Hygienic - To Be or Not To Be? An Investigation into The Most Recent Evidence
Richard Hastings and Michail H. Karavolos*
BioCote Limited, Oak Court, Pilgrim's Walk, Prologis Park, Coventry, CV6 4QH, United Kingdom
*Corresponding Author: Michail H. Karavolos, BioCote Limited, Oak Court, Pilgrim's Walk, Prologis Park, Coventry, CV6 4QH, United Kingdom, Tel: 0044-7771375011;
Received: 04 October 2017; Accepted: 18 October 2017; Published: 25 October 2017
Article Information
View / Download Pdf Share at FacebookAbstract
The continual advancements in major technological sectors and their immediate relevance to society has led to significant changes in the way people interact in their homes and in society. Similarly, and consequently, advances in science have considerably improved our ability to study microorganisms and have brought about major developments in characterising microbial symbiotic populations in our body (microbiota) as well as the environment. The availability of antimicrobial technologies has led to improvements in hygiene and has introduced a new standard in developed and developing countries. It has been suggested, however, that improvements in hygiene, may have contributed directly or indirectly to a rise in our tendency to develop allergic diseases (atopy). In this review we will provide the most up to date evidence that will shed light into a new perspective surrounding this theory. We will provide insights into current research focusing on the role of hygiene especially in regard to the hygiene practice improvements made recently in industrialised society. Furthermore, we will discuss the evidence surrounding the reasons behind the unprecedented rise in atopic disease in relation to existing hygiene trends, particularly in domestic settings. This review will finally address the question whether a reduction in public hygiene would be beneficial for public health and relate this to current research evidence.
Keywords
Hygiene Hypothesis; Atopy; Microbiome; Epigenetics; Immunity; Antimicrobial; Antibiotic resistance
Article Details
Introduction
A hypothesis is a plausible explanation applied to clarify an observation. The term “Hygiene Hypothesis” [1, 2] was established and applied to certain trends in groups of human disease and it attempted to explain the connection between observed rises in atopic disease in children and reductions in numbers of infections. Rises in atopy were considerable and occurred over a short period of time in the latter half of the last century. Certainly, in one study, the occurrence of atopic diseases was found to be considerably higher in urban when compared to a similar population of rural children, despite the presence of higher levels of bacterial endotoxin in the environment where the rural children were living [3, 4]. The Hygiene Hypothesis accommodated the notion that improved hygiene in more developed societies was a cause of increased atopy. The clear implication of the Hygiene Hypothesis was that urban hygiene has gone too far and was counterproductive to children’s health. Since its appearance in 1989, the Hygiene Hypothesis has been joined by a considerable collection of evidence that may be useful to support or refute its premise [5, 6].
Observations of the marked rise in cases of diseases including Type 1 diabetes, asthma, hay fever, food allergies, Crohn’s, multiple sclerosis were inevitably made around the middle to late twentieth century [6, 7]. Generally, there was a concomitant decrease in the number of other, fundamentally different human diseases which did not escape the attention of public health officials - mumps, measles, tuberculosis amongst others [6, 7]. Importantly, the shifting epidemiological data was occurring in the same geographic regions, industrialised, developed countries. Reductions in infectious disease was far from a surprise; it was an expected and welcomed outcome of the systematic vaccination programmes. Alongside the prophylactic approach was the advent of antibiotic treatment for childhood infectious disease. And improvements in the standard of living for wide sections of the population was also regarded worthy of consideration. Of relevance to the Hygiene Hypothesis was the notion that standards of living were being driven up in developed countries due to improvements in public and personal hygiene. Developments were nicely captured by the question: Was the reduction in infectious disease in children somehow affecting the young immune system and promoting atopic disease? Hence, the Hygiene Hypothesis was conceived in order to elucidate the link between detected augmentations in atopic disease and corresponding decreases in numbers of infections in the population.
The Hygiene Hypothesis was immediately challenged scientifically due to the difficulty in elucidating the cause of epidemiological shifts in the populations involved [8]. To further complicate the task of addressing the accuracy of the hypothesis, it was regarded as unlikely that a single cause was going to be responsible for the observations [8]. This was a multi-factorial phenomenon. The epidemiological landscape was not shifting in a mildly curious manner ? the observations were of major and marked changes in the patterns of disease occurring over a relatively short period of time. The Hygiene Hypothesis was going to have to be supported by detailed, multidisciplinary, robust data that revealed the mechanistics behind the rise of atopy.
