Heterologous Prime-boost Immunisation with AdC68 and mRNA-based COVID-19 Vaccine Induced Strong and Durable Immune Responses in Mice
Miao Li1, †, Leitai Shi1, †, Hongshan Xu1, †, Shouchun Cao1, †, Yunpeng Wang1, †, Xingxing Li1, Wenjuan Li1, Qinghua Peng1, Enyue Fang1, 2, Jingjing Liu1, Xiaohong Wu1, Jia Li1, Danhua Zhao1, Lihong Yang1, Yanqiu Huang1, Ren Yang1, Yue Suo1, Hongyu Wang1, Guangzhi Yue1, Min Li3, *, Xinyu Liu1, *, Qiang Ye1, *, Yuhua Li1, *
Affiliation:
1Department of Arboviral Vaccine, National Institutes for Food and Drug Control, Beijing, China. 2Institute of Health Inspection and Quarantine,
Chinese Academy of Inspection and Quarantine,
Beijing, China
3Office of pharmaceutical science of biological products, Center for drug evaluation, NMPA, Beijing, China
†These authors contributed equally to this work.
*Corresponding author:
Min Li, Office of Pharmaceutical Science of Biological products, Center for Drug Evaluation, NMPA, Beijing, China
Xinyu Liu, Qiang Ye, Yuhua Li, Department of Arboviral Vaccine, National Institutes for Food and Drug Control, Beijing, China.
Induced Strong and Durable Immune Responses in Mice. Archives of Microbiology and Immunology. 7 (2023): 476-486.
Received: November 23, 2023; Accepted: December 04, 2023; Published: December 18, 2023
Article Information
Citation: Miao Li, Leitai Shi, Shouchun Cao, Yunpeng Wang, Xingxing Li, Wenjuan Li, Qinghua Peng, Enyue Fang, Jingjing Liu, Xiaohong Wu, Jia Li, Danhua Zhao, Lihong Yang, Hongshan Xu, Yanqiu Huang, Ren Yang, Yue Suo, Hongyu Wang, Min Li, Xinyu Liu, Qiang Ye, Yuhua Li. Heterologous Prime-boost Immunisation with AdC68 and mRNA-based COVID-19 Vaccine Induced Strong and Durable Immune Responses in Mice. Archives of Microbiology and Immunology. 7 (2023): 476-486.
View / Download Pdf Share at FacebookAbstract
Immune escape events caused by the emergence of new variants of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) continue to increase. The heterologous prime-boost immunisation strategy can improve the efficacy of vaccine protection. However, the persistent immune response after vaccination has rarely been investigated. In this study, we evaluated the immunogenicity of heterologous prime-boost protocols using the AdC68-based vaccine ChAdTS-S (Ad) and mRNA-based vaccine ARCoV (AR). Different groups of 4-week-old BALB/c mice were administered heterologous prime-boost or homologous prime-boost of Ad and AR, along with blank control phosphate-buffered saline vaccinations. We evaluated the immune response trend in these mice from 8 to 57 weeks after prime immunisation. Intranasal priming with Ad followed by an intramuscular booster with AR induced strong and durable humoral and local mucosal immune responses in all vaccination groups. The groups exhibited high IgG, IgA, and pseudovirus neutralising antibody titres and ACE2 binding inhibition from weeks 8 to 57 after prime vaccination. All Ad and AR vaccination groups showed a Th1-skewing cellular immune response. In conclusion, this study provides data to support coronavirus disease 2019 vaccination strategies and the existence of persistent immunity.
Keywords
<p>ChAdTS-S; ARCoV; heterologous prime-boost; durable protection; SARS-CoV-2</p>
Article Details
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, that started in 2019, continues to spread and has caused over 757 million infections and 6.85 million deaths as of 20 February 2023 [1]. COVID-19 is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a single positive-stranded RNA virus which belongs to the beta-coronavirus genus of the Coronaviridae family [2]. According to the World Health Organization, 171 COVID-19 vaccines are currently in preclinical studies. Some vaccines, such as BNT162b2, mRNA-1273, and AZD1222, have been approved for marketing in select countries, playing a key role in preventing virus transmission. However, with the emergence of multiple variants, previous data have shown that the protection provided by currently marketed vaccines against variant strains has decreased to varying degrees [3-5]. For example, unlike the first discovered strain, protection rates against which were > 90%, the efficacy of vaccines against the Omicron strain is below 50% [6].
Addressing the immune escape phenomenon and improving the immunogenicity of COVID-19 vaccines are urgent and imperative.
