COVID-19 Diagnosis based on Taste Disorders: A Case-Control Study
Kamil Adamczyk1, Michal Herman1, Janusz Fraczek1, Robert Piec2, Barbara Szykula-Piec2, Artur Zaczynski1, Rafal Wójtowicz3, Krzysztof Bojanowski1, Ewa Rusyan4, Zbigniew Król1, Waldemar Wierzba1,5, Edward Franek1,6*
1Central Clinical Hospital of the Ministry of the Interior and Administration in Warsaw, Poland
2The Main School of Fire Service, Warsaw, Poland
3Solec Hospital, Warsaw, Poland
4Warsaw Medical University, Warsaw, Poland
5UHE Satellite Campus in Warsaw, University of Humanities and Economics in Lodz, Poland
6Mossakowski Clinical Research Center of Polish Academy of Sciences, Warsaw, Poland
*Corresponding Author: Edward Franek, Mossakowski Clinical Research Center of Polish Academy of Sciences, Warsaw, Poland
Received: 04 December 2021; Accepted: 10 December 2021; Published: 14 December 2021
Article Information
Citation: Kamil Adamczyk, Michal Herman, Janusz Fraczek, Robert Piec, Barbara Szykula-Piec, Artur Zaczynski, Rafal Wójtowicz, Krzysztof Bojanowski, Ewa Rusyan, Zbigniew Król, Waldemar Wierzba, Edward Franek. COVID-19 Diagnosis based on Taste Disorders: A Case-Control Study. Journal of Environmental Science and Public Health 5 (2021): 516-535.
View / Download Pdf Share at FacebookAbstract
Background: We aimed to assess taste disturbances in COVID-19 patients. Additionally, we hypothesized that it is possible to establish a reliable and inexpensive screening method for SARS-COV-2 infection using a flavor test.
Methods: The ability to taste the sweet, salty, sour and bitter flavors was assessed in 52 COVID-19 patients and 36 controls using flavor tablets (sucrose, NaCl, ascorbic acid and grapefruit extract - 99% naringin) with flavor concentrations established previously in healthy subjects.
Results: Subjective smell or taste disturbances were reported by 65% of subjects in the test group compared with 8% in the control group. A numerical difference was demonstrated for all flavor tests recognitions between COVID-19 patients and the control group; however, the difference was only significant with the sweet and salty flavor tablets. Complete ageusia was very uncommon in the study group and occurred in only 1 out of 52 subjects. Only one predictive model – the medical questionnaire, consisting of questions regarding self-reported loss of taste, smell, or fever - displayed good performance in COVID-19 diagnosis (ROC AUC = 0.82). The performance of other predictive models, including the taste testers, was lower.
Conclusion: Among all tested flavors, the sweet and salty flavors perception was impaired in COVID-19 patients. Despite the objective taste tests being carried out, the medical questionnaire was the predictive model with the best performance, higher than any single taste test or even multiple tastes test.
Keywords
COVID-19; SARS-CoV-2; Taste; Flavor; Diagnostics
COVID-19 articles; SARS-CoV-2 articles; Taste articles; Flavor articles; Diagnostics articles
COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals SARS-CoV-2 articles SARS-CoV-2 Research articles SARS-CoV-2 review articles SARS-CoV-2 PubMed articles SARS-CoV-2 PubMed Central articles SARS-CoV-2 2023 articles SARS-CoV-2 2024 articles SARS-CoV-2 Scopus articles SARS-CoV-2 impact factor journals SARS-CoV-2 Scopus journals SARS-CoV-2 PubMed journals SARS-CoV-2 medical journals SARS-CoV-2 free journals SARS-CoV-2 best journals SARS-CoV-2 top journals SARS-CoV-2 free medical journals SARS-CoV-2 famous journals SARS-CoV-2 Google Scholar indexed journals Taste articles Taste Research articles Taste review articles Taste PubMed articles Taste PubMed Central articles Taste 2023 articles Taste 2024 articles Taste Scopus articles Taste impact factor journals Taste Scopus journals Taste PubMed journals Taste medical journals Taste free journals Taste best journals Taste top journals Taste free medical journals Taste famous journals Taste Google Scholar indexed journals Flavor articles Flavor Research articles Flavor review articles Flavor PubMed articles Flavor PubMed Central articles Flavor 2023 articles Flavor 2024 articles Flavor Scopus articles Flavor impact factor journals Flavor Scopus journals Flavor PubMed journals Flavor medical journals Flavor free journals Flavor best journals Flavor top journals Flavor free medical journals Flavor famous journals Flavor Google Scholar indexed journals Diagnostics articles Diagnostics Research articles Diagnostics review articles Diagnostics PubMed articles Diagnostics PubMed Central articles Diagnostics 2023 articles Diagnostics 2024 articles Diagnostics Scopus articles Diagnostics impact factor journals Diagnostics Scopus journals Diagnostics PubMed journals Diagnostics medical journals Diagnostics free journals Diagnostics best journals Diagnostics top journals Diagnostics free medical journals Diagnostics famous journals Diagnostics Google Scholar indexed journals smell disorders articles smell disorders Research articles smell disorders review articles smell disorders PubMed articles smell disorders PubMed Central articles smell disorders 2023 articles smell disorders 2024 articles smell disorders Scopus articles smell disorders impact factor journals smell disorders Scopus journals smell disorders PubMed journals smell disorders medical journals smell disorders free journals smell disorders best journals smell disorders top journals smell disorders free medical journals smell disorders famous journals smell disorders Google Scholar indexed journals SARS-CoV-2 virus articles SARS-CoV-2 virus Research articles SARS-CoV-2 virus review articles SARS-CoV-2 virus PubMed articles SARS-CoV-2 virus PubMed Central articles SARS-CoV-2 virus 2023 articles SARS-CoV-2 virus 2024 articles SARS-CoV-2 virus Scopus articles SARS-CoV-2 virus impact factor journals SARS-CoV-2 virus Scopus journals SARS-CoV-2 virus PubMed journals SARS-CoV-2 virus medical journals SARS-CoV-2 virus free journals SARS-CoV-2 virus best journals SARS-CoV-2 virus top journals SARS-CoV-2 virus free medical journals SARS-CoV-2 virus famous journals SARS-CoV-2 virus Google Scholar indexed journals patients articles patients Research articles patients review articles patients PubMed articles patients PubMed Central articles patients 2023 articles patients 2024 articles patients Scopus articles patients impact factor journals patients Scopus journals patients PubMed journals patients medical journals patients free journals patients best journals patients top journals patients free medical journals patients famous journals patients Google Scholar indexed journals
Article Details
1. Introduction
The novel coronavirus SARS-CoV-2 (severe acute respiratory syndrome corona-virus 2) is the cause of the ongoing pandemic. The outbreak of the novel coronavirus epidemic was first reported in the city of Wuhan in Hubei Province, China, but rapidly went on to affect almost all countries in the world [1]. The disease is characterized by fever, dry cough, dyspnea, chest pain and, in many cases, pneumonia; however, many other non-respiratory signs and symptoms have been described, such as general weak-ness, myalgia and arthralgia, or headache [2]. Other non-respiratory manifestations of COVID-19 include signs and symptoms arising from the gastrointestinal tract, such as taste and smell disorders, nausea, vomiting, diarrhea and anorexia. Such symptoms have been found in a significant percentage of patients, from below 10% to almost 70% [3].
