the world (RoW) with or without USA reports. VigiPoint analysis is useful to national pharmacovigilance centres since it examines ICSRs expectations, observations and highlights large differences [12]. VigiPoint analysis can compare performance of the national pharmacovigilance system ICSRs to ROW data, allowing for reflection on why differences are present, how the system has advantages and limitations in terms of signal hypothesis generation and opportunities for strengthening the national PV system to improve ability to detect signals [12, 13]. It can look at reporter profiles, overview of types of events reported and categories of medicines/vaccines and types of patients most likely to be included in the data [13].
Objective of the Study: The objective of this study was to explore vigiPoint differences in the medicines and vaccines ICSRs reporting patterns of Zimbabwe reports compared to the RoW with and without the USA reports. The differences included patients’ age range, ICSRs VigiGrade completeness score, types of AEs profiles, co-suspected drugs and their seriousness submitted to VigiBase from January 1998 to July 2022.
2. Methods and Materials
This study used vigiPoint, method of analysis of deduplicated Zimbabwe ICSRs (ADRs/SAEs /AEFIs) key features in VigiBase that are identified using odds ratios subjected to statistical shrinkage compared to RoW reports with and without USA Reports [12]. Such odds ratios were found by other studies to be advantageous over alternative measures of association such as mutual information, relative risk (including proportional reporting ratios (PRR) and information component (IC) values), and cosine similarity of working well for common as well as for rare covariates [8, 12, 14]. Figure 1 below adapted from Juhlin K. et al 2017 and Wakao R. et al 2019) shows the vigiPoint equation in relation to the Zimbabwe VigiBase data(subset of interest) and RoW(comparator, the data can be summarised in a standard 2 × 2 contingency Table 1 below, ) including each potential interest [8, 12].
Table 1: 2x2 Contingency table for Zimbabwe ICSRs subset of interest compared with rest of the World (RoW) ICSRs data.
|
Feature + (Yes) |
Feature – (No) |
||
|
Zimbabwe (subset of interest) |
a |
b |
a +b |
|
Rest of World (RoW) (comparator) |
c |
d |
c +d |
|
a + c |
b +d |
a +b+c+d |
The equation that vigiPoint uses is the following, where the 0.01*(a+b) is the shrinkage factor and the variables are:
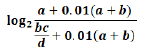
Figure 1: The Zimbabwe VigiBase data and the RoW vigiPoint equation, adapted from Juhlin K. et al 2017 and Wakao R. et al 2019 [8, 12].
vigiPoint data-driven analysis method is an open-ended exploration in VigiBase/pharmacovigilance that compares a report subset to one or more reference subsets in terms of the relative frequency of a wide range of covariates, for example, patient sex, age, reported medicines, vaccines AEs, and reporting country [12, 14]. All non-categorical covariates based on the ICSR reports data in VigiBase are automatically divided (without selection bias) into relevant groups using the holistic inbuilt disproportionate analysis tools stated above such as IC before VigiPoint analysis such as patient age, suspected medicines/vaccines , type of AEs, seriousness, quality of reports (VigiGrade completeness), and reporter types [8, 12, 13]. VigiPoint tool then analyses each comparison [e.g., the relative frequency of reports from Zimbabwe, or that of reports with Bacille Calmette-Guérin (BCG) vaccine] is essentially univariate and independent of the other [12, 14]. The comparisons are done using shrinkage log-odds ratios with 99% credibility intervals and require that the full credibility intervals be at least above 0.5 or below 0 in the log2 scale for the feature to be highlighted, which is roughly equivalent to a 40% difference in the shrunk odds ratio [12, 14]. The distance in the log scale between zero (0) and the credibility interval is referred to as the vigiPoint® score [12, 14].
The vigiPoint shrinkage together with the high thresholds applied for the credibility intervals and the 0.5 threshold ensures that only robust, differences are identified [12, 14]. The shrinkage odds ratio of vigiPoint is considered an observed-to-expected ratio and is obtained as the Bayesian posterior mean of an intensity parameter μ for a Poisson Po(μ · E)-distributed observed number of reports (O), where the expected value E is bc/d(8). With a Gamma prior distribution with hyperparameter k for μ: G (k; k), the corresponding posterior distribution for μ is also Gamma (but with parameters O + k and E + k), and the shrunk odds ratio is computed as follows adapted from Wakao R. et al 2019 and Norén GN et al 2013 [8, 13]:
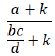
Credibility intervals that indicate a range of values of μ compatible with data can be calculated by the inverse of the Gamma cumulative distribution function [8]. The lower C% credibility interval limit of the shrunk log-odds ratio (or the upper C% confidence interval limit in the case of negative associations) is referred as the vigiPoint score, where C is the size of the credibility interval [8]. When this exceeds the pre-defined threshold T (or falls below − T for negative association), the corresponding covariate value or range is highlighted as a key feature [8]. For transparency, odds ratios presented as part of the results are unshrunk [7], In this study we used the standard implementation of vigiPoint with C = 99, k = 0.01 × n (where n is the size of the subset of interest; in the case of the Zimbabwe reports and T = 0.5 [8, 12].
