Biomonitoring of Urinary 1-Hydroxypyrene as an Indicator of PAHs Exposure in the Adult Population of West Bengal, India
Anupa Yadav1,2, Aniruddha Mukhopadhayay2, Amit Chakrabarti1, AsimSaha1 and Pritha Bhattacharjee2*
1ICMR - Centre for Ageing and Mental Health (I-CAM),Division of Non-Communicable Diseases (NCD), Indian Council of Medical Research (ICMR);Block - DP-1, Sector V, Salt Lake Kolkata - 700091, India.
2Department of Environmental Science, University of Calcutta, Kolkata-700019, India.
*Corresponding Author: Dr. Pritha Bhattacharjee, Assistant Professor Environmental Toxicogenomics, Molecular Biology and Human Genetics, Department of Environmental Science, University of Calcutta, Kolkata-700019, India.
Received: 12 September 2023; Accepted: 19 September 2023; Published: 28 November 2023.
Article Information
Citation: Anupa Yadav, Aniruddha Mukhopadhayay, Amit Chakrabarti, AsimSaha and Pritha Bhattacharjee. Biomonitoring of Urinary 1-Hydroxypyrene as an Indicator of PAHs Exposure in the Adult Population of West Bengal India. Journal of Environmental Science and Public Health. 7 (2023): 185-199.
View / Download Pdf Share at FacebookAbstract
Background: Exposure to environmental Polycyclic Aromatic Hydrocarbons (PAHs) among the general population is a global issue. PAHs exposure causes various health issues that depend on Intensity, exposure duration, age, health status, and genetic susceptibility.
Aim: To evaluate PAHs exposure by biomonitoring of urinary 1-hydroxypyrene (1-OHP) in residents of West Bengal, India.
Method: Urine samples were obtained from 100 individuals living in urban and rural areas. 1-OHP was quantified by high-pressure liquid chromatography (HPLC). A structured questionnaire was used to collect daily exposure-related information.
Results: The mean value of 1-OHP was 0.44μg/L (median 0.26μg/L) in total individuals. It was higher in urban and male than rural and female individuals. The 95th percentile value of 1-OHP was found higher than reference value 1.03μg/L established by German Environment Agency. The highest 1-OHP was observed in age group 40->40years (0.49μg/L). Low income group (LIG), smoker males and females involved in cooking practice have significantly high 1-OHP (p<0.0001; p=0.0002; p=0.0002 respectively). Females who were using biomass cooking fuel had significantly high 1-OHP(p=0.002). 1-OHP in smoker travellers was 3.4-fold higher than non-smoker nontraveller individuals. Rural females staying indoors had significantly higher (p=0.0003)1-OHP than urban females.
Conclusion: High PAHs exposure was found in our study individuals compared to other countries. In urban environments, traffic emissions and tobacco smoke were found as the prime contributors, while in rural areas biomass fuel smoke was a major contributing factor. Therefore, there is an urgent need to reduce PAHs exposure at regulatory and individual levels.
Keywords
Environmental PAHs,Urinary 1-hydroxypyrene, Urban area, Rural area, Smoker and traveller, Biomass fuel.
Article Details
1. Introduction
PAHs are persistent organic pollutants (POPs). These are Omni present and emitted into the environment during incomplete combustion of organic substances such as coal, oil, gas and wood [1, 2, 3, 4]. These pollutants are also present in cigarette smoke, automobile exhaust, and other sources of air pollution [5, 6, 7]. PAHs are among the most commonly studied compounds emitted during the burning of biomass fuels such as wood, dry dung and coal [8, 9, 10, 11, 12]. Biomass fuel burning is a major source of indoor air pollution at the global level, and it is responsible for about 1.6 million early deaths globally [13]. World’s half population depends on biomass fuels such as wood, dung, dry leaves, crop residue and charcoal for their domestic energy need [14, 15]. Generally, these solid biomass fuels are used in metal and clay stoves, has less oxygen supply which causes incomplete combustion of carbonaceous fuel and consequently emission of smoke containing a mixture of toxic pollutants including PAHs [16]. PAHs exposure among general public occurs regularly in their daily life from various sources of exposure via inhalation (major way), ingestion and dermal absorption [17, 18, 19]. Dietary PAHs exposure is mainly reported from western food like pasta, hamburger [20]. High pyrene 1.6-3.7ng/m3 and <0.07-0.41ng/m3 was found in indoor air using biomass fuel [20, 21]. Huang W et al. 2006 found exposure to environmental tobacco smoke (ETS) results in high 1-OHP in urine of non-smokers, while Scherer G et al. 2000 does not support this fact [22,66]. High level of urinary 1-OHP in Indian children 14.02µg/g, 13.32µg/g and 11.9µg/g was positively associated with polluted indoor air, traffic pollution and tobacco smoke respectively [6]. PAHs are carcinogenic, mutagenic, genotoxic and teratogenic in nature [23, 24, 25]. Non-carcinogenic effects include, but is not limited to, dermatological, pulmonary, gastrointestinal, renal system, and cardiopulmonary mortality [26, 27, 28, 29, 30, 31, 32]. These fatal effects draw more attention of researchers in evaluating the body burden of these organic compounds [33, 34, 35, 36, 37, 38]. Ruiz-Vera et al. 2015found a significant association between vascular function and concentration of 1-OHP [8]. Other biomonitoring studies also reported an association between urinary 1-OHP and poor semen quality, alteration in thyroid hormone (homeostasis) and cancer development [39, 40]. Once PAHs get absorbed in the human body, it metabolized into two phases. First of all, oxidized by cytochrome P450 enzyme (CYP1A1) to a more reactive form, and then further changed to diol-epoxides to initiate cell transformation. In the second phase, the product formed during the last reaction combines with glucuronic acid and sulfates (endogenous hydrophilic compounds) to produce sufficiently hydrophilic compounds that can easily be excreted through urine or faeces [41, 1, 42]. 1-OHP is the main urinary metabolite most commonly studied for biomonitoring of PAHs preferentially excreted in urine [43]. Although, it's a urinary metabolite of pyrene alone; It is also an indicator of exposure to total PAHs, because environmental concentrations of different PAHs correlate with each other [20]. The half-life of 1-OHP in human urine is 6-35hours [19]. Pyrene is the most commonly found compound in the class of PAHs, that is why pyrene and its urinary metabolite (1-OHP) are most widely used in human biomonitoring studies for assessment of PAHs exposure [44-49, 1, 5, 8, 19]. 1-OHP is a highly reliable, robust and established exposure biomarker of PAHs from analytical point of view since more than two decades [50], therefore, widely used in occupational, environmental and epidemiological studies globally for biomonitoring of PAHs [45, 51-65].Worldwide human biomonitoring studies have been carried out in general populations such as Germany [66], Canada [67], Ukraine [68], United States [70, 1], United Kingdom [71], Korea [72], and Iran [5]. In South India Guo et al. 2013 monitored urinary1-OHP (0.7µg/L). Many more studies have reported exposure to PAHs in children also by monitoring urinary 1-OHP [6, 7, 73-76]. The American Conference of Governmental Industrial Hygienists (ACGIH) suggested biological exposure indices (BEI) value for 1-OHP is 2.5µg/L in urine. An environmental survey in Germany established reference values (95th percentiles) of 1-OHP for smokers (0.73µg/g creatinine, 1.03µg/L) and non-smokers (0.30µg/g creatinine, 0.53µg/L) [65,77]. To our knowledge, there are only a limited number of studies that have examined PAHs exposure in the Indian population, where environmental conditions favour more gaseous forms of PAHs and promote more inhalation intake. A few studies conducted in South India - in adult population, in children - and one from North India - among professional restaurant cooks - found high 1-OHP [73, 6, 61]. Guo et al. 2013 monitored intake (from all possible sources of exposure, measured by urinary metabolites of PAHs and pyrene) of total PAHs (39.5µg/day) and pyrene (11.6µg/day) in Indian population [73]. The total PAHs daily intake among Indians was 2.9, 1.5, 1.4 and 4.5-fold higher than in Japan, Korea, Kuwait and Malaysia respectively. While the intake of pyrene in Indians was 5.8, 4.1, 2.3 and 7.7-fold higher than Japan, Korea, Kuwait and Malaysia respectively. Therefore, Indian population might be at a higher risk of adverse health effects associated with exposure to PAHs and pyrene. This situation urged for biomonitoring of PAHs body burden in Indian population. However, No such study for monitoring PAHs exposure in adult population of West Bengal (Eastern India) has been reported till now. Therefore, biomonitoring of urinary 1-OHP was required to evaluate the body burden of PAHs among residents of West Bengal, Eastern India. Moreover, in India, no information is available on reference value for urinary 1-OHP to orient public health policies for reduction of environmental exposure to PAHs. In light of the above facts, the present work was planned for the biomonitoring of PAHs exposure, by assessing urinary 1-OHP among general public – those who were occupationally unexposed to PAHs, living in urban and rural areas of West Bengal, Eastern India. The various socioeconomic and lifestyle factors such as age, gender, family income, smoking, cooking, and travelling activities influencing PAHs exposure were also considered. Indeed, this is a one of a kind study which evaluates the association between socioeconomic, lifestyle factors, and urinary 1-OHP as exposure biomarker tracers to PAHs in West Bengal residents.