Our previous research showed that intranasal administration of the AdC68-based vaccine ChAdTS-S (Ad) followed by an intramuscular injection of the mRNA vaccine ARCoV (AR) induced a higher humoral, mucosal, and cellular immune response in BALB/c mice than homologous vaccination with Ad or AR [7]. However, few studies have reported the persistent response to heterologous sequential immunisation and the level of cross-reactive antibodies against variants. Meanwhile, this information is critical for dealing with constantly evolving new variants. Long-term sustenance of high levels of neutralising antibody (NAb) is important for persistent vaccines protection and reduction of vaccination doses. The mucosal immune response induced by intranasal immunisation with Ad is indispensable in preventing respiratory virus infection [8, 9]. High secretory IgA (SIgA) levels in the mucosal immune response can significantly enhance neutralisation efficacy [10]. Hassan [11] found that a single intranasal injection of the chimpanzee adenovirus vector COVID-19 vaccine (ChAd-SARS-CoV- 2-S) induced higher, broader, and longer-lasting immunity in mice than an intramuscular injection. This improvement in immunity manifested as high levels of IgG, IgA, and neutralising antibody responses. In addition, intranasal vaccination almost completely protected the upper and lower respiratory tracts against multiple new coronavirus variants from six weeks to nine months after primary vaccination [12]. Therefore, improving the level of vaccine-induced mucosal immune response may play a key role in preventing the emergence of multiple novel coronavirus variants [13]. In this study, we aimed to provide a basis for optimising COVID-19 vaccination strategies by exploring the persistence of the systemic and local mucosal immune responses induced by the combination of intranasal and intramuscular injections and heterologous inoculation with Ad and AR COVID-19 vaccines in mice. In conclusion, this study provides data support for the persistence of the cross-immune response.
Methods
Animals and Vaccines
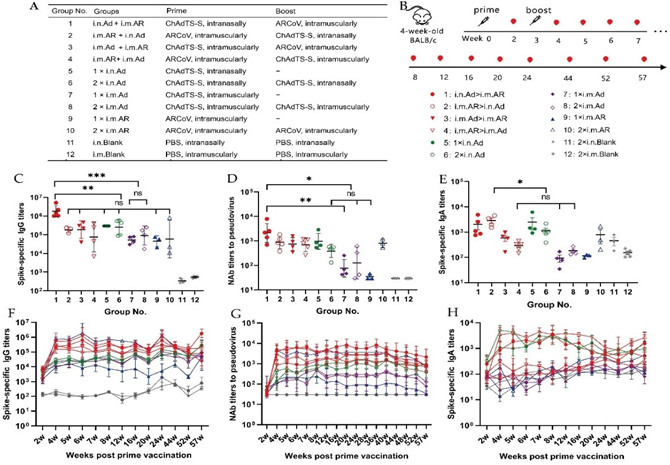
All animal experiments were approved by the Experimental Animal Welfare and Ethics Committee of the Chinese National Institutes for Food and Drug Control (NIFDC) and were conducted in conformance with the guidelines of this committee. 6-week-old, pathogen-free female BALB/c mice were provided and housed by the Animal Experimentation Centre of NIFDC. The chimpanzee adenovirus vector SARS-CoV-2 vaccine ChAdTS-S (5 × 1010 vp/0.5 mL; Walvax, Yunnan, China), which encodes the Wuhan-Hu-1 strain spike (S) protein [14], designated Ad; and the mRNA vaccine ARCoV (15 µg/0.5 mL; Abogen, Suzhou, China), which encodes the Wuhan-Hu-1 strain S RBD [15], designated AR were evaluated in this study. The mice were randomly assigned to groups 1~4, 5~10, and 11~12 (n = 3~5 per group) that received heterologous prime-boost, single dose or homologous prime-boost, and phosphate-buffered saline (PBS, blank control) vaccinations, respectively (Figure 1A and B). Each vaccination consisted of 5 × 109 vp of Ad or 6 μg of AR, initial vaccination was administered on day 0, and booster vaccination was administered at 21 days after prime.
Enzyme-linked Immunosorbent Assay (ELISA)
SARS-CoV-2 S protein-specific IgG and IgA titres were measured using ELISA. 96-well ELISA plates (Corning, NY, USA) were coated overnight with 0.2 μg SARS- CoV-2 S protein (Sino Biological, Beijing, China), washed with PBST (PBS containing 0.05% Tween 20) and blocked with PBST containing 1% bovine serum albumin at 37°C for 1 hour. After washing the plates six times with PBST, a 4-fold serial dilution of serum was added to the wells. Plates were washed six times with PBST and then incubated with horseradish peroxidase (HRP)-conjugated goat anti- mouse IgG antibody (1:10,000 dilution; ZSGB-BIO, Beijing, China) or HRP-conjugated goat anti-mouse IgA antibody (1:10,000 dilution; Abcam, Cambridge, UK) for 1 h at 37 °C. After washing, 3,3',5,5'-tetramethylbenzidine (TMB, Beyotime, Jiangsu, China) was applied and the antibody titres were determined by measuring the absorbance at 450 and 630 nm. The IgG or IgA titres in the serum was calculated using the software GraphPad Prism v9 and the serum antibody titres value was determined as the reciprocal of the highest dilution 2.1 times higher than the absorbance value of the negative control.
Recombinant Vesicular stomatitis Virus (VSV)-based Pseudovirus NeutralisatioAnssay
The neutralisation assay was carried out as previously described [16]. Mice serum samples were inactivated at 56°C for 30 minutes. Mix serially diluted sera with 650 TCID50 of luciferase expressing VSV virus-based SARS-CoV-2 pseudovirus and incubate at 37°C for 1 hour. Vero cells were added and incubated at 37°C and 5% CO2 for 24 hours. Relative luciferase activity was measured using a luciferase assay system. A cellular control and a viral control were also set up. Calculate the percentage neutralisation and the sample half neutralisation titre EC50 using the Reed-Muench method.