Perhaps a constructive place to start the pursuit of the hypothesis was a ruling out of genetic predisposition as a causative factor [6]. On a basic, but compelling level, the timescales were too short. Furthermore, population genetics data failed to show significant differences and hence the efforts were focused on environment and lifestyle factors [6]. Whilst diverse and extensive in scope, the influences of the environment and lifestyle were a better fit for the timescales the Hygiene Hypothesis was built on. Singular issues that were relatively easy to be addressed and clarified to reveal the actual phenomenon. For example, house dust mites were accused of promoting atopy in children and few argued against the emergence of the mites in the centrally-heated built environment at the right time [9]. At best, the case of the dust mites was an indication that the Hygiene Hypothesis had merit, for as a single factor, it was a long way from the body of evidence that was needed to establish the hypothesis. The passing of time saw the Hygiene Hypothesis focus on the suspicion that a decline in the exposure of children to microbes in general, not just certain infections, was the real culprit behind rises in atopic disease [9]. After all, developed societies were breaking many traditional connections between young humans and infectious agents. Consider the advent and ubiquity of clean water and fit for consumption food, progress in public health sanitation, common administration of antibiotics and vaccines and changing birth practices not omitting the widespread urbanisation of populations.
Over time, the range of candidate factors supporting the Hygiene Hypothesis has expanded. It was understandably difficult for researchers to neglect emerging or previously unrecognised factors from their considerations when there was sufficient overlap between the new factors and the definitions of the existing factors. So, the Hygiene Hypothesis was obliged to capture more dynamism in human behaviour, such as smaller family sizes, changing diets and what had been defined as “improved household amenities and higher standards of personal cleanliness” or “cleaner homes”. A notable outcome reflecting numerous scientific research studies was published by the International Scientific Forum on Home Hygiene [8]. This organisation addressed the possible implications that the Hygiene Hypothesis may have on domestic hygiene. In this review we will firstly examine the clarity of the evidence supporting a causal link between a decline in microbial exposure and the rise in atopic disease. Secondly, we will evaluate the evidence demonstrating to what extent might cleaning and hygiene, as distinct from other influences on microbial exposure, be a significant factor in the rise of atopic disease.
An evaluation of the clarity of the evidence
Current evidence show a generally waning increase in the numbers of atopy cases [10, 11]. Some studies have been able to draw conclusions that certain key diseases have plateaued or are falling in reported numbers. For example, in a variety of European Union countries, asthma, bronchitis and childhood allergies are not being reported by the health care system in the same volumes of numbers as previously [10-12].
Few doubt the biological plausibility of the Hygiene Hypothesis. However, with time and with the availability of new technologies and hence, evidence, scientific communities have begun to produce pioneering data that subsequently question the foundations of the Hygiene Hypothesis. Suffice to say, there is immunological (largely, serological) evidence supporting the Hygiene Hypothesis and for the purpose of this review the biological plausibility of the Hygiene Hypothesis is acknowledged [4, 13]. One has to consider, however, that the models relied upon to generate the immunological-type evidence change continuously. Additional research is needed in order to elucidate the contribution of the immunological data in supporting or refuting the hypothesis and how the immunological landscape of the population fits with the causes of atopy.
The proxy parameters utilised to pursue the Hygiene Hypothesis have included family size and/or structure and its relation to atopy. In an example study the evidence supported the lack of a discernible relationship between reduced family size in industrialised countries (from the early twentieth century) and the rise in atopy [14]. Indeed, one study estimated that smaller families accounted for just 1% of the rise in observed atopy in the period 1961 to 1991 [14]. Various studies have also examined sub-divisions of familial parameters and their input to the development of atopy. For example, evaluating birth order, sibship size and gender data against those of atopy in the same populations failed to yield a robust conclusion [15]. Differences in atopy along gender and birth order lines were initially promising but did not reach a credible explanation [15].