COVID-19 may also have neurological manifest-tations. Initially, it was suspected that this may be due to the fact that coronavirus enters the central nervous system via the peripheral nerves or the olfactory bulb and olfactory sensory neurons [4]. However, more recent MRI studies [5-7] prove the presence of transient changes in the olfactory bulb in anosmic patients. The mechanism of these changes, however, is related to the infection of the cells of the vascular pericytes of the olfactory bulb by the virus. Experimental studies have shown high activity of ACE2 coronavirus receptors in the pericytes of the olfactory bulb vessels [7].
One of the neurological manifestations of SARS-CoV-2 virus infection is smell and taste disorders. It is still not clear if these disturbances are transient or permanent. Ac-cording to one study from Iran, 75% of patient reported improvement in olfacto-ry/gustatory disturbances within 2 weeks from diagnosis [8]. In another study, 75% out of 237 patients reported olfactory/gustatory disorders after 7.2 days from diagnosis [9]. In yet another, Israeli study, the mean duration of taste disorder was 18.6 ± 19 days and in 7% of cases after 208 days taste changes were still unresolved [10]. Moein et al. described smell impairment assessed with a well-validated test in 98% of patients, among whom 58% were either anosmic or severely microsmic [11]; however, the frequency of self-reported olfactory disorders may be lower. In a relatively large cohort of a multicentre European study, the frequency of self-reported olfactory dysfunction was 88% [12]. In another study, the self-reported frequency of anosmia was 67.8% compared with 16% of SARS-CoV-2-negative subjects [13]. Taste disorders have also been reported and, although some reports regard single patients only, dysgeusia and ageusia appear to constitute two common symptoms of COVID-19, occurring in 10% to almost 90% of patients, [12, 14-16] with the majority of meta-analyses reporting the frequency of taste disorders in the range of 30-50% [17-28]. However, taking into account the fact that most of the studies conducted were based on questionnaires, and some studies indicate that objective tests detect more infected people with an olfactory disorder than the questionnaire studies [29], it can be assumed that there are more COVID-19 patients with taste disorders than reported. Due to the prevalence of olfactory and taste disturbances in COVID-19, these symptoms are considered to be the best predictor (olfactory disorder) and the second-best predictor (taste disorder) of SARS-CoV-2 infection [30]. However, it is still unclear whether the olfactory and taste disorders in COVID-19 are associated with upper respiratory symptoms or not. According to Santos et al. of the thirteen studies solely in the meta-analysis, only six reported individuals presenting changes in smell and taste who also experienced nasal obstruction [31].
Similarly to olfactory dysfunction, most studies regarding taste disorders in SARS CoV-2 positive patients did not assess individual taste disturbances, but only the overall presence or absence of taste disturbance. Among those studies that assessed in-dividual taste disturbances, the most disturbed tastes according to individual authors were: salty and sweet [32-34], sweet and salty [35], sweet and sour [36], sweet and bitter [37].
Therefore, the present study aimed to conduct a more precise assessment of gustatory dysfunction in COVID-19 patients. Specifically, disorders in sweet, sour, and salty and bitter flavors in a group of young, otherwise asymptomatic, or mildly symptomatic subjects were quantitatively assessed and compared with a properly matched SARS CoV 2-negative control group. The flavor tablets used in the study contained generally available food substances with a specific smell, for example, sucrose, table salt, ascorbic acid, or grapefruit extract. This test, therefore, evaluated the taste disturbance along with the retronasal olfaction component.
2. Materials and Methods
2.1 Pilot study to determine the flavor test compositions
A pilot study was conducted on 25 young, healthy male adults with a mean age of 21.11 years (min 19, max 26) with no concomitant diseases, who tested negative for SARS-CoV-2 according to the PCR results of a nasopharyngeal swab test within two days from the study. Each subject received 20 flavor tablets (five different concentrations of four flavors: sour, sweet, salty and bitter). The subject decided on the order in which to take the tablets.