We defined the study reporting pattern subsets of the Zimbabwe ICSRs of interest for which the permutation analysis of 99% credibility interval of the shrunk log-odds ratios above 0.5 or below −0.5 were flagged as key features. The shrinkage was set at the vigiPoint default corresponding to 0.018% of the size of the Zimbabwe data subset since at the time of the analysis the Zimbabwean reports made up 0.018% of the VigiBase database. This study conducted vigiPoint analysis of 5213 ICSRs from Zimbabwe versus RoW* with and RoW** without USA reports from 1998 to July 2022. The vigiPoint analysis was run on the VigiBase Zimbabwe unduplicated ICSRs dataset as the subset of interest and the discrepancy between the vigiPoint RoW with and without USA reports as the primary comparator and sensitivity analysis. The covariates of interest were patient age groups, AEs as MedDRA preferred terms (PTs), serious and non-serious AEs suspected medicines and vaccine as mainly WHO Drug active ingredients names, type of reporter, country of origin and quality of the ICSRs information as measured by the VigiGrade completeness score(9). High vigiGrade high completeness was score was defined as a score of 0.8 to 1.0 [9].
3. Results
Zimbabwe had higher reporting from nurses (75.3% vs 17.3%) compared with the RoW with and without USA reports, see Table 2 showing that the proportion of reports submitted by physicians is much lower in Zimbabwe compared to RoW and RoW excluding USA, 19.2% vs. 43.4%/32.9%. The proportion of reports submitted by pharmacists’ reports were less when compared to RoW and RoW without USA, 5.1% vs. 12.9%/10.3%. Consumer reports formed a negligible contribution to reports in Zimbabwe unlike RoW* with USA where such reports form a considerable proportion of total reports, 0.4% vs. 43.1%. In Table 3, most Zimbabwe reports were serious 71.6% vs 35.8%, and a larger fraction were fatal 5.3% vs 2.6%. Zimbabwe serious reports, most patients were recovered after hospitalisation and some AE might be due to suspected medicines or vaccines or underlying disease or other causes. However, the cause of death was not always evident since causality assessment is not part of the vigiPoint analysis due to the inherent limitation of the information contained in most VigiBase reports. In Table 3, most Zimbabwe ICSRs had higher quality known as VigiGrade completeness score 52.9% compared to RoW with and without USA 20.1% and 31.9% respectively. In Table 4 most Zimbabwe patient demographics age range reports were for young people aged 18 −44 years (43.1% vs. 30.7%), infants and young children 1−23 months (13.8% vs. 3.0%) and older children 2− 11 years (12.1% vs. 4.0%). Globally, reports for 45−65 years old patients were 21.1% vs 33% and 65-74 years 2.2% vs. 15%.
See Table 5 that shows the top 20-odd ADRs reported in Zimbabwe selected for comparison with RoW with and without USA reports showed that the most frequently reported MedDRA AE terms in Zimbabwe were rash (12.5% vs. 5.9%), peripheral neuropathy (4.9 %vs. 0.2%), gynaecomastia (3.5% vs. 0.1%), injection site abscess (2.3% vs. 0.1%) and anaemia (2.2% vs. 0.7%). The least reported reactions were pyrexia 3.3% vs. 6.5%, headache 2.3% vs. 7.2% and pruritus 2.3% vs. 5.8% but compared to RoW with the USA reports, headache was 2.3% vs. 6.1%, dyspnoea 0.5% vs. 3.1%, pain in extremity 0.5% vs. 1.9% and nausea 0.4% vs. 6.2%. Table 6 shows top 21 list of co-suspected medicines and vaccines although it was not always possible to single out a specific medicine or vaccine since 36.8% were given in 3-5 medicines or vaccines combinations.