ACE2-binding Inhibition (Neutralisation) Using ELISA
The SARS-CoV-2 ACE2 neutralisation kit (Meso Scale Discovery (MSD), Panel 18 (ACE2) kit, K15570U) was used to quantitatively measure titres of antibody that blocked the binding of ACE2 to its cognate ligands (S proteins from Wuhan-Hu-1, B.1.1.7, B.1.351, B.1.526.1,
Figure 1: Design and characteristics of potent immune response of prime-boost with ChAdTS-S-S and ARCoV. (A, B) Overall scheme of the group design, vaccination, and immunological characterization. indicates vaccination, 5 × 109 VP of ChAdTS-S-S or 6 μg of ARCoV were used for each vaccination. (C-E) The serum spike-specific binding IgG titers (C), pseudovirus NAb titers (D), and spike-specific binding IgA titers (E) on week 57 after prime immunisation (n = 3-5 per group). (F-H) Temporal changes in the serum spike-specific IgG titers (F), Pseudovirus NAb titers (G), and spike-specific IgA titers (H) for up to 57 weeks after the prime immunisation (n =3-5 per group). In the VSV- based pseudovirus assay, NAb titers less than 30 were recorded as 30 when plotting the figures. Bars represent the geometric mean ± geometric SD *p < 0.05; **p < 0.01; ***p < 0.001; ns: p > 0.05.
B.1.617, B.1.617.1, B.1.617.2, B.1.617.3, P.1, P.2 strains).
Plates were coated with the specific antigen and the bound antibodies in the samples (1:100 dilution) were detected using an MSD instrument that measures the light emitted from the tag. ACE2 binding inhibition equals 1 - (average electrochemiluminescence signal of the sample / average electrochemiluminescence signal of the blank control) × 100%
IFN-γ Secretion Levels Using ELISpot Assay
Mice were euthanised and soaked in 75% ethanol. Spleens were isolated and transferred into a 40-μm cell strainer with 4~5 mL mouse lymphocyte separation medium (Dakewe, Beijing, China), and ground with a syringe piston. The suspension of splenic lymphocyte was immediately transferred to a 15 mL centrifuge tube, covered with 1 mL RPMI 1640 medium (Hyclone, Logan, UT, USA), and centrifuged at 800g for 30 minutes at room temperature. After centrifugation, the liquid in the 15 mL centrifuge tube was divided into four layers from the top to bottom: the RPMI 1640 medium covering, lymphocyte, separation fluid, erythrocyte and cell fragment layers. The lymphocyte layer was transferred to a fresh tube, and 10 mL RPMI 1640 medium was added. After centrifugation at 250g for 10 min at room temperature, the lymphocytes were collected. The supernatant was discarded and the cells were suspended in serum-free medium. IFN-γ positive cells were detected using the Mouse IFN-γ ELISpot kit (Mabtech, Sweden). 96-well PVDF plates were washed four times with 200 µL of PBS and then closed with RPMI- 1640 medium containing 10% fetal bovine serum for at least 2 hours at room temperature. Freshly isolated lymphocytes (2.5 × 105 cells) were transferred to the wells and stimulated at 37 °C for 24 hours with a peptide library (1 µg/mL per peptide; Genscript, Nanjing, China) of the entire S protein of SARS-CoV-2. The plates were incubated with anti-mouse IFN-γ antibody at room temperature for 2 hours and then with streptavidin-HRP (1:1000 dilution, Dakewe) for 1 hour. After washing, 100 μL TMB substrate solution per well was added and developed for 5 min until distinct spots emerged. Spots were imaged and counted using an ImmunoSpot® S6 Universal instrument (Cellular Technology Limited, Shaker Heights, OH, USA).
Intracellular Cytokine Staining
Splenic lymphocytes were isolated and then stimulated for 8 hours at 37 °C with 2 μg/mL of the S protein peptide library and brefeldin A (1:1000 dilution; Biolegend, San Diego, CA, USA), to block cytokine secretion. Following stimulation, lymphocytes were washed and stained with a mixture of the following antibodies against lineage markers: BV421 hamster anti-mouse CD3e antibody, BV510 rat anti-mouse CD4 antibody, and FITC rat anti-mouse CD8a antibody as well as the fixable viability stain 780 (BD Biosciences, San Jose, CA, USA) to distinguish between live and dead cells. After two washes with PBS, cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences), washed with Perm/Wash buffer (BD Biosciences), and stained with PE-conjugated rat anti-mouse IFN-γ, BV605 rat anti-mouse interleukin (IL)-2, PE-Cy7 rat anti-mouse IL-4, APC rat anti-mouse IL-10, and BB700 rat anti-mouse tumour necrosis factor (TNF) (BD Biosciences). Cells were washed with Perm/Wash buffer and PBS, resuspended in PBS, and subjected to flow cytometry using a FACS Lyric analyser (BD Biosciences). At least 200,000 events were collected for each sample. Data were analysed using FlowJo software (TreeStar, Ashland, OR, USA). CD8+ and CD4+ T cells were gated from single cells (FSC-A vs. FSC-H), lymphocytes (FSC-A vs. SSC-A), and live CD3+ T cells (CD3+ vs. LD780−); detection data are presented as percentages of cytokine-positive cells among CD8+ or CD4+ T cells.