2. Materials and Methods
2.1 Study population
This cross-sectional study was conducted in West Bengal during November 2021 - February 2022 and included individuals aged 20 to 80years (mean age 49years). Total 100 healthy individuals (44 males and 56 females) participated from rural and urban areas, those who had been residing in the study area for more than one year and did not have any medical history or health problems. Both working and non-working individuals were included and they did not have any occupational exposure to PAHs. The exclusion criteria were, participants living in industrial zone, staying at current address less than one year. We evaluated urinary 1-OHP as an exposure biomarker of PAHs and impact of various socioeconomic and lifestyle factors on 1-OHP from routine life activities among study individuals. This study protocol was approved by Institutional Ethical Committee (approval number 15thIEC/CNCD/5.5 dated 15.07.2021.) All the participants were informed about research aim and then only their consent for study participation was obtained. The confidentiality of the study data was well ensured.
2.2 Data collection and variables’ description
To enroll the study participants, we went to their door step and they were interviewed face-to-face using a structured questionnaire and information related to their lifestyle and health were collected. The questions on personal and family history, socio-demographic and anthropometric characteristics, employment history, residential history, and lifestyle factors such as smoking, travelling, cooking practice etc were asked to collect relevant information. Individuals were grouped by age (<35years, 35-50years, >50-65years and >65years), cooking activity (NA and Yes), smoking habit (Yes and No), travelling activity (Yes and No), and study area (rural and urban). They were classified into low-income group (LIG) earning <20K/Month, middle-income group (MIG) earning up to 50K/Month, and high-income group (HIG) earning >50K/Month based on their monthly family income. Women were again divided into two groups based on type of fuel used for cooking 1) using LPG and 2) wood/dry leavess etc. Men were also divided into two groups based on their smoking and travelling activity 1) smoker traveller and 2) non-smoker non-traveller. These classifications were done to better understand PAHs exposure from ambient environment via tobacco smoke, traffic pollutants and cooking fuel smoke.
2.3 Urine Sampling and Analysis
The morning first (mid-stream) urine sample (100mL) was collected from all individuals for estimation of 1-OHP. The sterilized polypropylene urine containers (Tarson) was used to collect the urine samples. After completion of sample collection, each sample container was labeled with a code that represented the participant details and sample collection date. After that all the collected samples were brought to laboratory under refrigerated (4oC) conditions and stored at -20oC till further analysis. To estimate urinary 1-OHP, it was extracted from urine as described by Ifegwu et al. 2012. [78]. In brief, urine sample (1mL), 1mL of the sodium acetate buffer solution (pH 4.6) and enzyme beta-glucoronidase (2000units) were mixed and incubated at 38oC overnight (about 18hours) to hydrolyze the conjugated 1-OHP. Then the incubated samples were shaken thoroughly to homogenize. This whole content (about 3mL) was passed through C18 solid phase extraction (SAX) cartridges. These cartridges were pre-conditioned with 3mL methanol and 3mL distilled water (HPLC grade). After that the SAX cartridge was again washed with 3mL distilled water to remove water soluble compounds from the sample matrix. Finally, the SAX cartridge was desorbed with 2mL methanol (eluting solvent). This final eluted solvent was injected (20µl) to HPLC for analysis. Chromatographic analysis was performed by HPLC (Shimadzu, Japan) coupled with system controller (Model: SLC-10 A), connected to an automated liquid sampler (Model: LC-10AT), Detector (Model: RF-10AXL), column oven, and a monitor. The mobile phase used for this analysis consisted of methanol and water (80:20V/V). It was filtered prior to analysis by passing through 47mm, 0.2µm, hydrophilic polypropylene membrane filter. Chromatographic condition was flow rate 1mL/min; reverse phase column dimensions 250mm, 4.6mm, and 5µm (ODS-2 Hypersil) and oven temperature was 400C. Chromatogram of 1-OHP was detected by fluorescence detector, excitation at 242nm and emission at 388nm wavelengths. The run time of whole linear gradient programme was 20 min for each sample, retention time (RT) of 1-OHP was at 8.3minutes [78]. The precision observed for 1-OHP was 1-4% at calibration concentration 0.25µg/L. Study recovery range between 98-114% at spiked concentration 3×LOQ. The limit of quantification was 1.6µg/L. The results below LOD (i.e. 0.5µg/L) were taken as instrument-measured concentration, as referred in other study [79, 80].
2.4 Estimation of Urine Creatinine
The creatinine concentration in urine has been determined by Jaffe method based on photometric measurement of creatinine reaction with picric acid at 37oC (Peake and Whiting, 2006; Weber and van Zanten, 1991). The reagent kit for creatinine estimation was supplied by Arkray (ARKRAY, India). The photometric measurement was done with UV-visible spectrophotometer (Model: Lambda 45, Perkin Elmer, USA) at 520 nm.
2.5 Statistical Analysis
The analysis of the collected data was performed using Microsoft Excel. The levels of urinary 1-OHP were expressed as µg/g creatinine and µg/L. The urine samples having creatinine below 0.3g/L and above 3g/L were rejected, according to American Conference of Governmental Industrial Hygienists (ACGIH) recommendation [81]. Creatinine concentration in urine between 0.3g/L to 3.0g/L represents normal hydrates in an adult [82]. Descriptive statistical analysis included interquartile values (25th and 75th percentiles) and the 95th percentile values were performed by SPSS software. The statistical significance of the difference between various factors among the study individuals was analyzed using Mann–Whitney U test (for nonparametric data).
3. Results
Socio-demographic characteristics of study individuals are described in Table 1. The study initially involved a total of 121 individuals. However, 21 individuals have to be excluded from the study due to inadequate urine samples, damaged sample containers and urine creatinine falling below <0.3g/L or exceeding >3.0g/L as per ACGIH recommendation [81]. Consequently, the final sample size consisted of 100 individuals who were able to meet the study criteria. Majority of individual were 35-50years age, 47%. Most of these individuals were High School Graduates, 28%. Highest number of individual falls in LIG category, 84%. Individuals involved in cooking were 51%. Daily travellers account for 29%. Smoking prevalence was only in 12% of the total study individuals.
Table 1: Socio-demographic information of study individuals (N=100)
|
Variables |
Rural N (%) |
Urban N (%) |
Total N (%) |
|
All |
40(40%) |
60(60%) |
100 (100%) |
|
Gender |
|||
|
Male |
16(40%) |
28(47%) |
44(%) |
|
Female |
24(60%) |
32(53%) |
56(%) |
|
Age Group (years) |
|||
|
<35 |
07 (17%) |
10 (16%) |
17 (17%) |
|
35-50 |
14 (35%) |
33 (55%) |
47 (47%) |
|
50-65 |
09 (23%) |
09 (15%) |
18 (18%) |
|
>65 |
10 (25%) |
08 (14%) |
18 (18%) |
|
Education |
|||
|
No Formal Education |
12 (30%) |
16 (27%) |
28 (28%) |
|
Primary |
15 (37%) |
11 (18%) |
26 (26%) |
|
High School |
7 (18%) |
21 (35%) |
28 (28%) |
|
S Sec. School |
2 (5%) |
6 (10%) |
8 (8%) |
|
College |
4 (10%) |
6 (10%) |
10 (10%) |
|
Family Income |
|||
|
LIG |
36 (90%) |
48 (80%) |
84 (84%) |
|
MIG |
4 (10%) |
9 (15%) |
13 (13%) |
|
HIG |
0 (0%) |
3 (5%) |
3 (3%) |
|
Cooking |
|||
|
NA |
18 (45%) |
31 (52%) |
49 (49%) |
|
Yes |
22 (55%) |
29 (48%) |
51 (51%) |
|
Travelling |
|||
|
Yes |
4 (10%) |
25 (42%) |
29 (29%) |
|
No |
36 (90%) |
35 (58%) |
71 (71%) |
|
Smoking |
|||
|
Yes |
2 (5%) |
10 (17%) |
12 (12%) |
|
No |
38 (95%) |
50 (83%) |
88 (88%) |
Abbreviations: LIG, low income group; MIG, middle income group; HIG, high income group; NA, not applicable
3.1 Distribution of Urinary 1-OHP
Table2a summarizes 1-OHP (µg/L) results without creatinine correction, the mean urinary 1-OHP was 0.44µg/L, median 0.26µg/L and ranged 0.01 to 1.97µg/L. The mean value of 1-OHP was higher in male and urban individuals than in female and rural individuals but this was not statistically significant for any of the groups. The 95th percentile value of 1-OHP was found 1.51µg/L for total study individuals, 1.55µg/L for males, 1.4µg/L for females, 1.21µg/L for urban individuals, and 1.31µg/L for rural individuals. Table2b presents creatinine corrected 1-OHP(µg/g) results. The mean urinary 1-OHP was 0.30µg/g, median 0.26µg/g and ranged 0.01 to 1.40µg/g. The mean concentration of urinary 1-OHP in females (0.32µg/g) was higher than in males (0.28µg/g) but the difference was not significant. However, urinary 1-OHP was significantly higher (p=0.002) in urban individuals than rural ones. The 95th percentile value of urinary 1-OHP was 1.06µg/g, 1.23µg/g, 0.92µg/g, 0.82µg/g and 1.25µg/g for total study individuals, males, females, urban, and rural individuals respectively.