Table 2: Key features of notifier (reporter) higher relative reporting rates for nurse reporters, and relatively lower notifiers physicians, pharmacists and consumers, for Zimbabwe reports compared with VigiBase® RoW with and without the USA, ICSRs.
|
Notifier |
Zimbabwe n(%) |
RoW* n(%) |
Odds ratio |
vigiPoint Score |
RoW without USA n(%) |
Odds ratio |
vigiPoint Score |
|
|
Higher Relative Reporting Rates in Zimbabwe Subset |
||||||||
|
Nurses |
3839(75.3) |
4014893 |
14.5 |
3.619 |
2538744(19.6) |
12.51 |
3.438 |
|
|
Lower Relative Reporting Rates in Zimbabwe Subset |
||||||||
|
Pharmacist |
261(5.1) |
2391420(10.3) |
0.47 |
-0.961 |
1680449(12.9) |
0.36 |
-1.302 |
|
|
Physician |
980(19.2) |
7614338(32.9) |
0.49 |
-1.005 |
5630068(43.4) |
0.31 |
-1.637 |
|
|
Lawyer |
0(0) |
502619(2.2) |
0 |
-1.667 |
||||
|
Unknown |
112(2.1) |
6314471(21.4) |
0.08 |
-3.137 |
3250494(20) |
0.09 |
-3.019 |
|
|
Consumer/Non-Health Professional |
21(0.4) |
9975100(43.1) |
0.01 |
-5.738 |
||||
RoW* =Rest of the World with the USA reports.
RoW ** =Rest of the World without the United States of America (USA) reports.
Table 3 : Zimbabwe ICSRs seriousness and VigiGrade completeness score characteristics compared to RoW* with and without the USA** ICSRs.
|
Reported Cases |
Zimbabwe N(%) |
RoW* n(%) |
Odds ratio |
vigiPoint Score |
RoW without USA n(%) |
Odds ratio |
vigiPoint score |
|
Higher relative reporting rates in Zimbabwean subset. |
|||||||
|
Serious (E2B only) |
3578(71.6) |
9300571(37.5) |
4.2 |
2.006 |
4344196(35.8) |
4.53 |
2.107 |
|
High vigiGrade score (>=0.8) |
2479(52.9) |
5923100(20.1) |
4.47 |
2.06 |
5167678(31.9) |
2.4 |
1.222 |
|
Lower Relative Reporting Rates in Zimbabwean Subset |
|||||||
|
Non-serious (E2B only) |
1420(28.4) |
15506846(62.5) |
0.24 |
-2.031 |
7802569(64.2) |
0.22 |
-2.138 |
|
Low vigiGrade score (<0.8) |
2204(47.1) |
23515127(79.9) |
0.22 |
-2.133 |
11027583(68.1) |
0.42 |
-1.244 |
|
No statistical difference or statistically different result is too small to be considered. |
|||||||
|
Reported Fatal |
276(5.3) |
1258159(4.3) |
1.25 |
0.269 |
425239(2.6) |
2.08 |
0.827 |
Table 4 : Zimbabwe patient age range characteristics compared to RoW* with and without the USA** ICSRs.
|
Age Group |
Zimbabwe n(%) |
RoW* n(%) |
Odds ratio |
vigiPoint Score |
RoW without USA** n(%) |
Odds ratio |
vigiPoint Score |
|
Higher Relative Reporting Rates in Zimbabwean Subset |
|||||||
|
28 days to 23 months |
701(13.8) |
580941(2.7) |
5.74 |
2.112 |
413071(3.0) |
5.26 |
2.024 |
|
2 to 11 years |
616(12.1) |
804830(3.8) |
3.53 |
1.561 |
562030(4.0) |
3.3 |
1.485 |
|
12 to 17 years |
306(6) |
629087(2.9) |
2.12 |
0.865 |
374443(2.7) |
2.33 |
0.967 |
|
18 to 44 years |
2192(43.1) |
6123123(28.6) |
1.89 |
0.892 |
4296473(30.7) |
1.71 |
0.754 |
|
Lower Relative Reporting Rates in Zimbabwean Subset |
|||||||
|
45 to 64 years |
1070(21.1) |
7238959(33.8) |
0.52 |
-0.906 |
4591553(32.8) |
0.55 |
-0.841 |
|
65 to 74 years |
111(2.2) |
3360257(15.7) |
0.12 |
-2.584 |
20709759(14.8) |
0.13 |
-2.489 |
|
More than 75 years |
43(0.8) |
2614237(12.2) |
0.06 |
-2.987 |
1653138(11.8) |
0.06 |
-2.935 |
|
Unknown |
133(2.6) |
8072749(27.4) |
0.07 |
-3.412 |
2229717(13.7) |
0.16 |
-2.219 |
|
No statistical difference or statistically different result is too small to be considered. |
|||||||
|
0 to 27 days |
41(0.8) |
47119(0.2) |
3.687 |
0.5591 |
36855(0.3) |
3.082 |
0.5101 |
Table 5: Relative reporting rates for specific adverse events (AE) MedDRA preferred Terms for Zimbabwe compared to RoW*’s ICSRs with and without USA reports.