MSD Th1/Th2 Cytokine Profiling
The supernatant of the stimulated splenocytes ELISpot plate was collected. Concentrations of TNF-α, IL-2, IL-4 and IL-10 were measured using the V-PLEX Multifactor Assay (Mouse) Kit. Cytokine levels were determined using a MESO QuickPlex SQ 120 (MSD). Cytokine concentrations were calculated using a standard curve.
Statistical Analysis
All graphs and statistics were analysed using GraphPad Prism v9 software. Data are expressed as geometric mean ± geometric standard deviation. Statistical differences between multiple groups were determined using one-way ANOVA.
*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, no significant differences.
Results
Heterologous Inoculation of ChAdTS-S and ARCoV Induces a Strong, Durable Humoral Immune Re- sponse in Mice
The vaccination scheme administered using the Ad and AR vaccines is illustrated in Figure 1A. Serum samples were collected from the 2nd week after the prime immunisation until the 57th week (Figure 1B). Spike-specific binding antibody IgG in the serum was detected by ELISA to evaluate the adaptive immune response induced by different immunisation strategies in mice. At week 57 after prime immunisation, all groups, except the negative control group, maintained high IgG titres. The i.n. Ad > i.m. AR group showed the highest IgG level with a geometric mean titres (GMTs) of 1,796,438 which was significantly higher than that of the other groups (Figure 1C), followed by the 1×i.n. Ad and 2×i.n. Ad groups, with GMTs of 298,521 and 259,705, respectively. NAb levels may be highly correlated with the protective efficacy of the vaccines. At 57th week post-priming, the level of NAb against wild-type SARS-CoV-2 was measured using a recombinant VSV-based pseudovirus neutralisation assay (Figure 1D). Among all groups, heterologous immunisation with i.n. Ad > i.m. AR induced the highest NAb level of 2,140, which was 2.4-fold, 2.9-fold, and 3.1-fold higher than those of the i.m. AR > i.n. Ad, i.m. Ad > i.m. AR, and i.m. AR > i.m. Ad groups, respectively. However, none of these increases were significantly different. In the homologous vaccination groups, intranasal Ad inoculation induced higher NAb titres than intramuscular injection. Ad > i.m. AR induced significantly higher Nab levels than 1×i.n. Ad, 2×i.n. Ad, 1×i.m. Ad, and 2×i.m. Ad. The immunological effect of Ad > i.m. AR was significantly higher than that observed with homologous Ad inoculation. In addition, we observed a 23-fold increase in NAb levels following two injections of AR relative to a single injection. However, no significant increase was observed following two injections of Ad relative to a single injection. The NAb level after two injections of nasal vaccination was lower than that after a single injection. The serum IgA antibody levels induced by the different immunisation strategies (Figure 1E) were evaluated by ELISA at week 57 after priming. Regardless of homologous or heterologous immunisation, the experimental group administered i.n. Ad showed relatively higher IgA levels. The i.m. AR > i.n. Ad group had the highest IgA titre (GMTs: 2629), which was 5.4- and 9.7-fold higher than the titres observed in the i.m. Ad > i.m. AR and i.m. AR > i.m. Ad groups, respectively. Among the four Ad homologous groups, the two intranasal groups showed higher IgA levels. This finding indicates that intranasal Ad administration can more effectively activate the body's mucosal immune response.