Table 2a: Urinary 1-OHP (µg/L) in total individuals according to gender and study area wise
|
N (%) |
Mean |
Range (µg/L) |
Median |
CI 95% lower L- Upper Limit |
p-value |
Percentile |
|||
|
(µg/L) |
25th |
75th |
95th |
||||||
|
Total |
100(100%) |
0.44±0.41 |
0.01 - 1.97 |
0.26 |
0.36 -0.53 |
0.11 |
0.75 |
1.51 |
|
|
Male |
44(44%) |
0.42±0.31 |
0.02 - 1.67 |
0.26 |
0.30- 0.54 |
>0.05 |
0.16 |
0.79 |
1.55 |
|
Female |
56(56%) |
0.46±0.21 |
0.01 - 1.97 |
0.27 |
0.33-0.58 |
0.07 |
0.74 |
1.4 |
|
|
Urban |
60 (60%) |
0.4±0.2 |
0.01 - 1.97 |
0.27 |
0.33-0.56 |
>0.05 |
0.11 |
0.69 |
1.21 |
|
Rural |
40 (40%) |
0.43±0.23 |
0.01 - 1.51 |
0.26 |
0.30-0.56 |
0.11 |
0.76 |
1.31 |
|
Table 2b: Creatinine corrected 1-OHP concentration (µg/g) in study participants (N=100)
|
N (%) |
Mean 1-OHP (µg/g) |
Range 1-OHP (µg/g) |
Median |
CI 95% lower Limit- Upper Limit |
p-value |
Percentile |
|||
|
25th |
75th |
95th |
|||||||
|
Total |
100(100%) |
0.30±0.30 |
0.01 - 1.40 |
0.26 |
0.24 - 0.36 |
0.08 |
0.49 |
1.06 |
|
|
Male |
44(44%) |
0.28±0.31 |
0.01 - 1.40 |
0.16 |
0.19 - 0.37 |
>0.05 |
0.04 |
0.35 |
1.23 |
|
Female |
56(56%) |
0.32±0.31 |
0.01 - 1.25 |
0.22 |
0.24 - 0.40 |
0.07 |
0.49 |
0.92 |
|
|
Urban |
60 (60%) |
0.27±0.28 |
0.91 - 1.40 |
0.14 |
0.20 - 0.34 |
0.002 |
0.07 |
0.34 |
0.82 |
|
Rural |
40 (40%) |
0.43±0.43 |
0.01 - 1.25 |
0.26 |
0.30 - 0.56 |
0.1 |
0.61 |
1.25 |
|
3.2 Variation in urinary 1-OHP with
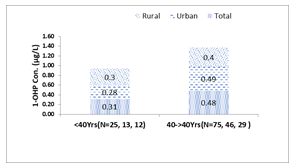
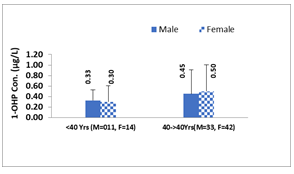
Age: The sample size of this study was insufficient to allow for categorization of study participants into multiple age groups, therefore they were classified only into two age groups, <40 years and ≥40years of age. The highest level of 1-OHP (0.48µg/L, 0.49µg/L and 0.4µg/L) was found in individuals of the age group ≥40years for total individuals, urban, and rural respectively (Figure 1a). Conversely, the lowest level of 1-OHP (0.31µg/L, 0.28µg/L and 0.3µg/L) was found in individuals of the age group <40years for total individuals, urban, and rural respectively. 1-OHP concentration increased from <40years to ≥40years with increase in age. That variation trend in 1-OHP was similar in all study individuals, irrespective of study area. We also evaluated the sex differences in excretion level of urinary 1-OHP across both the above-mentioned age groups and found that in age group of <40years male have higher levels of 1-OHP than females, while female have higher 1-OHP than male in age of ≥40years, as shown in Figure 1b. Although, this difference was not significant in any of the age groups.
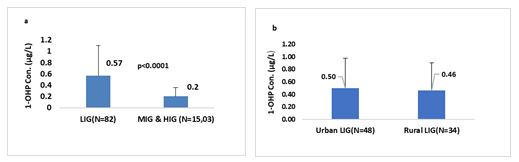
Family income: The association between family income and pollution exposure have very important aspect when we talk about human health risks. The number of individuals in the HIG was less, so the MIG and HIG were merged for this particular analysis. The results presented in Figure3a, showed that the participants in LIG had higher 1-OHP (0.57µg/l) than those in the merged MIG and HIG (0.2µg/L), and this difference had strong statistical significance (<0.0001). In order to evaluate the impact of study areas environment LIG individuals were further categorized as urban and rural LIG. Results showed that urban LIG had higher 1-OHP (0.50µg/l) than rural LIG individuals (0.46µg/l), but the difference was not significant (Figure 2b.)
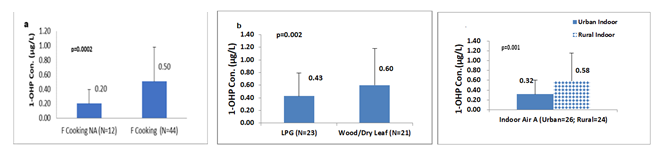
Cooking practice: Female individuals were predominantly involved in cooking practice. Smoke emitted during fuel burning in the cooking process was a very important source of PAHs exposure via inhalation for females. There were 56 female participants, they were grouped into two categories based on their involvement in cooking food - those who were involved in cooking (N=44) and those who weren’t involved in cooking (N=21). Furthermore, they were divided into two groups based on the fuel type they were using for cooking - those who used clean fuel (N=23) and those who used solid biomass (N=21). The females who were involved in cooking food and using solid biomass fuel had significantly (p=0.0002, and p=0.002) higher urinary 1-OHP 0.50µg/L and 0.60µg/L respectively, than those who were not involved in cooking and used clean fuel, figure4a, b summarizes these results.
- Work environment: To investigate the effect of indoor air on urinary 1-OHP in female individuals who were staying at home, in both urban and rural areas, were classified into two groups: urban and rural homemakers. 1-OHP was significantly (p=0.001) higher in rural homemakers (0.58µg/L) than urban homemakers (0.32µg/L). In rural areas indoor air might have higher PAHs concentrations leading to higher urinary 1-OHP in rural homemaker females figure 3c.
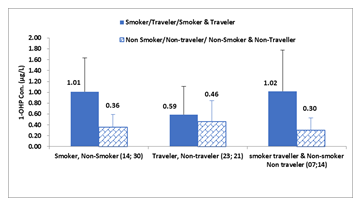
- Travelling activity: To examine the relationship between urinary1-OHP and exposure to tobacco smoke and traffic pollutants during travel in male individuals, they were categorized as i) smoker and non-smoker, ii) traveller and non-traveller, iii) smoker travel & non-smoker non-traveller. Notably, there were no smokers among rural individuals and we did not find frequent travelling to consider the potential effects of traffic pollution in rural individuals. The males who were smokers, daily travellers, and smoker travellers had higher urinary 1-OHP (1.01µg/L, 0.59µg/L, and 1.02µg/L, respectively) than their counterparts (Figure4). The difference in 1-OHP was significantly higher in smoker and smoker travellers (p=0.0002 and p=0.0003, respectively) than in non-smoker and non-smoker non-travellers males. These observations indicate that tobacco smoke and traffic emissions had a significant contribution to PAHs exposure in urban smokers and smoker traveller males.