Each tablet had an identical volume of 0.33 mL and was made of 3.3% gelatin and 0.33% agar dissolved in distilled water. After adding flavor to the solutions, triangular tablets were formed. The tablets had the natural odor of the substances they were com-posed of and contained different flavoring substances in the following concentrations:
- sour (ascorbic acid): 3.125, 6.25, 12.5, 18.75, 25 mg/mL
- sweet (sucrose): 20, 40, 60, 80, 106.4 mg/mL
- salty (sodium chloride): 7.75, 13.5, 17, 27, 34.75 mg/mL
- bitter (grapefruit extract- 99% naringin): 20, 30, 40, 50, 60 mg/mL
2.2 Testing procedure
The subjects were instructed to rinse their mouths with water. After placing a flavor tablet in their mouth, they waited until it had dissolved and then named the perceived flavor. Each subject was asked to indicate the flavor and estimate its strength on a scale of 1 to 5. If the subject misidentified a flavor or did not identify it at all, the response was assigned a value of 0. The lowest and the highest flavor concentrations selected were the ones perceptible by 90% of subjects. For the lowest concentration, 23 of the 25 participants in the pilot study recognized the flavor correctly, while for the highest, 23 of the 25 participants in the pilot study did not mark the next concentration as higher than the previous one. During this pilot study, difficulties in identifying the intensity of the bitter flavor were observed; therefore, it was decided to reject the diagnostic values of various bitter flavor concentrations, leaving only the lowest perceptible concentration.
Finally, based on the criteria above, the following flavor tablets were selected along with the names assigned to each flavor test:
- Sour:
- Sour 1 – 6.25 mg/mL
- Sour 2 – 12.5 mg/mL
- Sweet:
- Sweet 1 – 40 mg/mL
- Sweet 2 – 80 mg/mL
- Sweet 3 – 106.4 mg/mL
- Salty:
- Salty 1 – 13.5 mg/mL
- Salty 2 – 17 mg/mL
- Salty 3 – 27 mg/mL
- Bitter:
- Bitter 1 – 40 mg/mL
2.3 Main study
Due to the early epidemiological situation, after detecting a SARS-CoV-2 corona-virus outbreak in the Main School of Fire Service in Warsaw, Poland, students living together in a dormitory were tested for SARS-CoV-2 using the RT-PCR test of a nasopharyngeal swab according to the WHO standards. Students with a positive test result were isolated in specially adapted hotel rooms. Students who tested negative were placed in individual rooms in a school dormitory. Students from both groups were placed under the supervision of the Central Clinical Hospital of the Ministry of the Interior and Administration in Warsaw, which had been transformed into an infectious diseases hospital.
The main study was conducted on 92 students who fulfilled the inclusion criteria (confirmed SARS-CoV-2 infection, aged over 18 years, and provided signed informed consent) and exclusion criteria (lack of informed consent, smell or taste disturbances lasting more than three months on the day of the test, any neurological disease including head trauma in the past, or any disease or factor that in the opinion of an investigator may have influenced the tests, such as chronic upper respiratory disease, including chronic rhino sinusitis, vision problems that would make it difficult to read the test instructions and the lack of knowledge of the Polish language in which the test instructions were written). None of the subjects was excluded based on the above criteria. Eighty-eight students completed the study. Of these students, 51 were in the study group (100% included in the study) and 37 were in the control group (90% included in the study; four study subjects did not return the questionnaire). The flow diagram of the stages of the study is shown in Figure 1.
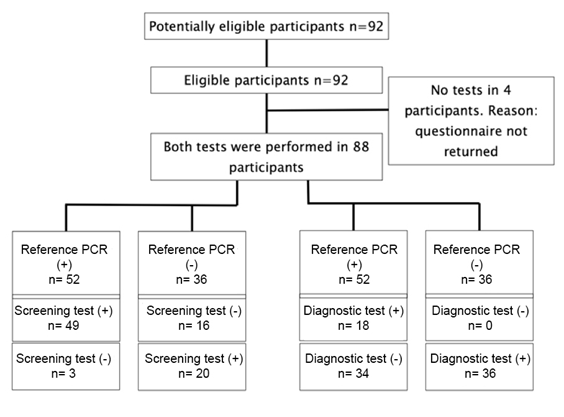
Figure 1: Study flow diagram and post-hoc derivative prediction models: screening test and diagnostic test results.
There were no inconclusive test results
- reference PCR (+) - sample positive - SARS- CoV-2 detected
- reference PCR (-) - sample negative - SARS- CoV-2 not detected
- screening/diagnostic test (+) positive - flavor test results suggest SARS-CoV-2 infection
- screening/diagnostic test (-) negative - flavor test results suggest no SARS CoV-2 infection
- screening test positive when questionnaire positive (occurrence of any of the symptoms described in the questionnaire: self-reported loss of taste, smell, or fever) or Sweet 1 flavor misidentified
- diagnostic test positive when any of the following flavors misidentified: Salty 1 or Salty 2 or Sweet 3
On the day of the examination, an additional swab was collected, and a PCR test performed on each student, both in the study and control groups to confirm SARS-CoV-2 infection. Therefore, at the time of the data collection, the researchers knew the results of the first PCR test but not the second. Only one person from the control group tested positive for SARS-CoV-2 after re-examination. This result was confirmed with a PCR test and the subject was transferred from the control group to the test group. Therefore, the final number of subjects in the control group was 36 and in the study, group was 52. RT PCR tests were conducted in the National Institute of Public Health - National Institute of Hygiene (NIPH-NIH), which is the main governmental public health research institute and the reference center for the national network of sanitary epidemiological service [38].
The GeneFinder COVID-19 PLUS RealAmp Kit was used, detecting RdRp, E and N genes with a sensitivity of 10 copies of these genes, and cut-off point of ≤ 43 cycles for a positive result. The assessors of the PCR (reference) tests were not aware of any clinical information regarding the subjects and the flavor test results. The study was approved by the Ethics Commission for the Central Clinical Hospital of the Ministry of the Interior and Administration in Warsaw (decision no. 82/2020). No external funding or support was available, and the researchers were entirely inde-pendent. The publication was prepared according to the Standards for Reporting of Di-agnostic Accuracy Studies (STARD 2015) [39].
A full gustatory function assessment with four flavors was performed in all subjects. The flavor tablets were prepared in a manner analogous to the pilot study. Each tablet was placed in a separate package with a corresponding sample number. Each subject received a set containing a collecting bag with 10 tablet packages: one tasteless reference and nine flavor tables, and a one-page study instruction with an answer sheet. Neither the subjects nor the researchers knew the flavor of the individual samples. The subjects were instructed to rinse their mouths with water and to begin testing the flavors with a tasteless sample that was clearly marked with a number and subsequently proceed to the flavor samples in the order that the tablet packages were taken out of the collecting bag. The subjects were instructed to wait until the tablet dissolved in their mouth, then to name and write down the perceived flavor on the answer sheet.