Heterologous Prime-booster with ChAdTS-S and ARCoV Induced Durable Broad-spectrum Neutral- ising Activity against SARS-CoV-2 Variants in Mice
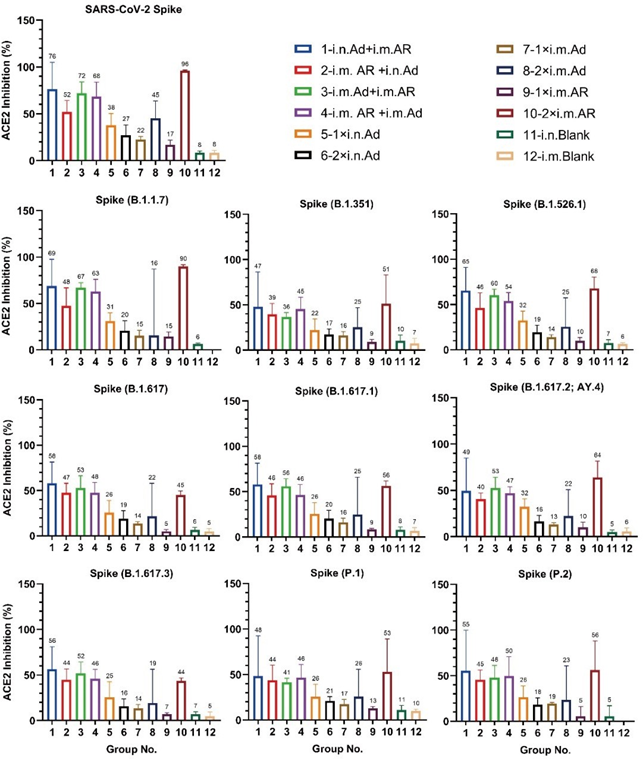
The serum ACE2-binding inhibition rates (i.e., neutralising activity) against the S protein (prototype, B.1.1.7, B.1.351, B.1.526.1, B.1.617, B.1.617.2; AY.4, B.1.617.3, P.1, and P.2) were measured. Heterologous prime-booster with i.n. Ad > i.m. AR, i.m. AR > i.n. Ad, i.m. Ad > i.m. AR, i.m. AR > i.m. Ad, and 2×i.m. AR induced relatively higher ACE2 inhibition activity, with an inhibition rate between 36% and 96% (Figure 2). However, homologous immunisation with i.n. Ad or i.m. Ad and a single injection of AR is relatively low, with an inhibition rate between 5% and 45%. Homologous immunisation with 2×i.m. AR induced ACE2-binding inhibitory activities against the prototype and B.1.1.7 S proteins, which were 96% and 90%, respectively. The inhibitory activities against other variants were significantly reduced but remained between 44% and 68%. This decreasing trend was consistent with that reported in the literature. Among the four heterologous immunisation groups, the i.n. Ad > i.m. AR group showed balanced ACE2 binding and neutralising activity against ACE2 binding by the prototype, B.1.1.7, B.1.351, B.1.526.1, B.1.617, B.1.617.1, B.1.617.2; AY.4, B.1.617.3, P.1, and P.2 S proteins, the binding inhibition rates were 76%, 69%, 47%, 65%, 58%, 58%, 49%, 56%, 48%, and 55%, respectively. The ACE2- binding inhibition rate in each immune group was consistent with the NAb detected using the VSV pseudovirus method.
Intramuscular Prime-booster with ChAdTS-S or ARCoV Induces High Level of Long-term Cellular Immune Responses
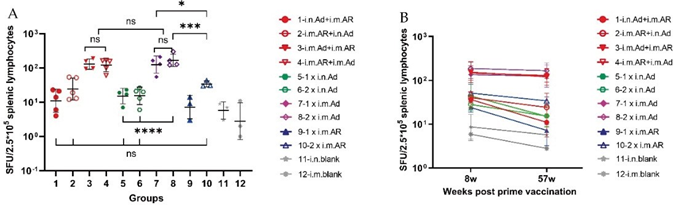
Spleen lymphocytes of mice were isolated at week 57 after primary immunisation and stimulated with SARS- CoV-2 S protein for 24 hours. Then, an ELISpot assay was performed to detect the secretion level of IFN-γ. The T cell immune response was stronger in the i.m. Ad > i.m. AR, i.m. AR > i.m. Ad, 1×i.m. Ad, and 2×i.m. Ad groups than in the other groups, reaching 129, 121, 125, and 166 spot- forming units per 2.5×105 spleen lymphocytes, respectively. No significant differences were found among the four groups (Figure 3A). In addition, 1×i.m. Ad induced a higher T cell immune response than 1×i.n. Ad (P = 0.0025), and 2×i.m. Ad induced a higher T cell immune response than 2×i.n. Ad (P < 0.0001). However, homologous immunisation with 1×i.m. or 2×i.m. Ad induced a stronger T cell immune response than 2×i.m. AR (P = 0.03 and P = 0.0009), but no significant difference was found between the four groups administered intranasal Ad and the 2×i.m. AR group. This finding indicates that intramuscular injection of Ad can induce a stronger and more durable cellular immune response than intranasal administration. This response remained high at 57 weeks post-priming (Figure 3B), which is important for the specific killing and clearance of SARS-CoV-2 infected cells.