4. Discussion
In this study, individuals were occupationally not exposed to PAHs and smoking prevalence was also low (12%). The results found the mean value of 1-OHP in smoker travellers was 3.4-fold higher than in non-smoker non-traveller individuals. When results of our study were compared with globally available literature, we found that mean 0.44µg/L and median 0.26µg/L (0.30µg/g; median 0.26µg/g) values of 1-OHP found in our study individuals was higher than found in general population of Iran (0.36µg/L) [5], Northern India (0.38 µg/L) [61], Southern India (0.42 µg/L) [73], United States (0.05µg/L) [1], Ukraine (0.30µg/L) [68] and Korea (0.10µg/L) [69]. However, 1-OHP found in our study individuals was lower than found in the general population of Afghanistan (1.65µg/L) [83] and China (1.2µg/L) [39]. This comparison illustrates that our study population had higher levels of urinary 1-OHP than individuals in some other countries, but lower levels than individuals in Afghanistan and China. Higher levels of 1-OHP in our study individuals were supported by Guo et al. 2013, they reported high (>10µg/day) total daily intakes (DIs) of PAHs in South Indian population [73]. It was well reported that lifestyle, environmental factors and sampling time influences urinary 1-OHP [84, 74, 19, 85]. In our study, urinary 1-OHP was found below the recommended BEI (2.5µg/L) by ACGIH [25]. The reported 95th percentile values of 1-OHP in our study were 1.51µg/L, 1.55µg/L, 1.4µg/L, 1.21µg/L, and 1.31µg/L for total individuals, males, females, urban, and rural populations, respectively. These values were found higher than those established after Germany Survey for smokers (1.03µg/L) and non-smokers (0.53µg/L) [65,77]. These values are 4-times higher than those found in firefighters before their involvement in any fire extinguishing operation [44]. In present study, 33% of the individuals had 1-OHP higher than the recommended value (0.53µg/L) for the unexposed general population [65]. Ruiz-Vera et al. 2015 also observed the same results in Mexican general population [8].
4.1 Daily pyrene intake (µg/Day) by study individuals
Urinary biomonitoring studies provided a means of evaluating the total daily intake of certain environmental chemicals including PAHs. Urinary 1-OHP is prime metabolite of pyrene alone although it is commonly used for biomonitoring of PAHs. Therefore, we used urinary 1-OHP data to find out total daily exposure to pyrene among study population. The daily intake (DIs) of pyrene among our study individuals was estimated by the following equation:
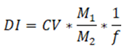
Where DI is the total daily intake of PAHs or Pyrene (μg/day), C is the urinary 1-OH concentration (μg/L), V is the human daily excretion volume of urine (L/day) (assumed a volume of 2.0 L), M1 and M2 are the respective molecular weights of parent PAHs and its metabolite (g/mol) (i.e. Pyrene and 1-OHP respectively), and f is the ratio of 1-OH excreted in urine relative to the total exposure dose (values of 6.8% was used for pyrene) [73]. The estimated DI of Pyrene among our study individuals was 12.9, 12.4, 13.5, 13.2 and 12.6μg/day for all individuals, males, females, urban and rural respectively. Daily total intake of pyrene in our study individuals was higher than that measured by Guo et al. 2013 and Bulder et al. 2013 in population of China, Soutern India (Chennai, Tamil Nadu), Japan, Korea, Kuwait, and Malaysia, while similar to that was find in Vietnam (12.6μg/day) [73,87]. Although the value of DI for pyrene measured in our study was well below than the reference dose (30μg/day) of DI for pyrene recommended by US EPA [ 87, 73, 129]. Our results showed that urban individuals had higher urinary 1-OHP than rural. Although, this difference was not significant. The elevated 1-OHP in urban individuals might be attributed to their higher exposure to PAHs emitted from vehicular and industrial sources in urban environments. Additionally, differences in lifestyle and dietary habits between urban and rural individuals might also contribute to this exposure difference. Several studies reported food as a significant source of PAHs exposure, particularly through consumption of grilled and barbecued foods [5, 88, 89, 90]. For larger PAHs such as pyrene, major route of exposure was ingestion; while for lower-weight PAHs inhalation was main route of exposure [1,43]. In urban individuals, consumption of charred food, second-hand tobacco smoke, frequent use of indoor incense and traffic pollution might cause elevatied 1-OHP, similar results were observed in other studies [5, 91, 92]. On the other hand, in rural areas, use of solid fuels such as wood, dry dung, and dry leaves for domestic purposes might cause higher PAHs exposure. Various works have reported high emission of PAHs in indoor air of rural areas due to use of solid biomass fuels [9, 10, 11, 12, 93]. We also found that females had higher 1-OHP than male individuals. That difference might be attributed to the fact that of 56 females, 79% (N=44) were involved in cooking food and among them about 50% (N=21) used to cook with solid biomass fuel. It is a well-established fact that exposure to cooking fumes and solid biomass fuel combustion emission leads to high PAHs exposure, that contribute to the higher urinary1-OHP in females [60, 94]. Moreover, the lower excretion of urinary creatinine in females might also caused higher levels of 1-OHP in them [75]. Our findings for higher 1-OHP in females are consistent with several studies [68, 97, 98], while inconsistent with others [5, 72]. Studies revealed that gender is an important physiological parameter to monitor pollutant exposure and related health effects, our results also align with this fact [99, 100, 101]. Further, the present study findings highlighted the importance of gender in predicting PAHs exposure through the measurement of 1-OHP levels in the urine of the general population. Consideration of gender differences was further imperative in interpreting biomarkers of PAHs exposure due to variations in body composition and hormone levels [102]. High proportion of adipose tissue in females might lead to increased susceptibility for lipophilic compounds such as PAHs [102, 103]. Females might also be more exposed to PAHs through cooking activities and spending more time indoors where higher levels of PAHs [6, 12, 104. 105]. In our study, 79% of the total female participants were involved in food cooking activities, which might explain the higher levels of 1-OHP observed in females. The findings of our study favored that sex-specific differences should be considered when interpreting biomarkers of PAHs exposure [94, 60, 97, 98, 68, 5, 72, 99, 100, 101]. Various studies have explored the link between urinary 1-OHP levels and age in different populations, with a particular focus on children and adolescents [1, 5, 106, 107]. This association in adult population was very less studied particularly in general population. We have explored this angle and found a positive relationship between age and urinary 1-OHP, higher levels were observed in individuals aged ≥40years. This trend was same across all the study individuals, irrespective of their location (urban or rural). This might be attributed due to more number of individuals (almost 3-times, N=75) in that age group, and they had high environmental PAHs because of their daily routine and job activities, which involve both indoor and outdoor tasks. Furthermore, our analysis of sex differences in the age groups of <40years showed that males had higher 1-OHP than females, while in ≥40years age group females had higher 1-OHP than males. However, these differences were not significant in either of the age group. This difference could potentially be explained by the fact that in both age groups, females were directly and consistently exposed to cooking practices without any gaps, and spend more time indoors compared to males. That was due to their traditional roles as caregivers and homemakers, which often require them to spend extended periods of time for cooking in enclosed spaces. The duration of exposure is a crucial factor in the determination of health risks. Therefore, the increased exposure to indoor pollutants might contributed to the higher levels of 1-OHP observed among females. In contrast, males have gaps in their outdoor environmental exposure (such as traffic exposure) due to weekend offs, which could explain the gender-based differences in the same age group. Our study is an evidence to the significant relationship between gender-related age and levels of urinary 1-OHP in adults. Furthermore, it was equally worth noting that due to the limited sample size individuals were classified in only 2 age groups, further research with a larger sample size and more age categories might be necessary to fully understand the relationship between age, sex, and urinary 1-OHP levels. The study findings highlighted the need for more targeted exposure reduction strategies for individuals in age group ≥40years, those exhibited the high environmental PAHs exposure. Our findings revealed that LIG individuals had significantly (p<0.0001) higher 1-OHP than the merged MIG and HIG. Furthermore, urban LIG had higher 1-OHP than rural LIG individuals, although this difference was not significant. Notably, LIG individuals were found at a higher risk of exposure to environmental PAHs and this risk was more aggravated by their living conditions in urban commercial areas such as highly-trafficked roads, flyovers, employed as house servants, labourers, street hawkers, and tea-sellers. While, rural LIG were using non-clean fuels for cooking purposes that might be the main source of PAHs exposure. However, we could not find literature showing the association