Some studies have produced conclusions that unambiguously support the Hygiene Hypothesis, that is, close personal contact appears to promote protection against atopy. A notable example is the sharing of beds, cots and other sleeping facilities by small children. The Hygiene Hypothesis is supported here simply because we can assume this type of close contact between children will more effectively share and distribute microorganisms capable of inducing an immune response [2]. Again, outcomes of finer scale studies examining similar parameters have been less clear. Some children attending day care facilities very early in life have been revealed to acquire protection against atopic disease whilst the same protective effects are not apparent in other children subjected to comparable conditions.
What about rural environments and atopies? There are data suggesting children in rural communities benefit from some degree of protection absent in their urban counterparts [3]. Observations in line with that statement do not appear to apply to the adult populations in the same environmental settings. There are no robust epidemiological datasets describing a reduced risk of adult atopy that can be attributed to living in rural communities. In actual fact, farmers have an elevated risk of occupational allergic disease, for example lung disease is an occupational hazard of long-term exposure to microbial allergens in rural settings. There is also little substantive evidence of protection against atopy for occupations such as waste disposal and sewage workers.
Despite the difficulties of conducting robust science to discern relationships directly between parameters of most interest, there have been studies attempting to elucidate a relationship between atopy and microbial exposure/infection [16]. From this perspective, the relationship of food-borne and gastrointestinal infections and the development of atopy has recently received scientific attention [17]. For such studies to be able to support the Hygiene Hypothesis, it is necessary to produce evidence supporting an inverse relationship between the exposure to pathogens and rates of atopy. The Hygiene Hypothesis has not been supported in any meaningful manner by these studies because their conclusions have failed to reveal such a relationship [18]. Bearing this in mind, there are occasional suggestions of an atopy protective effect for some sub-populations, but this type of outcome is far from widespread or reproducible [17]. Furthermore, the Hygiene Hypothesis is undermined by the uncertainties associated with these studies. Atopy protective effects are often considered as independent of other key parameters - age, sibship size, birth order. Quite apart from the data generated, the gastrointestinal microbe needs to be viewed with caution. Is the microbe acting as a marker for poor orofaecal hygiene and exposure to other, more important gastrointestinal pathogens? [17, 18]. Opinions among researchers are contradicting and some provide a conclusion only for discrete populations studied (e.g. individuals who carry a particular variant of the gene involved in viral gut infection) [19]. To summarise, these studies provide a complicated outcome that can only be interpreted with reservations [18].
Examining non-gastrointestinal evidence reveals a similar, confusing, often contradictory picture. Studies have shown a Hygiene Hypothesis-supporting relationship between measles infection and atopy numbers [20, 21]. Other studies refuted the hypothesis in their conclusions [22, 23]. A set of Mycobacterial infection data have supported the Hygiene Hypothesis [21, 24], while a different set of data refute the same hypothesis [24-26]. In another example it was demonstrated that Respiratory Syncytial Virus, is often implicated in triggering asthma rather than protecting against it [27]. Finally, studies conducted to elucidate the connection between malaria and the development of atopy yielded contradictory results and did not reach a credible conclusion [28, 29]. Clearly, the evidence generated from scientific studies utilising these parameters has resulted in contradictory results. Sometimes, the evidence supports the Hygiene Hypothesis because, in broad terms, there appears to be a protective association between childhood exposure to microbes and reduction in atopy cases. On the other hand, the Hygiene Hypothesis is refuted by those studies dismissing any protective effect and even demonstrating an increased risk of atopy following early infections. Hence, there is a continual need to re-evaluate the causative factors of atopy. One approach was to consider that the overall exposure to microorganisms, rather than specific types of microbes, is the key to immunological development that is relevant to rates of atopy [30-33].
Evidence supporting the Hygiene Hypothesis, require to show a reduction of infectious disease in a suitable period prior to associated rise in atopy. These events are relying on the assumption that immunological processes are not deferred between the two concepts. A second assumption is that the stimulation of the immune system (or lack of it) must be confined between pregnancy and early childhood. To support the Hygiene Hypothesis the evidence needs to present as a noticeable reduction in infections followed by a noticeable rise in atopy.