2.4. Statistical analysis
Statistical analyses were conducted with PQStat version 1.8.0.392 and IBM SPSS Statistic version 21. As the entire accessible student population was tested, no prior sample calculation was performed. There were no undetermined results or missing values. The descriptive statistics for the quantitative variables are given as the mean ± SD. The statistical analysis of different taste dysfunctions and questionnaire items between the subgroups was performed using the Fisher's exact two-tailed test. The level of statistical significance was set at P ≤ .05 with a 95% confidence interval. The diagnostic test confidence interval (sensitivity, specificity, predictive values, and accuracy) was calculated at the 95% confidence intervals using the Clopper–Pearson method for a single proportion. Area under the ROC Curve (AUC) analysis was per-formed to assess the ability of prediction models to distinguish SARS-CoV-2 positive subjects from SARS-CoV-2 negative subjects. AUC-values were considered as follows: excellent 1.00–0.90, good 0.80–0.90, moderate 0.70–0.80, poor 0.60–0.70, and fail <0.60 [40]. In addition to the AUC, the corresponding P-value indicates if the AUC is significantly larger than an AUC of 0.5.
3. Results
The characteristics of COVID-19 patients and controls are listed in Table 1. The groups did not differ in terms of age, height, or weight. Almost all subjects were male. There were no significant differences concerning the percentage of cigarette smokers or electronic cigarette smokers between both groups. The median time between the first positive SARS-CoV-2 PCR test result and the study was five days. From the symptoms characteristic of COVID-19, listed in Table 1, only fever was significantly more frequent in the SARS-CoV-2 positive group than self-reported smell and taste disorders. In both groups, rhinitis and/or sore throat were relatively frequent.
Subjective smell or taste disturbances were reported by 65% of subjects in the test group compared with 8% in the control group. A numerical difference was demonstrated for all flavor tests recognitions between COVID-19 patients and the control group; however, the difference was only significant with the Sweet 1 (p < 0.004) and Salty 2 (p < 0.009) flavor tablets. Complete ageusia was very uncommon in our study group and occurred in only 1 out of 52 subjects (see Supplementary Materials for participants’ responses). Detailed data is shown in Table 2.
|
Parameter |
COV group (n = 52). Mean (min; max) value |
Controls (n = 36). Mean (min; max) value |
|
Age [years] |
21.7 (19; 26) |
20.8 (19; 24) |
|
Male sex [n] |
51 |
34 |
|
Height [cm] |
181 (170; 198) |
181 (170; 197) |
|
Weight [kg] |
78.5 (60; 112) |
78.5 (62; 93) |
|
Time from the first positive PCR [days] |
5 (1; 8) |
NA |
|
Symptoms [last 30 days]: Fever [n] Cough [n] Shortness of breath [n] Rhinitis and/or sore throat |
21* 15 8 22 |
2 5 1 13 |
|
Current smokers (cigarettes) |
4 |
7 |
|
Current smokers (electronic cigarettes) |
3 |
1 |
|
Self-reported smell disorders |
25* |
3 |
|
Self-reported taste disorders |
32* |
2 |
*p<0.05 denotes significance.
Table 1: Basal characteristics of subjects with SARS-CoV-2 infection (COV) and controls.
The diagnostic values of the questionnaire and selected taste tests are shown in Table 3. The questionnaire, being a self-reporting survey, consisted of thirteen questions including demographics, smoking habits, symptoms experienced during illness (listed in Table 1), and subjective rating of smell and taste. Participants were asked to rate their subjective perception of smell and taste acuity before and during COVID-19, using a numerical scale ranging from 0 (no sense of smell or taste) to 10 (excellent sense of smell or taste). Smell or taste disorder was considered to be present if participants rated it at least 1 point lower than at pre-illness levels. It was regarded as positive when at least one statistically significant symptom (self-reported loss of taste, smell, or fever) was present within the previous 30 days. The primary models included a questionnaire and selected taste tester with the highest sensitivity (Sweet 1) and specificity (Salty 1, Salty 2, Sweet 3) in COVID-19 diagnostics. Derivative models consisted of two or more primary prediction models.
|
Tested variable |
Indication |
COV group (n) |
Controls (n) |
p |
|
Sweet 1 (40 mg/mL) |
Correct |
15 |
22 |
0.004* |
|
Incorrect |
37 |
14 |
||
|
Sweet 2 (80 mg/mL) |
Correct |
43 |
32 |
0.546 |
|
Incorrect |
9 |
4 |
||
|
Sweet 3 (106.4 mg/mL) |
Correct |
46 |
36 |
0.077 |
|
Incorrect |
6 |
0 |
||
|
Salty 1 (13.5 mg/mL) |
Correct |
46 |
36 |
0.077 |
|
Incorrect |
6 |
0 |
||
|
Salty 2 (17 mg/mL) |
Correct |
43 |
36 |
0.009* |
|
Incorrect |
9 |
0 |
||
|
Salty 3 (27 mg/mL) |
Correct |
50 |
35 |
1 |
|
Incorrect |
2 |
1 |
||
|
Sour 1 (6.25 mg/mL) |
Correct |
26 |
24 |
0.133 |
|
Incorrect |
26 |
12 |
||
|
Sour 2 (12.5 mg/mL) |
Correct |
44 |
34 |
0.189 |
|
Incorrect |
8 |
2 |
||
|
Bitter 1 (40 mg/mL) |
Correct |
42 |
33 |
0.225 |
|
Incorrect |
10 |
3 |
*p<0.05 denotes significance.