between family income and urinary 1-OHP in general public or occupational settings. It is worth noting that our study was one of its kind to examine the association between family income and levels of 1-OHP in urine of general public from Eastern India. Our results provided valuable information for understanding the relationship between economic status and urinary 1-OHP levels in general population. Our findings underscored the need to consider family income as a key factor for assessment of exposure to environmental pollutants and in development of public health policies to mitigate the risks associated with PAHs exposure. Females who were involved in cooking practices using solid biomass fuel had significantly (p<0.01) higher levels of urinary 1-OHP than those who were not involved in cooking or those were using clean fuel. Emission of PAHs in indoor air by combustion of solid biomass was well reported (Zhang and Smith, 2007). In our study approximately 51% (N=21) of females were using wood and dry leaves as cooking fuel and spent about 2-3hours per day for cooking food for their family. That regular prolonged exposure to cooking fumes and smoke might lead to higher urinary 1-OHP. Studies have reported that fumes produced during cooking processes (frying and grilling) were significant sources of PAHs exposure [62, 63, 61, 64, 108]. Deep frying generates higher levels of PAHs compared to other cooking methods [62, 63]. Therefore, cooking is a significant indoor and outdoor source of PAHs exposure. PAHs had been quantified in exhaust stacks of restaurants [2, 94]. Indoor air polluted with PAHs resulted in increased urinary 1-OHP 14.02µg/g [6]. In our study those females were involved in cooking had higher 1-OHP (0.50µg/L) than that in firefighters(0.31µg/L) after their first operation, while lower than those found in Mexican women (4.3µg/L, in 2011), (0.82µg/L, in 2015) and African individuals (1.5 µmoles/moles creatinine) who used wood as cooking fuel [8, 14, 109]. Interestingly, rural females staying indoors had significantly (p<0.01) higher 1-OHP than those of urban females. In the field survey, it was observed that in most of the rural houses’ kitchen was attached to the living room and not properly ventilated, that might cause elevated levels of indoor PAHs. Moreover, in all the households of our study incense were used for Pooja twice a day and incense smoke (fumes) is well reported significant source of indoor air PAHs [110, 104, 105]. Pruneda-Alvarez et al. 2012 reported rural and suburban areas females were the prime victims of indoor PAHs exposure because they spend several hours a day for cooking in front of traditionally used open fire stoves [12]. We observed that females were regularly exposed to cooking fume and smoke during cooking, as this was routine practice for them twice a day. In India, females start intermittent cooking from adolescent age, and regular cooking typically starts after the age of 20years, particularly if they belong to LIG. Therefore, our study highlighted that cooking might be a significant source of exposure to PAHs in females, but additional research is required to establish a more robust association between cooking and 1-OHP levels. Other factors such as cooking place ventilation, the frequency and duration of cooking also have an impact on the level of exposure to PAHs. Females who spent extended periods of time engaging in indoor household activities, particularly cooking, were at elevated risk of exposure to PAHs, which might lead to various health disorders in the female individuals of our study. Singh et al. 2016 reported an increased cancer prevalence among women who had chronic exposure to cooking pollutants [61]. Particularly in context of Indian women who were using biomass fuel for cooking, increased platelet activation, platelet-leukocyte complexes, pro-inflammatory mediators, systemic inflammation, and oxidative stress had been observed [111, 112,113]. Furthermore, chronic exposure to wood smoke has been associated with headache, respiratory infections, asthma, blindness [114, 115, 116], cervical cancer in females [117], low birth weight, lung cancer, cataracts, and haematological disorders [95, 118, 119]. According to the World Health Organization (WHO,2009) report, indoor pollution caused by the burning of solid biomass fuel was responsible for 64% of deaths globally [120]. The combustion emission is responsible for approximately 28,000 deaths per year and around 40 million cases of acute respiratory illness [121, 122]. To mitigate cooking fuel emission exposure the Indian government had launched Pradhan Mantri Ujjwala Yojana (PMUY) in 2016, and the National Biomass Cookstoves Initiative (NBCI) in 2009 to provide clean cooking fuel and improve cooking stoves. Our study found inadequate implementation of these government schemes in the studied area, highlighting the urgent need for their effective implementation. Biomonitoring can be an essential tool to assess the extent of PAHs’ body burden and safeguarding the health of female (those are a particularly vulnerable segment of society) from the adverse effects of PAHs exposure. Our results indicated smokers had significantly (p<0.01) higher 1-OHP than non-smokers, similar observations were made by Hoseine et al. (2018) [18]. Furthermore, 1-OHP in our study smokers were similar to (1.01µg/L) - established by a German population survey; While in non-smoker it was lower (0.36µg/L) than in German non-smokers (0.53µg/L) [65,77]. Environmental tobacco smoke (ETS) is a significant source of PAHs exposure for the non-smoker and significantly (p<0.05) associated with elevated urinary 1-OHP in general population [18, 88, 22]. Here, it was worth noting that in each cigarette tobacco content varies from 1-1.5g and single cigarette contains approximately 50ng of pyrene [18, 123]. Furthermore, second-hand tobacco smoke was also found as a potential factor for elevated urinary 1-OHP [6,7]. However, in some studies, the association between tobacco smoke exposure and the excretion of urinary 1-OHP remains controversial [22], while not in others [66]. Our results also indicated that tobacco smoke was a significant contributor to elevated 1-OHP in smoker individuals. In our study routine travel was uncommon among rural individuals, the study mainly focused on travel-related PAHs exposure in urban male by adjusted smoking. We found travellers had higher 1-OHP than non-travellers, although that difference was not significant. Studies from other part of India (Chennai, Tamil Nadu) also reported weak association between 1-OHP and traffic-related PAHs [6, 124]. While, other studies have reported a positive association between traffic density and urinary 1-OHP in unexposed population [6, 125, 126, 96]. Significantly high urinary1-OHP was found in individuals living in high traffic and industrial zone than those who were living in residential and low traffic area zone [127,128, 74]. Likewise, our results also reported an association between elevated 1-OHP and traffic-related emission exposure in daily traveller individuals. These findings suggest that traffic emissions and tobacco smoke are important contributors to PAHs exposure among the general population. The study highlighted the importance of reducing exposure to PAHs from tobacco smoke and traffic emissions, particularly for urban males who smoke and travel frequently.
5. Limitations
Our study sample size may not be fully representative of the West Bengal population. Furthermore, the use of HPLC instead of more sensitive techniques such as LC/MS may limit the applicability of the findings to other populations. Therefore, these limitations should be considered when interpreting the implications of the study's results
6. Conclusions
In urban residents, smoking and travelling were major contributors to PAHs exposure, while in rural residents emissions from biomass fuel were prime contributors to PAHs. Inhalation was the primary route of exposure in study individuals. Females using wood/dry leaves as cooking fuel have higher 1-OHP than those who were using clean fuel. Females were at more risk of developing health issues than males, as their routine engaged in cooking. The study also witnessed inadequate implementation of the Pradhan Mantri Ujjwala Yojana (PMUY) and the National Biomass Cookstoves Initiative (NBCI) scheme in the study area. The study lacks data on dietary PAHs exposure and monitoring of air PAHs in the study area. However, this study found that the combination of sociodemographic and lifestyle factors with urine 1-OHP analysis provided valuable information on PAHs exposure in the general population during their routine life activities. The results determined in this study from the small sample size should be interpreted with caution, because people’s lifestyle, environmental factors, and sampling time influence the concentration of urinary 1-OHP. Overall, our study provided baseline data on urinary 1-OHP in West Bengal's adult population, which might be used for future epidemiological studies and also for policy-making efforts aimed at reducing environmental PAHs emissions at both the ministerial and personal levels.
Acknowledgement
The authors are thankful to the director in charge and officer in charge of the Indian Council of Medical Research - Centre for Ageing and Mental Health, Kolkata (India), to support this work. We are also grateful to the Department of Environmental Science, University of Calcutta, West Bengal, India for supporting the study execution. I would like to thank my colleagues, fellow scholars and office staff.
Conflict of Interest
The Authors declare no conflict of interest
References
- Li Z, Sandau CD, Romanoff LC, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ Res 107 (2008): 320-331.