|
MedDRA Preferred Term |
Zimbabwe (%) |
ROW* (%) |
Odds ratio |
vigiPoint Score |
ROW without USA** (%) |
Odds ratio |
vigiPoint Score |
|
Higher relative Reporting Rates in Zimbabwean subset |
|||||||
|
Rash |
651 (12.5) |
1307304 (4.4) |
3.07 |
1.4143 |
950962 (5.9) |
2.29 |
1.0652 |
|
Neuropathy peripheral |
255 (4.9) |
86428 (0.3) |
17.49 |
2.205 |
34650 (0.2) |
24.04 |
2.2938 |
|
Gynaecomastia |
183 (3.5) |
36671 (0.1) |
29.2 |
2.0119 |
9951 (0.1) |
59.3 |
2.0929 |
|
Injection site abscess |
122 (2.3) |
18926 (0,1) |
37.29 |
1.6545 |
16360 (0.1) |
23.75 |
1.6066 |
|
Anaemia |
115 (2.2) |
187936 (0.6) |
3.51 |
0.9791 |
114387 (0.7) |
3.18 |
0.9212 |
|
Stevens-Johnson syndrome |
91 (1.7) |
40355 (0.1) |
12.96 |
1.2767 |
30108 (0.2) |
9.56 |
1.2169 |
|
Lipodystrophy acquired |
90 (1.70 |
8934 (0.0) |
57.94 |
1.4068 |
8373 (0.1) |
34.03 |
1.3777 |
|
Rash pruritic |
89 (1.7) |
121982 (0.4) |
4.18 |
0.9439 |
74934 (0.5) |
3.74 |
0.896 |
|
Skin hyperpigmentation |
84 (1.6) |
11950 (0.0) |
40.37 |
1.3304 |
6311 (0.0) |
42.1 |
1.3327 |
|
Jaundice |
68 (1.3) |
34434 (0.1) |
11.3 |
1.0485 |
24245 (0.1) |
8.83 |
1.0073 |
|
Lower Relative Reporting Rates in Zimbabwean subset |
|||||||
|
Pyrexia |
172 (3.3) |
1050685 (6.5) |
0.49 |
-0.084 |
|||
|
Headache |
118 (2.3) |
1811836 (6.1) |
-1.1821 |
1175919 (7.2) |
0.3 |
-1.4045 |
|
|
Pruritus |
118 (2.3) |
938562 (5.8) |
0.38 |
-1.1014 |
|||
|
Dyspnoea |
27 (0.5) |
904958 (3.1) |
0.16 |
-1.453 |
509170 (3.1) |
0.16 |
-1.4775 |
|
Pain in extremity |
26 (0.5) |
5719054 (1.9) |
0.25 |
-0.986 |
|||
|
Nausea |
23 (0.4) |
1814243 (6.2) |
0.07 |
-2.3876 |
1210156 (7.5) |
0.05 |
-2.6483 |
|
Pain |
22 (0.4) |
830102 (2.8) |
0.15 |
-1.452 |
|||
|
Arthralgia |
19 (0.4) |
696118 (2.4) |
0.15 |
-1.3232 |
391832 (2.4) |
0.15 |
-1.3462 |
|
Chest pain |
19 (0.4) |
492110 (1.7) |
0.22 |
-0.9816 |
318116 (2.0) |
0.18 |
-1.1343 |
|
Fatigue |
13 (0.2) |
733900 (4.5) |
0.05 |
-2.1983 |
|||
|
Palpitations |
12 (0.2) |
227294 (1.4) |
0.16 |
-0.9759 |
|||
|
Chills |
8 (0.2) |
575675 (3.5) |
0.04 |
-2.0205 |
|||
Table 6: Co-suspected medicines and vaccines commonly reported for most Zimbabwe reactions ICSRs compared to RoW with and without the USA reports.