Table 2: Gustatory disorders in subjects with SARS-CoV-2 infection (COV) and controls.
|
Prediction Model |
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy |
ROC-AUC |
p* |
|
Primary Models |
|||||||
|
Questionnaire |
0.77 (0.63– 0.87) |
0.86 (0.70– 0.95) |
0.89 (0.76– 0.96) |
0.72 (0.42– 0.75) |
0.81 (0.70-0.88) |
0.82 (0.72-0.91) |
<0.001* |
|
Sweet 1 |
0.71 (0.57–0.83) |
0.61 (0.43– 0.77) |
0.73 (0.58– 0.84) |
0.59 (0.42–0.75) |
0.67 (0.56-0.77) |
0.67 (0.56-0.78) |
0.003* |
|
Salty 1 |
0.12 (0.04–0.23) |
1 (0.9–1) |
1 (0.54–1) |
0.44 (0.33–0.55) |
0.48 (0.37-0.59) |
0.56 (0.44-0.68) |
0.359 |
|
Salty 2 |
0.17 (0.8–0.3) |
1 (0.9–1) |
1 (0.66–1) |
0.46 (0.34–0.57) |
0.51 (0.40-0.62) |
0.59 (0.47-0.70) |
0.169 |
|
Sweet 3 |
0.12 (0.04–0.23) |
1 (0.9–1) |
1 (0.54–1) |
0.44 (0.33–0.55) |
0.48 (0.40-0.59) |
0.56 (0.44-0.68) |
0.359 |
|
Derivative Models |
|||||||
|
Screening test |
0.94 (0.84– 0.99) |
0.55 (0.38– 0.72) |
0.75 (0.63– 0.85) |
0.87 (0.66– 0.97) |
0.78 (0.68-0.86) |
0.75 (0.64-0.86) |
<0.001* |
|
Diagnostic test |
0.34 (0.22– 0.49) |
1 (0.90– 1) |
1 (0.81–1) |
0.51 (0.39– 0.64) |
0.61 (0.50-0.72) |
0.67 (0.56-0.78) |
0.006* |
*p<0.05 indicates that the AUC is significantly larger than an AUC of 0.5.
PPV = positive predictive value; NPV = negative predictive value; ROC-AUC = the area under a receiver operating characteristic (ROC) curve.
Table 3: Prediction models performance for sample size n = 88.
A primary model with the highest sensitivity (77%) was the medical questionnaire - the subjective assessment of a subject’s symptoms. An objective flavor test with the highest sensitivity (71%) was the Sweet 1 flavor tablet test. The variables assessed in the study were selected and matched to two groups, with the highest sensitivity (a screening test) and highest specificity (a diagnostic test) to create derivative models. The screening test had 94% sensitivity and 55% specificity, while the diagnostic test had only 34% sensitivity and 100% specificity.
From different constructed derivative prediction models, two are shown in Table 3. Receiver Operational Characteristics (ROC) curves were constructed and presented in Figure 2 to evaluate the performance of the predictor models after selecting appropriate symptoms and taste testers. Only one predictive model – the medical questionnaire – displayed good performance (AUC = 0.82). Although the Sweet 1 taste tester was the most sensitive of all the taste tests, the performance of this model was poor (AUC = 0.67). In order to objectify the taste test, the combination of the Sweet 1 tester with the Questionnaire in Screening Test, resulted in moderate performance (AUC = 0.75). A diagnostic test created by combining the Salty 1, Salty 2 and Sweet 3 flavor testers showed poor performance (AUC = 0.67) in COVID-19 diagnostics, thus, its use seems to be unjustified.
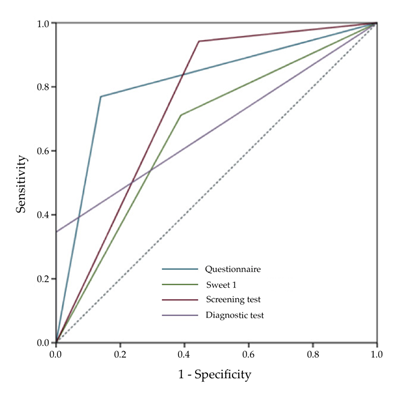
Figure 2: ROC curve plots for SARS-CoV-2 infection prognostic models. AUC for each prognostic model is displayed in Table 3. AUC = the area under a receiver operating characteristic (ROC) curve.
4. Discussion
This study confirms and demonstrates that chemo-sensory taste impairment can be regarded as a symptom of COVID-19. In the present study, almost 70% of young, otherwise healthy SARS COV 2 positive patients reported a smell disorder and more than 60% of them reported a taste disorder. These figures are about 10 times higher than the matched control group, in which over 8% and almost 6% of the subjects reported smell and taste disorders, respectively. It was shown that taste disturbances that were significantly more frequently recorded in SARS-CoV-2 positive subjects were sweet at the lowest flavor concentration (40 mg/mL) and salty at the medium flavor concentration (17 mg/mL). These findings are consistent with other reports showing that sweet [35-37, 41] and salty [32, 33] tastes perception may be particularly disturbed in COVID-19 patients. Sweet and salty tastes perception represent two different types of chemosensory function. Sweet taste perception, together with bitter and umami, is associated with G protein-coupled receptor (GPCR) [42]. On the other hand, the salty and sour taste transduction is ion channels dependent - Na+ for salty and H+ for sour [43]. The disturbance of both types of taste cells in the present work, as well as in the previous studies [32-37], indicates that the SARS-CoV-2 virus is either unspecific to the taste receptor type or that its activity is upstream of these receptors.
Taste and smell disturbances have been recognized as symptoms and listed as key diagnostic factors for COVID-19 [44-46]. WHO added anosmia and dysgeusia as symp-toms of COVID-19 in April 2020 [47]. Remarkably, research indicates that disturbances in smell and taste are some of the first symptoms that occur more frequently with SARS-CoV-2 asymptomatic infection [48]. Following this discovery, some institutions began to use questionnaires identifying loss of smell and taste as a screening for their visitors [49].