- Chen J-W, Wang S-L, Hsieh DPH, et al. Carcinogenic potencies of polycyclic aromatic hydrocarbons for back-door neighbors of restaurants with cooking emissions Science of the Total Environment 417 (2012): 68-75.
- Liaud C, Millet M, & Le Calvé S. An analytical method coupling accelerated solvent extraction and HPLC-fluorescence for the quantification of particle-bound PAHs in indoor air sampled with a 3-stages cascade impactor. Talanta 131 (2015): 386-394.
- Wang Y, Hu L, Lu G. Health risk analysis of atmospheric polycyclic aromatic hydrocarbons in big cities of China. Ecotoxicol 23 (2014): 584-588.
- Hoseini M, Nabizadeh R, Delgado-Saborit J M, et al. Environmental and lifestyle factors affecting exposure to polycyclic aromatic hydrocarbons in the general population in a Middle Eastern area. Environ Pollution 240 (2018): 781-792.
- Sivaswamy S, Sambandan S, Puttusamy N, et al. Urinary 1-Hydroxypyrene levels among children with asthma in Chennai South India Indian. J Sci Technol 12 (2020): 1316-1320.
- Wang IJ, Karmaus WJJ, Yang CC. Polycyclic aromatic hydrocarbons exposure oxidative stress and asthma in children. Int Arch Occup Environ Health 90 (2017): 297-303.
- Ruiz-Vera T, Pruneda-Álvarez L G, Ochoa-Martínez Á C, et al. Assessment of vascular function in Mexican women exposed to polycyclic aromatic hydrocarbons from wood smoke. Environ Toxicol Pharmacol 2 (2015): 423-429.
- Singh DP, Gadi R, Mandal TK, et al. Emissions estimates of PAH from biomass fuels used in rural sector of Indo-Gangetic Plains of India. Atmospheric Environ 68 (2013): 120-126.
- Adetona O, Li Z, Sjödin A, et al. Biomonitoring of polycyclic aromatic hydrocarbon exposure in pregnant women in Trujillo, Peru—comparison of different fuel types used for cooking. Environ Int 53 (2013): 1-8.
- Ding J, Zhong J, Yang Y, et al. Occurrence and exposure to polycyclic aromatic hydrocarbons and their derivatives in a rural Chinese home through biomass-fueled cooking. Environ Pollution 169 (2012): 160-166.
- Pruneda-Alvarez LG, Perez-Vazquez FJ, Salgado-Bustamante M, et al. Exposure to indoor air pollutants (polycyclic aromatic hydrocarbons toluene benzene). in Mexican indigenous women Indoor Air 2 (2012): 140-147.
- World Health Organization (WHO). World Health Organization Indoor Air Quality Guidelines: Household Fuel Combustion (2014).
- Riojas-Rodriguez H, Schilmann A, Marron-Mares AT, et al. Impact of the improved Patsari biomass stove on urinary polycyclic aromatic hydrocarbon biomarkers and carbon monoxide exposures in rural. Mexican women Environ Health Per 119 (2011): 1301-1307.
- Masera OR, Díaz R, & Berrueta V. From cookstoves to cooking systems: the integrated program on sustainable household energy use in Mexico. Energy Sus Develop 9 (2005): 25-36.
- Albalak R, Bruce N, McCracken JP, et al. Indoor respirable particulate matter concentrations from an open fire, improved cookstove, and LPG/open fire combination in a rural Guatemalan community. Environ Sci Technology 35 (2001): 2650–2655.
- Abdel-Shafy HI, Mansour MS. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egyptian J Petroleum 25 (2016): 107-123.
- Hoseini M, Yunesian M, Nabizadeh R, et al. Characterization and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in urban atmospheric Particulate of Tehran Iran. Environ Sci Pollution Res 23 (2016): 1820-1832.
- Li Z, Romanoff L C, Lewin M D, et al. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolites in the general population and comparison of spot first-morning and 24-h void sampling. J Exposure Sci Environ Epidemiol 20 (2010): 526-535.
- Suzuki K, Yoshinaga J. Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. Int Arch Occupational Environ Health 81 (2007): 115-121.
- Ohura T, Sugiyama T, Amagai T, et al. Simultaneous liquid chromatographic determination of 39 polycyclic aromatic hydrocarbons in indoor and outdoor air and application to a survey on indoor air pollution in Fuji Japan. J AOAC Inter 85 (2002): 188-202.
- Huang W, Caudill SP, Grainger J, et al. Levels of 1-hydroxypyrene and other monohydroxy polycyclic aromatic hydrocarbons in children: a study based on US reference range value. Toxicol Lett 163 (2006): 10-19.
- Rengarajan T, Rajendran P, Nandakumar N, et al. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer Asian Pacific. J Tropical Biomed 3 (2015): 182-189.
- Jongeneelen FJ. A guidance value of 1-hydroxypyrene in urine in view of acceptable occupational exposure to polycyclic aromatic hydrocarbons. Toxicol Lett 2 (2014): 239-248.
- American Conference of Governmental Industrial Hygienists (ACGIH). Polycyclic Aromatic Hydrocarbons (PAHS): BEI®, seventh ed. (Documentation. Cincinnati OH, USA) (2017).
- Jasso-Pineda Y, Diaz-Barriga F, Yanez-Estrada L, et al. DNA damage in Mexican children living in high-risk contaminated scenarios. Sci Total Environ 518 (2015): 38-48.
- Torres-Dosal A, Perez-Maldonado IN, Jasso-Pineda Y, et al. Indoor air pollution in a Mexican indigenous community: evaluation of risk reduction program using biomarkers of exposure and effect. Sci Total Environ 390 (2008): 362-368.
- Kim K-H, Jahan SA, Kabir E, et al. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60 (2013): 71-80.
- Brucker N, Charao MF, Moro AM, et al. Atherosclerotic process in taxi drivers occupationally exposed to air pollution and co-morbidities. Environ Res 131 (2014): 31–38.
- Brucker N, Moro AM, Charao MF, et al. Biomarkers of occupational exposure to air pollution inflammation and oxidative damage in taxi drivers. Sci Total Environ 463 (2013): 884-893.
- Feng Y, Sun H, Song Y, et al. A community study of the effect of polycyclic aromatic hydrocarbon metabolites on heart rate variability based on the Framingham risk score. Occupational Environ Med 71 (2014): 338-345.
- Xu X, Hu H, Kearney GD, et al. Studying the effects of polycyclic aromatic hydrocarbons on peripheral arterial disease in the United States. Sci Total Environ 461 (2013): 341-347.
- Diggs DL, Huderson AC, Harris KL, et al. Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 29 (2011): 324-357.
- Household use of solid fuels and high-temperature frying IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. World Health Organization Lyon France 95 (2010a).
- International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Air Pollution Part 1 Some nonheterocyclic polycyclic aromatic hydrocarbons and some related industrial exposures. World Health Organization Lyon France 92 (2010b).
- Rey-Salgueiro L, Martínez-Carballo E, García-Falcón MS, et al. Occurrence of polycyclic aromatic hydrocarbons and their hydroxylated metabolites in infant foods. Food Chemist 115 (2009a): 814-819.
- Rey-Salgueiro L, Martínez-Carballo E, García-Falcón MS, et al. Survey of polycyclic aromatic hydrocarbons in canned bivalves and investigation of their potential sources. Food Res Int 42 (2009b): 983-988.
- Okuda T, Okamoto K, Tanaka S, et al. Measurement and source identification of polycyclic aromatic hydrocarbons (PAHs) in the aerosol in Xi'an China by using automated column chromatography and applying positive matrix factorization (PMF). Sci Total Environ 408 (2010): 1909-1914.
- Xia Y, Han Y, Zhu P, et al. Relation between urinary metabolites of polycyclic aromatic hydrocarbons and human semen quality. Environ Sci Technol 43 (2009): 4567-4573.
- Zhu P, Bian Z, Xia Y, et al. Relationship between urinary metabolites of polycyclic aromatic hydrocarbons and thyroid hormone levels in Chinese non-occupational exposure adult males. Chemosphere 77 (2009): 883-888.
- Estabrook R, Saeki Y, Chacos N, et al. Polycyclic hydrocarbon metabolism: a plethora of phenomena. Advances in Enzyme Regulation 19 (1981): 3-17.
- Xue W, & Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol 1 (2005): 73-93.
- Ramesh A, Walker SA, Hood DB, et al. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol 23 (2004): 301-333.