Vaccination with ChAdTS-S Induces Durable Th1- biased Cellular Immune Responses
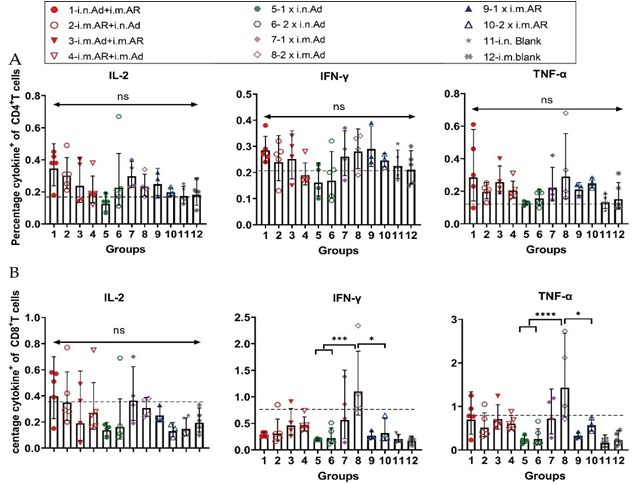
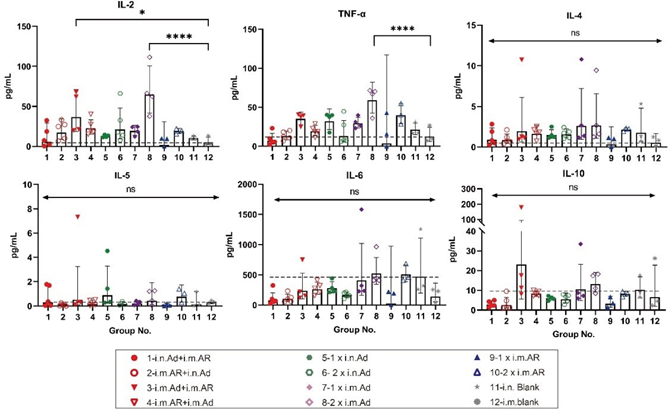
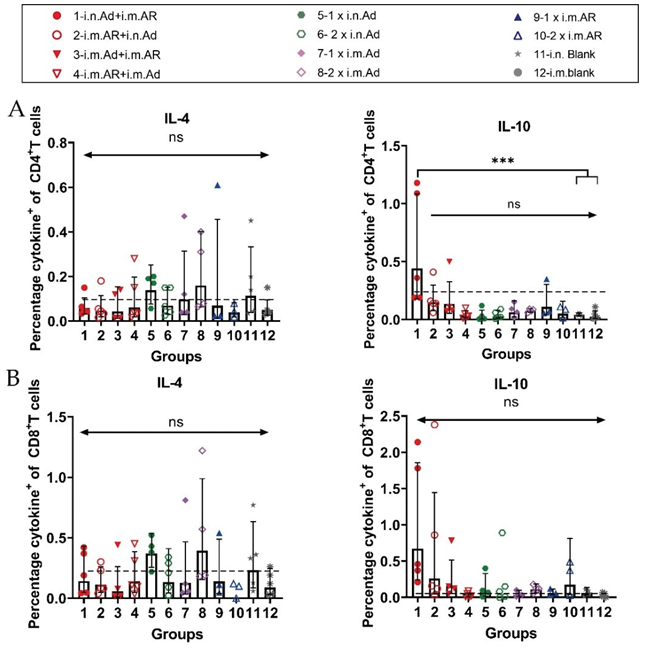
We further investigated the bias of each immunisation strategy to induce specific T-cell immune responses against the SARS-CoV-2 S protein. Splenic lymphocytes of mice were isolated at week 57 after primary immunisation, and the characteristics of T cell immune response cytokine secretion were evaluated by intracellular staining and MSD after stimulation with an overlapping S protein peptide library. Intracellular cytokine staining showed that heterologous inoculation of Ad and AR induced Th1-biased cytokines, such as IL-2, IFN-γ, and TNF-α, exhibited higher serum levels than that in the control group (Figure 4). Meanwhile, Th2-biased cytokine IL-4 had lower serum levels than that in the control group (Figure S1). Furthermore, 2×i.m. Ad induced CD8+ T cells secreted a higher proportion of Th1- biased cytokines such as IFN-γ and TNF-α (Figure 4B). However, Th2-associated cytokine levels, such as those of IL-4 and IL-10, were not significantly different from those in the control group (Figure S1). The overall level was higher than that in the control group, indicating that Ad mainly induces a Th1-biased T cell immune response. The Th1 and Th2 cytokine secretion levels in the i.n. Ad, i.m. AR, and heterologous immunisation groups were not significantly different from those in the control group. The Th1 immune response was higher in the 2×i.m. Ad group than that in the 1×i.n. Ad and 2×i.n. Ad groups, which was consistent with our previous findings. The levels of cytokines IL-2, IFN-γ, IL-4, and TNF-α were not significantly different between the heterologous inoculation groups (i.n. Ad > i.m. AR, i.m. AR > i.n. Ad, i.m. Ad > i.m. AR, and i.m. AR > i.m. Ad) and the control group. The responses of Th1 and Th2 cellular immunity were balanced. The results of MSD were comparable to those of intracellular cytokine staining, with the 2×i.m. Ad group still secreting higher levels of IL-2 and TNF-α at week 57 than the other groups. Other cytokine levels, such as that of IL-4, IL-5, IL-6, and IL-10, were not significantly different between groups, including the control group, indicating that the 2×i.m. Ad group induced a Th1- biased cellular immune response (Figure 5).
Discussion
Currently available COVID-19 vaccines [17-21] show varying degrees of reduced efficacy against multiple SARS- CoV-2 variants, and a durable and strong cross-immune response is essential for preventing infection with continuously updated variants. Previous studies have demonstrated that prime intranasal Ad followed by intramuscular AR induced a strong systemic and local mucosal immune response in BALB/c mice. In this study, we further investigated the
Figure 2: Neutralization capacity of sera by measuring inhibition of binding between angiotensin-converting enzyme 2 (ACE2) and SARS- CoV-2 spike proteins. Spike proteins from SARS-CoV-2 prototype and B.1.1.7, B.1.351, B.1.526.1, B.1.617, B.1.617.1, B.1.617.2, B.1.617.3, P.1, and P.2 strains measured 57 weeks after primary immunization. Negative ACE2-binding inhibition rates are shown as zero (n =3~5 per group). Bars represent geometric SD; numbers represent geometric mean of corresponding group.