The lowest concentration of sucrose (Sweet 1 tablet) had 71% incorrect identifica-tions in the COVID-19 subjects versus 39% in controls. The percentage of misidentifica-tion of this flavor among subjects in the control group was relatively high, considering the fact that this concentration was perceptible to more than 90% of healthy subjects in the pilot study. One of the reasons for this may be the relatively high percentage of subjects with rhinitis and sore throat in the control group (36%), which is known to increase flavor recognition threshold [50, 51]. It is also worth noting that this study was conducted in May 2020, which was the month of the peak of POVD (post-viral olfactory dysfunction) incidence [52]. However, it should be considered that if flavor identification is used as a part of a screening test, then the examined population will probably have similar frequency of rhinitis or influenza-like signs and symptoms as the control group.
Data regarding that issue in the literature is scarce. The study conducted on an American population by Yan et al. found self-reported taste disturbances among 71% of SARS-CoV-2 patients and 17% of non-infected subjects [13]. As with this study, many of the control subjects presented with influenza-like symptoms, including rhinitis and sore throat. These results seem to be comparable, as in the control group only 8% of patients reported subjective taste disturbances. However, almost 39% of the control group mis-takenly identified a low-intensity sweet flavor. In this case, gentle dysgeusia can be caused by olfactory loss from nasal obstruction, not from gustatory loss. This mechanism may overlap with the postulated mechanisms of taste disturbance in COVID-19-related impairment of the renin–angiotensin system [53].
Other researchers have identified a relatively similar frequency of taste disorders in COVID-19 patients. The highest prevalence of self-reported gustatory disorders identi-fied was 88% in a multicentre European study [12]. This figure is higher than the figure reported in the present research and may be due to the fact that the population described by Lechien et al. also included older subjects, up to 77 years of age, with concomitant diseases that could also affect taste and cause taste-threshold disturbances [12]. No control group was included in the study by Lechien et al. Interestingly, the prevalence of dysgeusia reported for the European and American populations appears to be much higher than in the Chinese SARS-CoV-2-infected patients (5.6%) as reported by Mao et al. [54], and Korean patients (15.3%) as reported by Lee et al., [55].
One study that objectively assessed gustatory dysfunction in COVID-19 patients used only single concentrations of particular flavors [36]. The patients in this study were considered to have ageusia if they were not able to identify any of the following four flavors: sweet, salty, sour or bitter. The frequency of ageusia according to this definition was 1.4% of study subjects, while 51.4% accurately identified all flavors. No control group was included in this study; in light of this, and due to differences in flavor preparation and concentrations, it is difficult to compare the results with those of the present study.
In the present study, the variable with the highest sensitivity in detecting SARS CoV 2 infection was the medical questionnaire. The medical questionnaire alone was also the predictive model with the best performance (ROC-AUC=0.82). This shows that the medical history itself can be used for detecting infected virus carriers, even though self-reported surveys demonstrate lower sensitivity in detecting taste disturb-ances in COVID-19 than the validated surveys [33]. A significant advantage of the questionnaire over the taste testers is the cost reduction for conducting the test – there is no need to prepare expensive flavor samples. The time of completing the questionnaire is also faster than that of the taste tests. To further facilitate the conducting of the questionnaire, it is possible to complete it in electronic form, for example, in public places such as airports with the benefit of having data immediately available in an electronic format.
The objective test with the highest sensitivity (71%) was the Sweet 1 test, although its performance as a stand alone test, measured using ROC-AUC tool is poor (ROC AUC = 0.67). After combining these both tests into a derivative model (the screening test), a moderate performance was achieved (ROC-AUC = 0.75) with a higher sensitivity (94%) than for each model separately. These results are similar to those found by Huart et al., i.e. 100% sensitivity of the sweet flavor test and 60% specificity in COVID-19 diagnosis [37].
It is important to find objective screening SARS-CoV-2 methods, because in subjective questionnaires, some people provide false information or fail to recognize symptoms of the disease. According to a study by Gostic et al., [56], only 5 to 25% of travelers report contact with the pathogen during inspection. According to another study by Gostic et al., screening questionnaires may miss more than 50% of COVID-19 cases [57]. The proposed screening model could theoretically be used in situations where an objective screening test for COVID-19 is required. However, it should be remembered that the combination of an objective taste test with a questionnaire reduces the performance of the entire test.
By combining three flavors that were only misidentified by COVID-19 patients: Salty 1, Salty 2 and Sweet 3, authors created another derivative model – the diagnostic test. Although this test demonstrated 100% specificity and 100% positive predictive value in diagnosing COVID-19, its performance measured using ROC-AUC tool was poor (ROC-AUC = 0.67). Therefore, despite the high specificity in the diagnosis of COVID-19, the authors believe that this test will not be of clinical utility.
Admittedly, this study has some limitations. The proposed screening models do not distinguish the basis of taste disturbances, so in the case of these disturbances resulting from infection with viruses other than SARS-CoV-2, these models will give a false positive result. Since this study was conducted on a population constituted of more than 60% of positive patients, the overall ROC-AUC values may be over-estimated. This implies the need for validation in a bigger population, which is planned in the future. Moreover, the taste tests require mental and physical cooperation in order to maintain a high performance, thus it will not be suitable for some children and elderly people. Further studies are needed to establish the exact effectiveness of the proposed test in these groups.
The fact that the majority of subjects in the examined and control groups in this study were men can also be regarded as a limitation. However, studies indicate that the frequency of smell and taste disturbances in COVID-19 is more common in women than in men [58, 59].
Another limitation of this study is the lack of an assessment of olfactory disorders. The use of odor tests in conjunction with flavor tests could increase the precision of the tests, given that the authors indicate that smell disorders are a better predictor of COVID 19 than flavor disturbances [30, 60].
5. Conclusions
Although vaccination for SARS-CoV-2 has already begun, the only method that can currently control the pandemic is still to screen the wider population for symptomatic, oligosymptomatic and asymptomatic SARS-CoV-2 carriers, isolate infected individuals and initiate early treatment of patients who develop symptoms of COVID-19. This re-quires an inexpensive, simple, fast, and sensitive screening method. The conducted experiment allowed to objectively assess the influence of the SARS CoV 2 virus on the perception of tastes in patients in the early post-infection pe-riod. Among all tested flavors, the sweet and salty flavors perception was impaired in COVID 19 patients.