|
Co-suspected medicine or vaccine |
Zimbabwe n (%) |
RoW* n (%) |
Odds ratio |
VigiPoint |
RoW** n (%) |
Odds ratio |
VigiPoint Score |
|
Higher Relative Reporting Rates in Zimbabwean Subset |
|||||||
|
Isoniazid |
662(12.7) |
46492(0.2) |
92.06 |
3.593 |
44617(0.3) |
52.76 |
3.468 |
|
Efavirenz |
586(11.2) |
27655(0.1) |
134.84 |
3.501 |
23444(0.1) |
87.54 |
3.442 |
|
Nevirapine |
447(8.6) |
24222(0.1) |
114.02 |
3.158 |
19127(0.1) |
79.48 |
3.114 |
|
Polio vaccine |
299(5.7) |
109633(0.4) |
16.3 |
2.319 |
40044(0.2) |
24.6 |
2.452 |
|
Stavudine |
267(5.1) |
15986(0.1) |
99.47 |
2.544 |
13508(0.1) |
64.8 |
2.507 |
|
Tenofovir |
239(4.6) |
37054(0.1) |
38.17 |
2.32 |
17101(0.1) |
45.55 |
2.346 |
|
Measles vaccine |
218(4.2) |
3853(0) |
333.78 |
2.358 |
3058(0) |
231.56 |
2.351 |
|
Zidovudine; Lamivudine |
188(3.6) |
11634(0) |
94.74 |
2.152 |
17638(0.1) |
19.44 |
1.475 |
|
Trimethoprim; Sulfamethoxazole |
177(3.4) |
95506(0.3) |
10.81 |
1.744 |
81642(0.5) |
6.95 |
1.564 |
|
Measles vaccine; Rubella vaccine |
172(3.3) |
7158(0) |
140.45 |
2.073 |
6917(0) |
80.02 |
2.049 |
|
Tetanus vaccine; HIB vaccine; Hepatitis b vaccine; Pertussis vaccine; Diphtheria vaccine |
170(3.3) |
39052(0.1) |
25.41 |
1.919 |
39052(0.2) |
13.97 |
1.791 |
|
Stavudine; Nevirapine; Lamivudine |
162(3.1) |
3856(0) |
245.1 |
2.023 |
13508(0.1) |
64.8 |
2.507 |
|
Retinol |
149(2.9) |
1135(0) |
764.02 |
1.945 |
999(0) |
477.93 |
1.942 |
|
Pyrazinamide |
140 (2.7) |
61191(0.2) |
13.26 |
1.6179 |
|||
|
Dolutegravir |
114(2.2) |
11077(0) |
59.46 |
1.6224 |
|||
|
Efavirenz; Lamivudine; Tenofovir |
113(2.2) |
17552(0.1) |
37.18 |
1.584 |
17538(0.1) |
20.48 |
1.52 |
|
Zidovudine |
108(2.1) |
21404(0.1) |
29.11 |
1.522 |
17638(0.1) |
19.44 |
1.475 |
|
Ritonavir; |
81(1.6) |
13612(0) |
34.16 |
1.291 |
9366(0.1) |
27.33 |
1.275 |
|
Lopinavir |
|||||||
|
Albendazole |
71(1.4) |
4423(0) |
91.99 |
1.221 |
4194(0) |
53.41 |
1.206 |
|
Praziquantel |
65(1.2) |
2610(0) |
142.56 |
1.157 |
2551(0) |
80.31 |
1.148 |
|
Lamivudine; Tenofovir |
49(0.9) |
1333(0) |
209.78 |
0.951 |
1316(0) |
117.01 |
0.946 |
4. Discussion
Most of the co-suspected medicines or vaccines with relatively high odd ratios and vigiPoint scores were in the youthful population since some studies observed that sub–Saharan Africa is home for 65 % to 85% of young people with HIV and TB globally(15, 16). Zimbabwe has a youthful demographic profile hence the higher fractional reporting for those younger than 44 years of age including infants and children. Zimbabwe had 0.14 physicians and 1.85 midwives/nurses per 1000 population below the Sustainable Development Goals (SDGs) index threshold of 4.45 midwives, nurses, and doctors per 1000 population, resulting in lower physicians reporting rates compared to RoW [17-19]. Also, Zimbabwe has higher reporting of serious reactions which could be accounted for several factors including comorbidities, limited primary health care services and probably immune reconstitution due to delayed antiretroviral therapy (ART) during the early years of limited availability of antiretrovirals (ARVs) [20, 21]. The Zimbabwe ICSRs contain a higher fraction of reports for co-suspected medicines such as stavudine or efavirenz that were phased out over the years. Some studies of efavirenz or nevirapine AEs in sub-Sharan Africa showed pharmacogenetic differences with 20% to 59% black ethnic populations being poor metabolizers of efavirenz or nevirapine due to the highly polymorphic cytochrome P450 2B6 (CYP2B gene with 516T allele) known to confer poor metabolism of both medicines [22-26]. Zimbabwe reporting patterns were maintained when USA data was excluded from the RoW comparisons showing the robust sensitivity of the vigiPoint and odds ratio analysis methodology. Further investigation is recommended of currently used ART cosuspected medicines such as dolutegravir related weight gain or tenofovir and kidney failure to ascertain the risk minimisation factors. There is a need to conduct in-depth risk-benefit mitigation measures of those medicines used currently for tuberculosis such as isoniazid known to cause liver toxicities and/or pellagra. Isoniazid is known to cause vitamin B3 deficiency, most likely due to its ability to interfere with niacin made cell-repair enzymes [27]. A study of Zimbabwe ICSRs that compared ADR profiles of patients on antiretrovirals (ART) versus patients on ART and anti-TBs in 2018 showed that co-administration of ART and