Figure 3: Cellular immune responses specific to SARS-CoV-2 spike proteins measured 57 weeks after primary vaccination. (A) Enzyme- linked immunospot (ELISpot) assays for IFN-γ after stimulation with SARS-CoV-2 spike protein, cells secreting IFN-γ were quantified using ELISpot assays (n = 3-5 per group; each point represents mean number of spots from two wells per sample). (B) Trends in cellular immune response levels from week 8 to week 57. Bars represent geometric mean ± geometric SD; *p < 0.05; ***p < 0.001; ns, p > 0.05.
Figure 4: Th1/Th2 skewing detected by intracellular cytokine staining in immunized mice. Lymphocytes were stimulated with SARS-CoV-2 spike peptide pools spanning the entire spike protein for 8 h. (A,B).Percentage of spike protein-specific IFN-γ, IL-2, TNF-α positive memory CD4+ T (A) and CD8+ T (B) cells, measured at week 57 after prime immunisation (n = 3-5 per group, one spot represents one sample). Bars represent the geometric means ± geometric SD, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, p > 0.05. The dashed lines represent the blank value.
Figure 5: Th1/Th2 skewing in immunized mice measured using MSD cytokine profiling. Lymphocytes were stimulated with SARS-CoV-2 spike peptide pools spanning the entire spike protein for 24 h. IL-2, TNF-α, IL-4, IL-5, IL-6, and IL-10 levels in supernatants were measured (n = 5 per group, one spot represents one sample). Bars represent geometric means ± geometric SD, *p < 0.05; ****p < 0.0001; ns, p > 0.05. The dashed lines represent the blank value.
persistent immune response to sequential immunisation with Ad and AR. We found that prime immunisation with intranasal Ad followed by intramuscular AR induced the highest levels of systemic and mucosal immune responses until at least 57 weeks after primary immunisation. This immunisation strategy induced high IgG, IgA, and pseudovirus-NAb titres and ACE2 binding inhibition (cross-neutralisation) against SARS-CoV-2 and its variants. This finding indicates that the prime immunisation strategy results in long-lasting immunogenicity and cross-neutralising potency, which may protect against emerging mutant strains. A durable and efficient immune response is highly related to the combined effects of systemic and local mucosal immune responses [22, 23]. Lapuente [24] found that in mice, intramuscular prime injection with a plasmid DNA or mRNA COVID-19 vaccine, followed by a booster of intranasal vaccination with an adenovirus type 5 or 19a vector-based vaccine, induced systemic and local mucosal immune responses. Primary immunisation with intramuscular mRNA, followed by nasal drip boost of Ad5-S, induced higher levels of IgA and lung- resident memory T cells than two intramuscular injections of mRNA neo-crown vaccine. This vaccination strategy also provided complete protection in mice against SARS-CoV-2 infection, which was consistent with our findings. However, our results revealed that nasal drip priming-muscle injection boosters induced higher levels of IgG, NAbs, and ACE2 binding inhibition and comparable IgA immune responses than the nasal drip priming-nasal injection booster strategy. The underlying immune mechanisms need to be explored further. The booster shot effectively increased the production of cross- reactive NAb. Pegu and collegues [25] found that single- shot vaccination with mRNA-1273 induced higher levels of cross-NAb against SARS-CoV-2 variants B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.429 (Epsilon), B.1.526 (Iota), and B.1.617.2 (Delta). However, booster vaccination induced higher levels of cross-NAbs for six months after vaccination, even though lower NAb levels were induced against strain B.1.351. This result is consistent with our observation of a higher neutralising potency response against all variants with two injections of AR. Conversely, a single injection of intramuscular AR induced lower amounts of cross-reactive antibodies. In addition, the nasal drip route induced more extensive and higher levels of cross-neutralising antibodies than intramuscular vaccination. This result is presumably due to the extensive neutralising effect of SIgA induced by the mucosal immune response [26, 27] and further suggests that the mucosal immune response induced by the neo- crown vaccine plays a crucial preventive role against the immune escape phenomenon of constantly emerging mutant strains [28, 29]. Cellular immunity is indispensable in the adjuvant humoral immune response. Multiple data suggest that the highly conserved epitope peptides in CD4+ and CD8+ T cells, compared to B cells, are critical for preventing infection by emerging variants [30, 31]. Various mRNA and adenoviral vector vaccines induce a biased Th1-type cellular immune response, which is important for the response to SARS-CoV-2 infection [32-34]. In addition, all vaccine induction groups induced a biased Th1-type cellular immune response compared to those eight weeks after the initial vaccination. All vaccination groups induced a Th1-type cellular immune response, with a decreasing trend in IFN- γ cytokine levels up to 57 weeks compared to eight weeks after primary vaccination, nasal Ad, and intramuscular AR but maintained levels comparable to mRNA vaccination.