The history of public health clearly describes considerable and widespread reduction in infections as a direct consequence to improved practices of what we might nowadays term biosecurity measures [34]. Historical evidence tells us that improved hygiene practices occurred too early in time to be directly or indirectly associated with the rise in atopy that prompted the Hygiene Hypothesis. An example is the decline, for example, in cholera and typhoid in industrialised countries [35]. These diseases were on the decline between the end of the nineteenth and beginning of the twentieth centuries ? representing a large gap before the atopy incidence volume increase. This pattern can also be observed in relation to other infectious childhood diseases showing a decline like, for example, rheumatic fever, measles, mumps, tuberculosis and hepatitis A which can be pinned chronologically to the 1940’s; more than a delay to the atopy serge witnessed decades later. Conversely, some diseases have only been in decline after the rise in atopy. The introduction of the measles vaccine in the UK was after the atopy rise, so too hepatitis A virus vaccine [36, 37]. Some infections such as tuberculosis are now considered to be increasing in metropolitan areas [38]. These broad-range observations on centre stage infections implicated in the Hygiene Hypothesis idea support the absence of a timely process of cause and effect as implied by the Hygiene Hypothesis. Similar to the case of rising numbers of tuberculosis infections, official statistics of public health in the UK, for example, reveal an increase in the number of reported gastrointestinal infections in the general population. Food poisoning has been on the rise since the 1970’s. Campylobacter, the main cause of bacterial gastroenteritis continues its upward trend[39] although there is a discussion to add here about raised awareness in community medicine and improvements in laboratory diagnosis of this infection that no doubt contribute to the number of reported cases. Everyone accepts that the number of reported food poisoning cases is something analogous to the tip of the iceberg and the figures under represent the true picture. The actual number of annual food poisoning cases in the general population and, more importantly here, the early childhood sub-group can only be guessed [39]. In addition, respiratory tract infections, save those controlled by vaccination, stubbornly refuse to decline in case numbers [40]. Like gastrointestinal infection rates, the true incidence of respiratory tract infections will be much higher than the proportion pursued to a laboratory diagnosis. For these diseases, generally, there was has been no significant epidemiological change preceding or during the rapid rise of atopic disorders.
Is there a connection between cleaning and hygiene?
One approach to the scientific research has been to consider the practice of and extent of cleaning that decontaminates the built environment and thus reduces the exposure of children to microorganisms. The use of cleaning agents constituted a convenient measuring parameter for a variety of studies. For the Hygiene Hypothesis to be credibly supported, the evidence would need to show a marked and continuous application of effective cleaning agents to those environments related to a significant increase in children’s atopy during their early years. However, comparing rates of atopy with deployment of soaps and detergents shows no such connection [41]. Comparisons of rates of asthma, eczema, and hay fever in 12 European countries in the 1990’s with per capita use of cleaning products, including soap and detergent, showed no correlation in support of the Hygiene Hypothesis [42]. Finer comparisons of the incidence of a specific disease in relation to the use of specific cleaning product types, fabric washing detergents, dishwashing detergents, toilet soaps and hard surface cleaners, resulted in contradictory data. Household bleach has been examined not least because of its high efficacy and universal use, but also because its popularity differs across European countries thus presenting as a variable, but measurable parameter. Bleach consumption in Europe tends to be highest per capita across Southern Europe whilst the Scandinavian region use considerably less bleach compared to, for example, Spain yet atopy rates are comparatively high in Scandinavia [43]. Once more, the varying volumes of bleach used in different industrialised countries does not present any significant correlation with the rates of atopy in these countries. One could, therefore, say that the evidence supporting the role of the Hygiene Hypothesis in relation to household cleaning product use is inconclusive.
Over the course of the twentieth century domestic hygiene practice changed considerably [44]. The first half of the century saw an emphasis on home hygiene through public health advice and governmental initiatives. The pre-antibiotic generation was taught of the benefits of clean walls, floors and ceilings. New consumable cleaning products were marketed that complimented the advice alongside a variety of affordable home cleaning aids. The first half of the twentieth century also saw other, social changes that might be expected to enhance the hygiene of a nation. Bathing and laundering at home became more frequent. Soap manufacture doubled in the United States between 1904 and 1919 (8.4 kg per capita to 16 kg) [44]. Showering became a popular replacement to bathing in the middle of century. For example, in the US the number of homes possessing a shower increased to 87% in 1960 from 61% twenty years previously [44]. Only slightly later Europe was following these social trends. This is useful, albeit proxy parameters, to set against the rate of atopy and thus re-evaluate the Hygiene Hypothesis. In all cases, the rise in atopy occurred at much the same time throughout the industrialized world and did not appear to follow hygiene practice change. This evidence in its entirety produced a considerable force refuting the validity of the Hygiene Hypothesis. This particular theme continues to produce more Hygiene Hypothesis-refuting evidence seemingly wherever it’s looked for and generated. The biggest increases in domestic cleaning products have been dishwashing products and hard surface cleaners (largely detergents based on synthetic surfactants rather than soap). These products came into general use in the 1950s, again pre-dating the rise in atopy [44].