Despite the objective taste tests being carried out, the medical questionnaire was the predictive model with the best performance, higher than any single taste test or multiple tastes test. The combination of the most sensitive objective taste test (Sweet 1) with a medical questionnaire (the screening test) objectified the test and increased its sensitivity, but on the other hand, decreased its performance from good to moderate.
For this reason, the authors recommend using this test only in selected clinical situations, when it is necessary to objectify the COVID-19 screening. The authors believe that a simple medical questionnaire consisting of questions regarding self-reported loss of taste, smell, or fever should be used widely to screen for SARS-CoV 2 infection.
6. Patents
Kamil Adamczyk, Michal Herman, Janusz Fraczek, Edward Franek and Artur Zaczynski: pending patent for a method of screening for increased risk of SARS-COV-2 infection, a diagnostic test kit, and implementing this method. Polish Patent Office No B.434028, Kamil Adamczyk, Michal Herman, Janusz Fraczek, and Artur Zaczynski: “Pending patent for system and method of detecting taste disturbances among the population with a viral infection, in particular, SARS-CoV-2 infection". Polish Patent Office submission number P.433465.
Supplementary Materials
Raw data with responses from individual study participants are available on request from the corresponding author.
Author Contributions
Conceptualization, K.A, E.F. and M.H; methodology, J.F , R.W. and Z.K.; resources, R.P., B.S., K.A.; data curation, R.P.; writing—original draft preparation, K.A., E.F., E.R, K.B. and A.Z.; writing—review and editing, E.F, W.W, Z.K and R.W.; supervision, E.F. and A.Z.; project administration, K.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Central Clinical Hospital of the Ministry of the Interior and Administration in Warsaw (decision no. 82/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data on the basis of which the statistical analysis was per-formed was attached to the work in the form of a supplement.
Acknowledgments
None.
Conflicts of Interest
Kamil Adamczyk, Michal Herman, Janusz Fraczek, Edward Franek and Ar-tur Zaczynski: pending patent for a method of screening for increased risk of SARS-COV-2 infec-tion, a diagnostic test kit, and implementing this method. Polish Patent Office No B.434028, Kamil Adamczyk, Michal Herman, Janusz Fraczek, and Artur Zaczynski: “Pending patent for system and method for detecting taste disturbances among the population with a viral infection, in particular, SARS-CoV-2 infection". Polish Patent Office submission number P.433465. Other Authors declare no conflict of interest.
References
- Helmy YA, Fawzy M, Elaswad A, et al. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. Journal of Clinical Medicine 9 (2020).
- Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. Journal of Medical Virology 92 (2020): 797-806.
- Xu G, Yang Y, Du Y, et al. Clinical Pathway for Early Diagnosis of COVID-19: Updates from Experience to Evidence-Based Practice. Clinical Reviews in Allergy and Immunology 59 (2020): 89-100.
- Bohmwald K, Gálvez NMS, Ríos M, et al. Neurologic Alterations Due to Respiratory Virus Infections. Frontiers in Cellular Neuroscience 12 (2018).
- Laurendon T, Radulesco T, Mugnier J, et al. Bilateral transient olfactory bulb edema during COVID-19–related anosmia. Neurology 95 (2020): 224-225.
- Politi LS, Salsano E, Grimaldi M. Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurology 77 (2020).
- Brann DH, Tsukahara T, Weinreb C, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Science Advances 6 (2020).
- Just J, Puth M-T, Regenold F, et al. Risk factors for a positive SARS-CoV-2 PCR in patients with common cold symptoms in a primary care setting – a retrospective analysis based on a joint documentation standard. BMC Family Practice 21 (2020).
- Kaye R, Chang CWD, Kazahaya K, et al. COVID-19 Anosmia Reporting Tool: Initial Findings. Otolaryngology–Head and Neck Surgery 163 (2020): 132-134.
- Klein H, Asseo K, Karni N, et al. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clinical Microbiology and Infection 27 (2021): 769-774.
- Moein ST, Hashemian SM, Mansourafshar B, et al. Smell dysfunction: a biomarker for COVID-19. International Forum of Allergy and Rhinology 10 (2020): 944-950.
- Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. European Archives of Oto-Rhino-Laryngology 277 (2020): 2251-2261.
- Yan CH, Faraji F, Prajapati DP, et al. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. International Forum of Allergy and Rhinology 10 (2020): 806-813.
- Gautier JF, Ravussin Y. A New Symptom of COVID-19: Loss of Taste and Smell. Obesity 28 (2020): 848..
- Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, et al. Smell and taste dysfunction in patients with COVID-19. The Lancet Infectious Diseases 20 (2020): 1015-1016.
- Giacomelli A, Pezzati L, Conti F, et al. Self-reported Olfactory and Taste Disorders in Patients With Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clinical Infectious Diseases 71 (2020): 889-890.
- Tong JY, Wong A, Zhu D, et al. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg 163 (2020): 3-11.
- Tsai ST, Lu MK, San S, et al. The Neurologic Manifestations of Coronavirus Disease 2019 Pandemic: A Systemic Review. Front Neurol 11 (2020): 498.
- von Bartheld CS, Hagen MM, Butowt R. Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis Reveals Significant Ethnic Differences. ACS Chem Neurosci 11 (2020): 2944-2961.
- Saniasiaya J, Islam MA, Abdullah B. Prevalence and Characteristics of Taste Disorders in Cases of COVID-19: A Meta-analysis of 29,349 Patients. Otolaryngol Head Neck Surg (2020): 194599820981018.
- Mair M, Singhavi H, Pai A, et al. A Meta-Analysis of 67 Studies with Presenting Symptoms and Laboratory Tests of COVID-19 Patients. Laryngoscope (2020).
- Kim JW, Han SC, Jo HD, et al. Regional and Chronological Variation of Chemosensory Dysfunction in COVID-19: a Meta-Analysis. J Korean Med Sci 36 (2021): e40.