antitubercular (anti-TB) medicines were associated with a higher frequency of medicine-induced liver toxicity, peripheral neuropathy, and that isoniazid preventative therapy was associated with a higher risk for psychosis and liver toxicity [4]. A descriptive study of the Zimbabwe vaccines ICSRs, including the causality assessment profiles, recommended more resources for post-mortem to ascertain cause of death after vaccination [1]. Also, a French study recommended the use of both qualitative causality assessment outcomes and quantitative signal detection methods for signal detection [28]. A scoping review study of VigiBase signal disproportionate analysis advised that signal detection should also be done with causality assessment data in addition to the IC analysis, PRR and ROR [29]. The VigiPoint data of top 21 cosuspected medicines included albendazole and praziquantel medicines for treatment of some tropical neglected diseases [30-32].There is, therefore, a need for further analysis of the Zimbabwe medicines ICSRs to include the causality assessment outcomes done by the National Pharmacovigilance Committee for further risk minimisation measures.
The advantages of using vigiPoint in this study is that it enabled describing patterns of reporting (i.e., identifying features or covariates for a specific set of reports as compared to other reports). It also employed the ROR with adaptive shrinkage hence was the same measure of association used for statistical signal detection but now with shrinkage. The advantage of the ROR over the other measures in this context was that it worked well for both rare and common covariates [12, 14]. vigiPoint uses adaptive shrinkage to protect against false-positive associations. The shrinkage applied was stronger as the aim was to detect patterns involving large numbers of reports. vigiPoint further used a 99% (instead of 95%) uncertainty interval for the ROR to only highlight covariates that deviate substantially between the reports that were compared [12].
5. Conclusion
The vigiPoint analysis study of the Zimbabwe’s reporting patterns compared to RoW with and without USA reports revealed key features such as differences in AEs profiles, demographic data depending on types of medicines and vaccines used over the years pharmacogenetics, comorbidities and clinical practice. This knowledge is essential in the global collaboration of risk minimisation including promotion of patient safety in Zimbabwe since it accounts for different medicines and vaccines used for various public health challenges encountered over the decades. Further studies of Zimbabwe medicines ICSRs pooled causality assessment outcomes are required for further risk minimisation measures in a resource-limited context including enhancement of consumer reporting using mHealth reporting tools.
Declarations
Ethics approval and consent to participate.
The study was approved by the Medical Research Council of Zimbabwe (MRCZ) ethical approval reference MRCZ/A/2268 and MRCZ ethical exemption (reference E/148) from consenting participants for passive medicines and vaccines ICSRs. Ethical approval was also obtained from the University of Cape Town (UCT) Human Research Ethics Committee (HREC 184/2020) for the vigiPoint study. The data was published in anonymised format.
Consent for Publication
The authors obtained consent for publication of the manuscript from the MCAZ National Pharmacovigilance Centre, the custodian of the Zimbabwe safety data since this is a work-related study.
Competing of interest
The authors declare no conflict of interest nor potential competing interest.
Funding:
The MCAZ funded the study in accordance with its mandate as the Zimbabwe National Pharmacovigilance Centre. However, additional funding was obtained from unrestricted technical research grants over the years from the WHO, Global Fund and UNICEF that assisted with enhancing pharmacovigilance programmes training health care workers (HCWs) countrywide in pharmacovigilance of medicines and vaccines since 2008 to 2022 that resulted in increased ICSRs reporting from 2008 to 2022.
Author contributions:
PPMN designed the study and wrote the main manuscript as the lead author. MSG and UCM supervised the study and reviewed the manuscript. PPMN analysed data and performed analysis including tables and figures with assistance from two data scientists at the Uppsala Monitoring Centre (UMC). All authors read and approved the final manuscript.