Correct hygienic practice is only transiently effective in decontaminating homes of microorganisms due to the continual potential for surface recontamination [8]. A series of more recent studies have confirmed the fact that pathogenic, commensal and environmental microbes are readily introduced into homes continually by the normal activities of people and their pets, by food, water and circulating air. The obvious conclusion is that modern home environments, despite their ‘clean’ appearance are always contaminated with microbes and the inhabitants are continuously exposed to immunological stimulation by those microbes [8, 45].
A controversial issue emerging from the above data also relates to the inability to set a standard load of exposure to immune-stimulating microorganisms (or lack of it) in order to prevent or allow the onset of atopy. There is still insufficient data exploring the role of, subclinical exposure, symptomatic infection or asymptomatic colonization in the development of atopy.
What is “clean”? People tend to perceive “cleanliness” particularly in the built environment, and especially in their homes, from a visual perspective. From a microbiological perspective, producing surfaces free from microbes, or surfaces at least tending towards higher levels of decontamination that may be used to support the Hygiene Hypothesis, all contaminating microorganisms must be physically removed ? thus a combination of water and cleaning agent is required to apply and wipe off the surfaces of interest [46]. Disinfectants can also be applied to kill or inactivate microorganisms without their specific removal. Whatever the efficacy claimed by the manufacturer of a cleaning agent, common sense suggests that there is a limited effect in decontamination of targeted surfaces [45]. No cleaning strategy (unless it is an industrialised chemically-based sterilisation procedure employed by healthcare facilities to sterilise whole indoor environments) is 100% effective [47]. Cleaning is decontamination and we may consider that we are never free from exposure to microorganisms, despite our best efforts and perception. The evidence from detergent-based domestic cleaning routines supports this view, that is, cleaning is decontamination and offers a limited effect in reducing exposure to microbes [46-48].
Additionally, there is cross contamination to consider. Utensils and materials used for cleaning are themselves contaminated by the microorganisms they target and they present the hazard of redistributing those microorganisms around the same or second environments during their continued use. Contamination can be easily distributed to other surfaces and to the hands of the person performing the cleaning. Cross contamination may be minimized in theory but it will always be questionable as to how much of the theoretical requirements are translated into reliable practice during busy cleaning schedules. Actually, the evidence indicates that minimizing cross contamination warrants a focus of attention [46]. Minimizing cross contamination in the home depends on the effectiveness of cleaning but also on when it happens. Prior to cleaning, 20% of selected hand and food contact sites in the kitchen, bathroom and toilet could be considered as hygienically clean (<10 colony forming units/25 cm2). After detergent-based cleaning, the figure rose to 68% [46]. Although disinfectant products were effective in reducing microbial contamination levels, the effects were relatively short lived. After 90 ? 180 minutes, most surfaces are decontaminated to pre-cleaning levels. Other longer-term studies (3 days - 9 months) concluded that daily or weekly cleaning with disinfectants is unlikely to reduce the risk of exposure to pathogens [49-51].