- Taeger D, Koslitz S, Kafferlein H U, et al. Exposure to polycyclic aromatic hydrocarbons assessed by biomonitoring of firefighters during fire operations in Germany. Int J Hygiene Environ Health 248 (2023):
- Demers PA, DeMarini DM, Fent KW, et al. Carcinogenicity of occupational exposure as a firefighter. Lancet Oncol 8 (2022): 985-986.
- Perez-Maldonado IN, Martínez-Salinas RI, Pruneda Alvarez LG, et al. Urinary 1-hydroxypyrene concentration from Mexican children living in the southeastern region in Mexico. Int J Environ Health Res 24 (2014): 113-119.
- Dominguez-Cortinas G, Diaz-Barriga F, Martinez-Salinas RI, et al. Exposure to chemical mixtures in Mexican children: high-risk scenarios. Environ Sci Pollution Res 20 (2013): 351-357.
- Trejo-Acevedo A, Rivero-Perez NE, Flores-Ramerez R, et al. Assessment of the levels of persistent organic pollutants and 1-hydroxypyrene in blood and urine samples from Mexican children living in an endemic malaria area in Mexico. Bulletin Environ Cont Toxicol 88 (2012): 828-832.
- Gunier RB, Reynolds P, Hurley SE, et al. Estimating exposure to polycyclic aromatic hydrocarbons: a comparison of survey biological monitoring and geographic information system-based methods. Cancer Epidemiol Biomarkers Prev 15 (2006): 1376-1381.
- Jongeneelen FJ. Biological monitoring of environmental exposure to polycyclic aromatic hydrocarbons; 1-hydroxypyrene in urine of people Toxicology Letters 72(1994): 205-211.
- Banks APW, et al. Characterising the exposure of Australian firefighters to polycyclic aromatic hydrocarbons generated in simulated compartment fires. International Journal of Hygiene and Environmental Health 231(2021): 113637.
- Cherry N, et al. Exposure and absorption of PAHs in wildland firefighters: a field study with pilot interventions Annals of Work Exposures and Health 2 (2021): 148-161.
- Hoppe-Jones C, Griffin SC, Gulotta JJ, et al. Dearmon-Moore D. Evaluation of fireground exposures using urinary PAH metabolites. J Exposure Sci Environ Epidemiol 5 (2021): 913-922.
- Rosting C, & Olsen R. Biomonitoring of the benzene metabolite sphenylmercapturic acid and the toluene metabolite s-benzylmercapturic acid in urine from firefighters. Toxicol Lett 329 (2020): 20-25.
- Casjens S, Brüning T, & Taeger D. Cancer risks of firefighters: a systematic review and meta-analysis of secular trends and region-specific differences International Archives of Occupational and Environmental Health 7 (2020): 839-852.
- Jalilian H, Ziaei M, Weiderpass E, et al. Cancer incidence and mortality among firefighters. Int J Cancer 10 (2019): 2639-2646.
- Fent KW, Toennis C, Sammons D, et al. Firefighters and instructors absorption of PAHs and benzene during training exercises. Int J Hygiene Environ Health 7 (2019): 991-000.
- Andersen MHG, et al. Assessment of polycyclic aromatic hydrocarbon exposure, lung function, systemic inflammation, and genotoxicity in peripheral blood mononuclear cells from firefighters before and after a work shift. Environ Mol Mutagenesis 6 (2018): 539–548.
- Keir JL, Akhtar US, Matschke DM, et al. Elevated exposures to polycyclic aromatic hydrocarbons and other organic mutagens in Ottawa firefighters participating in emergency on-shift fire suppression. Environ Sci Technol 21 (2017): 12745-12755.
- Kamal A, Cincinelli A, Martellini T, et al. Biomarkers of PAH exposure and hematologic effects in subjects exposed to combustion emission during residential (and professional) cooking practices in Pakistan. Environ Sci Pollution Res 23 (2016): 1284-1299.
- Singh A, Kamal R, Mudiam MKR, et al. Heat and PAHs Emissions in Indoor Kitchen Air and Its Impact on Kidney Dysfunctions among Kitchen Workers in Lucknow North India PLoS ONE 2 (2016): e0148641.
- Yao Z, Li J, Wu B, et al. Characteristics of PAHs from deep-frying and frying cooking fumes. Environ Sci Pollution Res 22 (2015): 16110-16120.
- Saito E, Tanaka N, Miyazaki A, et al. Concentration and particle size distribution of polycyclic aromatic hydrocarbons formed by thermal cooking. Food Chemist 153 (2014): 285-291.
- Sjaastad A K, Jorgensen R B, Svendsen K. Exposure to polycyclic aromatic hydrocarbons (PAHs) mutagenic aldehydes and particulate matter during pan frying of beefsteak. Occu Environ Med 4 (2010): 228-232.
- Wilhelm M, Hardt J, Schulz C, et al. New reference value and the background exposure for the PAH metabolites 1-hydroxypyrene and 1- and 2-naphthol in urine of the general population in Germany: basis for validation of human biomonitoring data in environmental medicine. Int J Hygiene Environ Health 3-4 (2008): 447-453.
- Scherer G, Frank S, Riedel K, et al. Biomonitoring of exposure to polycyclic aromatic hydrocarbons of non-occupationally exposed persons. Cancer Epidemiol Prev Biomarkers 9 (2000): 373-380.
- Viau C, Vysko_cil A, Martel L. Background urinary 1-hydroxypyrene levels in non-occupationally exposed individuals in the Province of Quebec Canada and comparison with its excretion in workers exposed to PAH mixtures. Sci Total Environ 16 (1995): 191-194.
- Mucha AP, Hryhorczuk D, Serdyuk A, et al. Urinary 1-hydroxypyrene as a biomarker of PAH exposure in 3-year-old Ukrainian children. Environ Health Per 4 (2006): 603-9.
- Kim H, Cho S-H, Kang J-W, et al. Urinary 1-hydroxypyrene and 2-naphthol concentrations in male Koreans. Int Arch Occupational Environ Health 74 (2000): 59-62.
- Grainger J, Huang W, Patterson Jr DG, et al. Reference range levels of polycyclic aromatic hydrocarbons in the US population by measurement of urinary monohydroxy metabolites. Environ Res 3 (2006): 394-423.
- Aquilina NJ, Delgado-Saborit JM, Meddings C, et al. Environmental and biological monitoring of exposures to PAHs and ETS in the general population. Environ Int 36 (2010): 763-771.
- Sul D, Ahn R, Im H, et al. Korea National Survey for Environmental Pollutants in the human body 2008: 1-hydroxypyrene 2-naphthol and cotinine in urine of the Korean population. Environ Res 118 (2012): 25-30.
- Guo Y, Senthilkumar K, Alomirah H, et al. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technology 47 (2013): 2932-2938.
- Fan R, Wang D, Mao C, et al. Preliminary study of childrens exposure to PAHs and its association with 8-hydroxy-22-deoxyguanosine in Guangzhou China. Environ Int 42 (2012): 53-58.
- Hansen AM, Raaschou-Nielsen O, Knudsen LE. Urinary 1-hydroxypyrene in children living in city and rural residences in Denmark. Sci Total Environ 363 (2006): 70-77.
- Kuo C T, Chen H W, Chen J L. Determination of 1-hydroxypyrene in children urine using column-switching liquid chromatography and fluorescence detection. J Chromatography B 805 (2004): 187-193.
- Becker K.Umwelt-Survey 1998 - Human-Biomonitoring. Umweltbundesamt, Berlin (2020).
- Ifegwu C, Osunjaye K, Fashogbon F, et al. Urinary 1-hydroxypyrene as a biomarker to carcinogenic polycyclic aromatic hydrocarbon exposure. Biomarkers Cancer 4 (2012): BIC-S10065.
- Gyllenhammar I, Glynn A, Jönsson BA, et al. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs? Environ Res 153 (2017): 48-54.
- Tranfo G, Pigini D, Paci E, et al. Biomonitoring of urinary benzene metabolite SPMA in the general population in Central Italy. Toxics 3 (2018): 37.
- American Conference of Governmental Industrial Hygienists.Threshold limit values (TLVs) for chemical substances and physical agents biological exposure indices for 2019. Cincinnati (2019).
- Bader M, Jäger T, Drexler H, et al. kreatininbezogenenAnalysenergebnissen. Beurteilungswerte in biologischem Material. MAK Collect. Occup Health Saf 4 (2020).
- Hemat H, Wittsiepe J, Wilhelm M, et al. High levels of 1-hydroxypyrene and hydroxyphenanthrenes in urine of children and adults from Afghanistan. J Exposure Sci Environ Epidemiol 22 (2012): 46-51.