Acknowledgements:
The authors acknowledge all the ICSRs reporters in Zimbabwe HCWs, clinics, hospitals, from both private and public health programmes MoHCC-ART and TB programmes, Zimbabwe Expanded Program on Immunisation (ZEPI-MoHCC), Medicines Control Authority of Zimbabwe management and national pharmacovigilance centre staff, the national Pharmacovigilance and Clinical Trials advisory Committee. Special thanks to Mr Nils Erlanson and Dr Henric Taavola, Uppsala Monitoring Centre, Sweden Data scientists who assisted in conducting the vigiPoint analysis of VigiBase database in accordance with the study objectives, method, materials, and results.
Limitations
- The study reviewed global country spontaneous ICSRS data only submitted to VigiBase yet some authors over the years claim that not all ICSRs are submitted to the national PV centres due to limited capacity for data management, under reporting or country policy in respect to VigiBase hence the reports may be relatively a small proportion of the total country safety data (2, 33).
- Each ICSR in VigiBase might contain more than one AE hence the number of MedDRA system organ classification (SOCs) may be more than the number of ICSRs. Double counting of combination fixed dose preparations for antiretrovirals, antitubercular, antimalarials and combination vaccines may lead to some products being over-represented in the count of products implicated in ICSRs.
Some data ICSRs might have low VigiGrade completeness score and might exclude causality assessment information.
References
- Nyambayo PP, Manyevere R, Chirinda L, Zifamba EN, Marekera SF, Nyamandi T, et al. Descriptive Research Study of the Adverse Events Following Immunization (AEFIs) Surveillance System in Zimbabwe (2023).
- Ampadu HH, Hoekman J, de Bruin ML, Pal SN, Olsson S, Sartori D, et al. Adverse drug reaction reporting in Africa and a comparison of individual case safety report characteristics between Africa and the rest of the world: analyses of spontaneous reports in VigiBase®. Drug Saf 39 (2016): 335-45.
- Suku CK, Hill G, Sabblah G, Darko M, Muthuri G, Abwao E, et al. Experiences and lessons from implementing cohort event monitoring programmes for antimalarials in four African countries: results of a questionnaire-based survey. Drug Saf 38 (2015): 1115-26.
- Masuka JT, Chipangura P, Nyambayo PP, Stergachis A, Khoza S. A comparison of adverse drug reaction profiles in patients on antiretroviral and antitubercular treatment in Zimbabwe. Clinical drug investigation 38 (2018): 9-17.
- Shaum A, Mujuru HA, Takamiya M, Ticklay I, Nathoo K, Sreenivasan N, et al. Enhanced surveillance for adverse events following immunization during the 2019 typhoid conjugate vaccine campaign in Harare, Zimbabwe. Vaccine (2022).
- Sandberg L, Taavola H, Aoki Y, Chandler R, Norén GN. Risk factor considerations in statistical signal detection: using subgroup disproportionality to uncover risk groups for adverse drug reactions in VigiBase. Drug Saf 43 (2020): 999-1009.
- Nyambayo PP, Manyevere R, Chirinda L, Zifamba EN, Marekera SF. Descriptive Research Study of the Adverse Events Following Immunization (AEFIs) Surveillance System in Zimbabwe. Clinical Case Reports and Studies. BRS Publishers (2023).
- Wakao R, Taavola H, Sandberg L, Iwasa E, Soejima S, Chandler R, et al. Data-driven identification of adverse event reporting patterns for Japan in VigiBase, the WHO global database of individual case safety reports. Drug Saf 42 (2019): 1487-98.
- Bergvall T, Norén GN, Lindquist M. vigiGrade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf 37 (2014): 65-77.
- Lagerlund O, Strese S, Fladvad M, Lindquist M. WHODrug: a global, validated and updated dictionary for medicinal information. Therapeutic Innovation & Regulatory Science 54 (2020): 1116-22.
- Heininger U, Holm K, Caplanusi I, Bailey S, Abdoellah SA, Arellano F, et al. Guide to active vaccine safety surveillance: Report of CIOMS working group on vaccine safety–executive summary. Vaccine 35 (2017): 3917-21.
- Juhlin K, Star K, Norén GN. A method for data-driven exploration to pinpoint key features in medical data and facilitate expert review. Pharmacoepidemiology and Drug Safety 26 (2017): 1256-65.
- Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res 22 (2013): 57-69.
- Ekhart C, van Hunsel F, van Puijenbroek E, Chandler R, Meldau E-L, Taavola H, et al. Post-Marketing Safety Profile of Vortioxetine Using a Cluster Analysis and a Disproportionality Analysis of Global Adverse Event Reports. Drug Saf 45 (2022): 145-53.