The present study has some limitations. First, due to resource limitations, we did not detect NAb titres against live SARS-CoV-2 virus [35]. The level of NAbs is closely related to the vaccine’s protective efficacy [31]. We need SARS-CoV-2 and variants to conduct challenge experiments to further demonstrate the protective efficacy of the initial Ad nasal drip and intramuscular AR vaccinations after a year. Second, we did not analyse S- IgA levels in the nasopharyngeal and bronchoalveolar lavage fluid, which is essential for preventing SARS- CoV-2 infection. Third, to evaluate the broad-spectrum nature of this immunisation strategy fully, we need to detect T-cell immunisation levels against other variants (such as B.1.1.7, B.1.351, P.1, and BA.1/2/3/4/5). In summary, intranasal priming with the AdC68-based vaccine ChAdTS-S and intramuscular injection of the mRNA vaccine ARCoV induced high and sustained humoral, cellular, and local mucosal immune responses. Sustained high levels of immune responses were observed until at least 57 weeks after priming, which provides preliminary data for subsequent immunisation strategies and optimisation.
References
- WHO Coronavirus (COVID-19) Dashboard (2022).
- Gorbalenya AE, et The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology 5 (2020): 536-544.
- Madhi SA, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the 1.351 Variant. New England Journal of Medicine 384 (2021): 1885-1898.
- Mahase E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African Bmj 372 (2021): n296.
- Tseng HF, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta Nature Medicine 28 (2022): 1063-1071.
- Pouwels KB, et Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nature Medicine 27 (2021): 2127- 2135.
- Li W, et al. Heterologous prime-boost with AdC68- and mRNA-based COVID-19 vaccines elicit potent immune responses in Signal Transduction and Targeted Therapy 6 (2021): 419.
- Fröberg J & Diavatopoulos DA. Mucosal immunity to severe acute respiratory syndrome coronavirus 2 Curr Opin Infect Dis 34 (2021): 181-186.
- Afkhami S, et al. Respiratory mucosal delivery of next- generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 185 (2022): 896-915.
- Li Y, Jin L & Chen The Effects of Secretory IgA in the Mucosal Immune System. Biomed Res Int (2020): 2032057.
- Hassan AO, et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in Cell Rep 36 (2021): 109452.
- H L, et Intranasal administration of a recombinant RBD vaccine induces long-term immunity against Omicron- included SARS-CoV-2 variants. Signal transduction and targeted therapy 7 (2022): 159.
- RF, O-R A, AM & DH Upper respiratory tract mucosal immunity for SARS-CoV-2 vaccines. Trends in molecular medicine null (2023).
- Li et al. Single-Dose Immunization with a Chimpanzee Adenovirus-Based Vaccine Induces Sustained and Protective Immunity Against SARS-CoV-2 Infection. Front Immunol 12 (2021): 697074.
- Zhang NN, et A Thermostable mRNA Vaccine against COVID-19. Cell 182 (2020): 1271-1283.
- Nie J. et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat Protoc 15 (2020): 3699-3715.
- Madhi SA, Izu A & Pollard AJ. ChAdOx1 nCoV-19 Vaccine Efficacy against the B.1.351 Variant. Reply. N Engl J Med 385 (2021): 571-572.
- Madhi SA, et Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med 384 (2021): 1885-1898, doi:10.1056/ NEJMoa2102214.
- Shinde V, et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med 384 (2021): 1899-1909.
- Collie S, Champion J, Moultrie H, Bekker LG & Gray
- Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med 386 (2022): 494- 496.
- Reis BY, et Effectiveness of BNT162b2 Vaccine against Delta Variant in Adolescents. N Engl J Med 385 (2021): 2101-2103.
- Kingstad-Bakke B, et al. Vaccine-induced systemic and mucosal T cell immunity to SARS-CoV-2 viral variants. Proc Natl Acad Sci U S A 119 (2022):
- Tang J, et Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci Immunol 7 (2022): eadd4853.
- Lapuente D. et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost Nat Commun 12 (2021): 6871.
- Pegu A, et Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 373 (2021): 1372-1377.
- Sterlin D, et IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 13 (2021).
- Wang Z, et al. Enhanced SARS-CoV-2 neutralization by dimeric Sci Transl Med 13 (2021).
- Ku MW, et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal Cell Host Microbe 29 (2021): 236-249.
- Hassan AO, et A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 183 (2020): 169-184.
- Le Bert N, et SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584 (2020): 457-462.
- Tarke A, et SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 185 (2022): 847- 859.
- Le Bert N, et SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584 (2020): 457-462.
- King RG, et Single-Dose Intranasal Administration of AdCOVID Elicits Systemic and Mucosal Immunity against SARS-CoV-2 and Fully Protects Mice from Lethal Challenge. Vaccines (Basel) 9 (2021).
- Corbett KS, et Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med 383 (2020): 1544-1555.
- Plotkin Correlates of protection induced by vaccination. Clin Vaccine Immunol 17 (2010): 1055- 1065.
Figure S1: Th1/Th2 skewing detected by intracellular cytokine staining on week 57 after primary immunization. Percentage of spike protein-specific IL-4- and IL-10-positive CD4+ (A) and CD8+(B) T cells, measured on week 57 after primary immunization (n =3-5 per group; one spot represents one sample). Bars represent the geometric mean ± geometric SD; ***p < 0.001; ns, p > 0.05. The dashed lines represent the blank value.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