Because of the types of studies described above and the evidence they have generated, it is reasonable to conclude that cleaning products, in general, have a limited impact on the overall exposure of humans to microbial contamination. What about compliance to home cleaning regimes that a professional would recognise as effective? There are studies that provide insight into these parameters that may be regarded as proxy parameters of the Hygiene Hypothesis. A notable study monitored the handwashing and other hygiene practices of mothers of recently vaccinated (polio) young children shedding the virus in their faeces [52]. The results were telling and suggest that poliovirus is not the only pathogen (to say nothing of microorganisms in general) in contaminating the environment under similar situations. Forty three percent of child carers washed their hands after changing a nappy compared with 76% who washed them after toilet visits. Nappy changing took place mainly in the living room of domestic homes and contact with adjacent surfaces and objects during nappy changing was frequent. Poliovirus was detected on 12% of living room surfaces, 10% of kitchen surfaces and 15% of bathroom sites. Hand contact sites were most frequently contaminated, such as bathroom taps, toilet flushes and door handles, soap dispensers and nappy changing equipment [52, 53]. Whilst this area of research offers limited insights into the degree and extent of microbial contamination in the built environment, they do support the well-recognised view that humans are never free from contact and interaction with microorganisms. The Hygiene Hypothesis has not provided a requirement for the degree and extent that that exposure and interaction should fall below to promote atopy disease. Without a defined threshold the evidence of naturally occurring contamination of environments people inhabit, cross contamination and myriad other factors tend to refute the Hygiene Hypothesis for the simple reason that people are always exposed to and contaminated by microbes from environmental sources. The other key issue question that needs to be addressed via the evidence is: do modern hygiene domestic hygiene and personal cleanliness sufficiently reduce exposure to microbes to adversely impact on the human immune system and give rise to atopic disease? An association between domestic hygiene and personal cleanliness and atopy, if there is one, is supported by, at best, weak evidence. We might consider our modern homes to be ‘clean’ but the evidence demonstrates they are not. Our homes are grossly contaminated with microbes of all types: bacteria, viruses and fungi, as well as dust mites and other insects. The undeniable consequence is that there is ample opportunity for humans to be exposed to microbes in domestic settings, particularly given that we spend so much time indoors [5]. Increased domestic and industrial consumption of cleaning products shows no compelling relationship to increases in atopy. Cleaned surfaces are rapidly re-contaminated and research shows to us that many microorganisms survive for lengthy period of time on environmental surfaces. Although the pattern of microbial exposure in the home may have changed, there is no irrefutable evidence that society’s modern preoccupation for cleanliness has resulted in a significant decline in overall microbial exposure. The Hygiene Hypothesis is again refuted and has not helped itself with data describing what ‘too clean’ actually means.
This article confines itself to a few aspects of the microbiology relevant to the Hygiene Hypothesis. Of course, the interaction of young people with microorganisms is one part of what is clearly a complicated and poorly understood phenomenon. The evidence offered by the microbiological studies generally struggles to promote and support the Hygiene Hypothesis in any robust and reproducible manner but other aspects of the changing face of atopy have been better received by the professionals. Atopy is undoubtedly more common in higher socio-economic groups [2]. Poverty and pollution are identified as risk factors for atopic disease, particularly asthma [5, 54-57]. Then we have the physiological angle. Evidence suggests increased body size of the foetus, resulting from improved maternal nutrition, can damage the development of the foetal immune system. Obesity is a considerable and growing problem in children in developed countries. The connection between adult obesity and asthma appears sound [58]. To consider obesity requires a consideration of modern diet and food trends in industrialised countries. Fast foods, lack of dietary milk, vegetables, fibre and foods rich in Vitamin E are issues that are considered as contributors to the rise in atopic disease [59-61]. The list of candidates that might be included on the list of contributions to the rise in atopy continues. For example, one needs to consider the trend for having children later in life in developed countries, changes to the manufacturing and decontamination processes of modern foods and the additives they contain, the widespread introduction of antibiotics and pesticides in the food chain and lack of exercise in modern society.
Perhaps the research focus needs to include additional evidence and consider not the environmental microorganisms that surround us as responsible for the thrust of the Hygiene Hypothesis, but the microorganisms inside and on us ? the gut microbiome. Research has more recently begun to shed fascinating light on how dependant we are on the health and stability of the communities of microorganisms that share our bodies [62]. Indeed, there is continuing evidence that atopy is connected to the stability of our microbiome from early age [63] which is itself influenced by a combination of the factors, and more, mentioned here. For example, gut microbiota population depletions and perturbed metabolic activity at 3 months are contributing to the development of childhood atopy and asthma [62, 64]. More recently, the relation between the infant microbiome and the risk of developing eczema has been highlighted [65].
More recently, advances in genomic research have produced credible evidence suggesting that epigenetic alterations of the human genome, resulting from gene-environment interactions, contribute significantly to the augmented incidence of atopic disease in populations [66]. Indeed, there is cumulative data supporting the hypothesis that epigenetic changes mediate alterations to food allergies and also asthma and allergic rhinitis [66-68]. It is now increasingly obvious that when exposed to constant environmental pressure, different forms of lifestyle, as well as a variety of constantly changing food habits, epigenetic traits will alter and lead to perturbations in atopy incidences [66, 67].