- Bygrave H, Mtangirwa J, Ncube K, Ford N, Kranzer K, Munyaradzi D. Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PloS one 7 (2012): e52856.
- Mavhu W, Willis N, Mufuka J, Bernays S, Tshuma M, Mangenah C, et al. Effect of a differentiated service delivery model on virological failure in adolescents with HIV in Zimbabwe (Zvandiri): a cluster-randomised controlled trial. The Lancet Global Health 8 (2020): e264-e75.
- Mabaso A, Museva T, Chivhenge E, Zingi GK, Chitongo L. Global COVID-19 Pandemic: A Strategic Opportunity for Operationalizing One Health Approach in Zimbabwe. The COVID-19-Health Systems Nexus: Emerging Trends, Issues and Dynamics in Zimbabwe: Springer (2023): 99-123.
- Furusa SS, Coleman A. Factors influencing e-health implementation by medical doctors in public hospitals in Zimbabwe. South African Journal of Information Management 20 (2018): 1-9.
- Chigudu S. The political life of an epidemic: cholera, crisis and citizenship in Zimbabwe: Cambridge University Press (2020).
- Dellière S, Guery R, Candon S, Rammaert B, Aguilar C, Lanternier F, et al. Understanding pathogenesis and care challenges of immune reconstitution inflammatory syndrome in fungal infections. Journal of Fungi 4 (2018): 139.
- Poizot-Martin I, Brégigeon S, Palich R, Marcelin A-G, Valantin M-A, Solas C, et al. Immune reconstitution inflammatory syndrome associated Kaposi sarcoma. Cancers 14 (2022): 986.
- Stöhr W, Back D, Dunn D, Sabin C, Winston A, Gilson R, et al. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antiviral therapy 13 (2008): 675-85.
- Mhandire D, Lacerda M, Castel S, Mhandire K, Zhou D, Swart M, et al. Effects of CYP2B6 and CYP1A2 genetic variation on nevirapine plasma concentration and pharmacodynamics as measured by CD4 cell count in Zimbabwean HIV-infected patients. Omics: a journal of integrative biology 19 (2015): 553-62.
- Atwine D, Bonnet M, Taburet AM. Pharmacokinetics of efavirenz in patients on antituberculosis treatment in high human immunodeficiency virus and tuberculosis burden countries: a systematic review. British Journal of Clinical Pharmacology 84 (2018): 1641-58.
- Seden K, Kiiza D, Laker E, Arinaitwe WJ, Waitt C, Lamorde M, et al. High prevalence and long duration of nervous system and psychiatric adverse drug reactions in Ugandan patients taking efavirenz 600 mg daily. Journal of Antimicrobial Chemotherapy 73 (2018): 3158-61.
- Maseng MJ, Tawe L, Thami PK, Moyo S, Kasvosve I, Novitsky V, et al. The role of CYP2B6 516G> T polymorphism on efavirenz/nevirapine toxicity. Implications on treatment outcomes: Lessons from Botswana. Medicine 101 (2022): e29066-e.
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatology, Photoimmunology & Photomedicine 37 (2021): 99-104.
- Berbain T, Pariente A, Miremont-Salamé G, Grandvuillemin A, Micallef J, Chouchana L, et al. Contribution of causality assessment for an automated detection of safety signals: an example using the french pharmacovigilance database. Drug Saf 43 (2020): 243-53.
- Sartori D, Aronson JK, Norén GN, Onakpoya IJ. Signals of Adverse Drug Reactions Communicated by Pharmacovigilance Stakeholders: A Scoping Review of the Global Literature. Drug Saf (2022): 1-12.
- Njomo DW, Tomono N, Muhoho Ne, Mitsui Y, Josyline KC, Mwandawiro CS. The adverse effects of albendazole and praziquantel in mass drug administration by trained schoolteachers. African Journal of Health Sciences 17 (2010): 10-4.
- Alvela-Suárez L, Velasco-Tirado V, Belhassen-Garcia M, Novo-Veleiro I, Pardo-Lledías J, Romero-Alegría A, et al. Safety of the combined use of praziquantel and albendazole in the treatment of human hydatid disease. The American journal of tropical medicine and hygiene 90 (2014): 819.
- Hong S-T. Albendazole and praziquantel: review and safety monitoring in Korea. Infection & chemotherapy 50 (2018): 1-10.
- Ampadu HH, Esseku Y, Dodoo AN. Evidence-Based Pharmacovigilance for Medicines Used in Public Health Programs in Africa. Evidence-Based Pharmacovigilance: Springer (2018): 185-99.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 