- Ibekwe TS, Fasunla AJ, Orimadegun AE. Systematic Review and Meta-analysis of Smell and Taste Disorders in COVID-19. OTO Open 4 (2020): 2473974X20957975.
- Hajikhani B, Calcagno T, Nasiri MJ, et al. Olfactory and gustatory dysfunction in COVID-19 patients: A meta-analysis study. Physiol Rep 8 (2020): e14578.
- Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral Manifestations in Patients with COVID-19: A Living Systematic Review. J Dent Res 100 (2021): 141-154.
- Abdullahi A, Candan SA, Abba MA, et al. Neurological and Musculoskeletal Features of COVID-19: A Systematic Review and Meta-Analysis. Front Neurol 11 (2020): 687.
- Agyeman AA, Chin KL, Landersdorfer CB, et al. Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis. Mayo Clin Proc 95 (2020): 1621-1631.
- Borsetto D, Hopkins C, Philips V, et al. Self-reported alteration of sense of smell or taste in patients with COVID-19: a systematic review and meta-analysis on 3563 patients. Rhinology 58 (2020): 430-436.
- Hannum ME, Ramirez VA, Lipson SJ, et al. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19–positive patients compared to subjective methods: A systematic review and meta-analysis. Chemical Senses (2020).
- Gerkin RC, Ohla K, Veldhuizen MG, et al. Recent Smell Loss Is the Best Predictor of COVID-19 Among Individuals With Recent Respiratory Symptoms. Chemical Senses 46 (2021).
- Santos REA, da Silva MG, do Monte Silva MCB, et al. Onset and duration of symptoms of loss of smell/taste in patients with COVID-19: A systematic review. American Journal of Otolaryngology 42 (2021).
- Salmon Ceron D, Bartier S, Hautefort C, et al. Self-reported loss of smell without nasal obstruction to identify COVID-19. The multicenter Coranosmia cohort study. Journal of Infection 81 (2020): 614-620.
- Parma V, Ohla K, Veldhuizen MG, et al. More Than Smell-COVID-19 Is Associated With Severe Impairment of Smell, Taste, and Chemesthesis. Chemical Senses 45 (2020): 609-622.
- Konstantinidis I, Delides A, Tsakiropoulou E, et al. Short-Term Follow-Up of Self-Isolated COVID-19 Patients with Smell and Taste Dysfunction in Greece: Two Phenotypes of Recovery. Orl 82 (2020): 295-303.
- Biadsee A, Biadsee A, Kassem F, et al. Olfactory and Oral Manifestations of COVID-19: Sex-Related Symptoms-A Potential Pathway to Early Diagnosis. Otolaryngology–Head and Neck Surgery (2020).
- Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID -19 patients: Single-center experience on 72 cases. Head and Neck 42 (2020): 1252-1258.
- Huart C, Philpott C, Konstantinidis I, et al. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology 58 (2020).
- EuroHealthNet | National Institute of Public Health - National Institute of Hygiene (2020).
- Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Bmj (2015).
- Metz CE. Basic principles of ROC analysis. Seminars in Nuclear Medicine 8 (1978): 283-298.
- Judkis M. What it's like to suffer from the coronavirus's weirdest symptom: Washington Post (2020).
- DuBois GE. Unraveling the biochemistry of sweet and umami tastes. Proceedings of the National Academy of Sciences 101 (2004): 13972-13973.
- Bachmanov AA, Bosak NP, Lin C, et al. Genetics of taste receptors. Curr Pharm Des 20 (2014): 2669-83.
- Center for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) - Symptoms (2020).
- Lost or changed sense of smell (2020).
- BMJ Best Practices. Coronavirus disease 2019 (COVID-19) - Symptoms, diagnosis and treatment (2020).
- Menni C, Sudre CH, Steves CJ, et al. Quantifying additional COVID-19 symptoms will save lives. The Lancet 395 (2020): e107-e108.
- Spadera L, Viola P, Pisani D, et al. Sudden olfactory loss as an early marker of COVID-19: a nationwide Italian survey. European Archives of Oto-Rhino-Laryngology (2020).
- Vazquez J. San Diego: UC Health - UC San Diego. Loss of smell and taste validated as COVID-19 symptoms (2020).
- Rydzewski B, Pruszewicz A. Assessment of Smell and Taste in Patients with Allergic Rhinitis. Acta Oto-Laryngologica 120 (2009): 323-326.
- Bozkurt G, Elhassan HA, Sözen E, et al. Assessment of taste functions in allergic rhinitis patients undergoing allergen-specific immunotherapy. European Archives of Oto-Rhino-Laryngology 276 ( 2018): 439-445.
- Konstantinidis I, Haehner A, Frasnelli J, et al. Post-infectious olfactory dysfunction exhibits a seasonal pattern. Rhinology 44 (2006): 135-139.
- Bigiani A. Gustatory dysfunctions in COVID-19 patients: possible involvement of taste renin-angiotensin system (RAS). European Archives of Oto-Rhino-Laryngology 277 (2020): 2395.
- Mao L, Wang M, Chen S, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study. Medrxiv- the preprint server for Health Sciences (2020).
- Lee Y, Min P, Lee S, et al. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. Journal of Korean Medical Science 35 (2020).
- Gostic KM, Kucharski AJ, Lloyd-Smith JO. Effectiveness of traveller screening for emerging pathogens is shaped by epidemiology and natural history of infection. eLife 4 (2015).
- Gostic K, Gomez ACR, Mummah RO, et al. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. eLife 9 (2020).
- Nowak B, Szymanski P, Pankowski I, et al. Clinical characteristics and short-term outcomes of coronavirus disease 2019: retrospective, single-center experience of designated hospital in Poland. Polish Archives of Internal Medicine (2020).
- Cocco A, Amami P, Desai A, et al. Neurological features in SARS-CoV-2-infected patients with smell and taste disorder. Journal of Neurology 268 (2020): 1570-1572.
- Rocke J, Hopkins C, Philpott C, et al. Is loss of sense of smell a diagnostic marker in COVID-19: A systematic review and meta-analysis. Clin Otolaryngol 45 (2020): 914-922.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 