- Hagedorn HW, Scherer G, Engl J, et al. Urinary excretion of phenolic polycyclic aromatic hydrocarbons (OH-PAH) in nonsmokers and in smokers of cigarettes with different ISO tar yields. J Analyt Toxicol 33 (2009): 301-309.
- Han IK, Duan X, Zhang L, et al. 1-Hydroxypyrene concentrations in first morning voids and 24-h composite urine: Intra- and inter-individual comparisons. J Exposure Sci Environ Epidemiol 18 (2008): 477-485.
- US Environmental Protection Agency Integrated Risk Information System (IRIS) (2022).
- Bulder AS, Hoogenboom LAP, Kan CA, et al. Initial risk assessment of polycyclic aromatic hydrocarbons (PAHs) in feed (materials) (2013).
- Shahsavani S, Dehghani M, Hoseini M, et al. Biological monitoring of urinary 1-hydroxypyrene by PAHs exposure among primary school students in Shiraz Iran. Int Arch Occup Environ Health 90 (2017a): 179-187.
- Alghamdi MA, Alam MS, Stark C, et al. Urinary metabolites of polycyclic aromatic hydrocarbons in Saudi Arabian schoolchildren in relation to sources of exposure. Environ Res 140 (2015): 495-501.
- Fasano E, Yebra-Pimentel I, Martínez-Carballo E, et al. Profiling distribution and levels of carcinogenic polycyclic aromatic hydrocarbons in traditional smoked plant and animal foods. Food Control 59 (2016): 581-590.
- Sun Z, Zhu D. Exposure to outdoor air pollution and its human health outcomes: A scoping review. PLoS ONE (2019): e0216550.
- Freire C, Abril A, Fernández MF, et al. Urinary 1-hydroxypyrene and PAH exposure in 4-year-old Spanish children. Sci Total Environ (2009): 1562-1569.
- Cavanagh JAE, Brown L, Trought K, et al. Elevated concentrations of 1-hydroxypyrene in schoolchildren during winter in Christchurch New Zealand. Sci Total Environ 1 (2007): 51-59.
- Abdullahi KL, Delgado-Saborit JM, Harrison RM. Emissions and indoor concentrations of particulate matter and its specific chemical components from cooking: a review. Atmos Environ 71 (2013): 260-294.
- Kamal A, Malik RN, Martellini T, et al. PAHs exposure biomarkers are associated with clinico-chemical changes in the brick kiln workers in Pakistan. Sci Total Environ 490 (2014): 521-527.
- Hansen AM, Mathiesen L, Pedersen M, et al. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studiesa review. Int J Hygiene Environ Health 211 (2008): 471-503.
- Morgan MK, Jones PA, Sobus JR, et al. Using urinary biomarkers to evaluate polycyclic aromatic hydrocarbon exposure in 126 preschool children in Ohio. Int J Environ Health Res 25 (2015): 628-639.
- Hu SW, Chan YJ, Hsu HT, et al. Urinary levels of 1-hydroxypyrene in children residing near a coal-fired power plant. Environ Res 8(2011): 1185-1191.
- Poli D, Mozzoni P, Pinelli S, et al. Sex Difference and Benzene Exposure: Does It Matter? Int J Environ Res Public Health 4 (2022): 2339.
- Messing K, Silverstein BA. Gender and occupational health Scandinavian. J Work Environ Health 35(2009): 81-83.
- Franconi F, Brunelleschi S, Steardo L, et al. Gender differences in drug responses. Pharmacol Res 55 (2007): 81-95.
- Arbuckle TE. Are there sex and gender differences in acute exposure to chemicals in the same setting? Environ Res101 (2006): 195-204.
- Clewell HJ, Tan YM, Campbell JL, et al. Quantitative interpretation of human biomonitoring data. Toxicol Applied Pharmacol 231 (2008): 122-133.
- Orecchio S. Polycyclic aromatic hydrocarbons (PAHs) in indoor emission from decorative candles. Atmospheric Environ 45 (2011): 1888-1895.
- Pagels J, Wierzbicka A, Nilsson E, et al. Chemical composition and mass emission factors of candle smoke particles. J Aerosol Sci 40 (2009): 193-208.
- Lazzer S, Bedogni G, Lafortuna CL, et al. Relationship between basal metabolic rate gender age and body composition in 8780 white obese subjects. Obesity 18 (2010): 71-78.
- Hubal EC, Sheldon LS, Burke JM, et al. Children's exposure assessment: a review of factors influencing Children's exposure and the data available to characterize and assess that exposure. Environ Health Per 8 (2000): 475-486.
- Sjaastad AK Svendsen K. Exposure to mutagenic aldehydes and particulate matter during panfrying of beefsteak with margarine rapeseed oil olive oil or soybean oil. Ann Occupational Hygiene 8 (2008): 739-745.
- Viau C, Hakizimana G, Bouchard M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int Arch Occu Environ Health 73 (2000): 331-338.
- Nethery E, Wheeler AJ, Fisher M, et al. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: a pilot study among pregnant women. J Exposure Sci Environ Epidemiol 22 (2012): 70-81.
- Ray MR, Mukherjee S, Roychoudhury S, et al. Platelet activation upregulation of CD11b/CD18 expression on leukocytes and increase in circulating leukocyte-platelet aggregates in Indian women chronically exposed to biomass smoke. Human Experiment Toxicol 25 (2006): 627-635.
- Barregard L, Sallsten G, Gustafson P, et al. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhalation Toxicol 18 (2006): 845–853.
- Dutta A, Ray MR, Banerjee. A Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicol Applied Pharmacol 261 (2012): 255-262.
- Miah Md D, Rashid HA, Shin MY. Wood fuel use in the traditional cooking stoves in the rural food plain areas of Bangladesh: a socioenvironmental perspective. Biomass and Bioenergy 1 (2009): 70-78.
- Rajvanshi AK. Ethanol fuel for rural households Maharashtra India: Nimbkar Agricultural Res Instit (2006).
- Ekici A, Eikici M, Kurtipek E, et al. Obstructive airway diseases in women exposed to biomass smoke. Environ Res 99 (2005): 93-98.
- Velema JP, Ferrera A, Figueroa M, et al. Burning wood in the kitchen increases the risk of cervical neoplasia in HPV-infected women in Honduras. Int J Cancer 4 (2002): 536-541.
- Pope DP, Mishra V, Thompson L, et al. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol Rev 1 (2010): 70-81.
- Al-Malki AL, Rezq AM, Al-Saedy MH. Effect of fire smoke on some biochemical parameters in firefighters of Saudi Arabia. J Occ Med Toxicol 3 (2008): 33.
- World Health Organization (WHO). Global Health Risks: Mortality and burden of disease attributable to selected major risks World Health Organization Geneva WHO Situation analysis of household energy use and indoor air pollution in Pakistan Discussion Paper on Child Health (2009).
- Harijan K, Mohammad AU. Potential of biomass conservation through dissemination of efficient cook stoves in Pakistan. APCBEE Procedia 5 (2013): 358-362.
- Colbeck I, Nazir ZA, Ali Z. Characteristics of indoor/outdoor particulate pollution in urban and rural residential environments of Pakistan Indoor. Air 1 (2009): 40-51.
- Knishkowy B, Amitai Y. Water-pipe (narghile) smoking: an emerging health risk behavior. Pediatrics 116 (2005): e113-e119.
- Migliore E, Berti G, Galassi C, et al. Respiratory symptoms in children living near busy roads and their relationship to vehicular traffic: results of an Italian multicenter study (SIDRIA 2). Environ Health 8 (2009).
- Rioux CL, Gute DM, Brugge D, et al. Characterizing urban traffic exposures using transportation planning tools: an illustrated methodology for health researchers. J Urban Health 87 (2010): 167-188.
- Rose N, Cowie C, Gillett R, et al. Weighted road density: a simple way of assigning traffic-related air pollution exposure. Atmospheric Environ 43(2009): 5009-5014.
- Lee M S, Eum K D, Zoh K D, et al. 1-Hydroxypyrene as a biomarker of PAH exposure among subjects living in two separate regions from a steel mill. Int Arch Occup Environ Health 80 (2007): 671-678.
- Gilbert NL, Viau C. Biological monitoring of environmental exposure to PAHs in the vicinity of a Soderberg aluminium reduction plant. Occup Environ Med 54 (1997): 619-621.
- Bevan R, Jones K, Cocker J, et al. Reference ranges for key biomarkers of chemical exposure within the UK population. Int J Hygiene Environ Health 2 (2013): 170–174.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 