In view of the increasing antibiotic resistance there is a strong urge to continue and improve good hygiene practice in all levels [69-72]. What is the rational, evidence-based benefit from such a strategy? Rather, would there not be real concerns about public health and infectious disease from a widespread lowering of hygiene standards? Arguably, hygiene should gain more attention to combat the modern globalised way of life [73]. A depiction of some of the major factors contributing to the development of atopy can be seen in Figure 1. International systems including food markets, travel and mass migration, are actively contributing in moving potentially harmful microorganisms around the globe on an unprecedented scale. Increased birth rates in developing countries and aging populations in more developed countries will very likely exacerbate the incidence of transmissible diseases [74-76]. Working parties in Europe and the UK seem to think so [77] and the evidence supports this as an effective way of significantly reducing the development of antibiotic resistance [78]. Therefore, the case for hygiene is compelling [73]. It is a cornerstone in the control of infectious disease ? Florence Nightingale taught us that many decades ago. Reduction in sanitation, cleanliness and established hygiene practices will naturally produce an increase in morbidity and mortality from infection [73]. There is, therefore, a need to establish a new perspective to the current outdated evidence supporting the Hygiene Hypothesis in view of continually emerging strong evidence that increasingly supports its inadequacy to explain recent findings especially in relation to microbiome research and early life exposure to microbes.
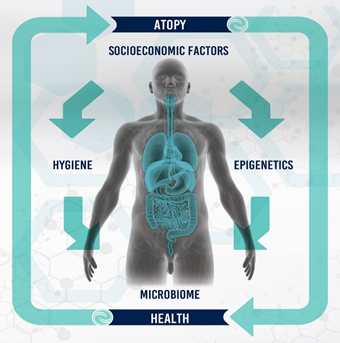
Figure 1: Diagrammatic representation of some of the major factors involved in the development of atopy in humans.
Conflicts of interest
The Authors would like to declare no conflicts of interest in relation to this publication.
Acknowledgements
The Authors would like to thank Megan Hughes and Dr. John Anastasopoulos for constructive revisions of the manuscript.
References
- Strachan DP. Hay fever, hygiene, and household size. BMJ 299 (1989): 1259-1260.
- Strachan DP. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax 55 (2000): S2-S10.
- Majkowska-Wojciechowska, B, et al. Prevalence of allergy, patterns of allergic sensitization and allergy risk factors in rural and urban children. Allergy 62 (2007): 1044-1050.
- von Mutius, E, et al. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy 30 (2000): 1230-1234.
- Platts-Mills TA, MC Carter and PW Heymann. Specific and nonspecific obstructive lung disease in childhood: causes of changes in the prevalence of asthma. Environ Health Perspect 108 (2000): 725-731.
- Platts-Mills TA. The allergy epidemics: 1870-2010. J Allergy Clin Immunol 136 (2015): 3-13.
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347 (2002): 911-920.
- Bloomfield SF, et al. Time to abandon the hygiene hypothesis: new perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect Public Health 136 (2016): 213-224.
- Teplitsky V, et al. House dust mites on skin, clothes, and bedding of atopic dermatitis patients. Int J Dermatol 47 (2008): 790-795.
- Fleming DM, et al. Declining incidence of episodes of asthma: a study of trends in new episodes presenting to general practitioners in the period 1989-98. Thorax 55 (2000): 657-661.
- Ronchetti R, et al. Is the increase in childhood asthma coming to an end? Findings from three surveys of schoolchildren in Rome, Italy. Eur Respir J 17 (2001): 881-886.
- Zöllner IK, et al. No increase in the prevalence of asthma, allergies, and atopic sensitisation among children in Germany: 1992-2001. Thorax 60 (2005): 545-548.
- von Mutius E. Infection: friend or foe in the development of atopy and asthma? The epidemiological evidence. Eur Respir J 18 (2001): 872-881.
- Wickens K, et al. The magnitude of the effect of smaller family sizes on the increase in the prevalence of asthma and hay fever in the United Kingdom and New Zealand. J Allergy Clin Immunol 104 (1999): 554-558.
- Matricardi PM, et al. Sibship size, birth order, and atopy in 11,371 Italian young men. J Allergy Clin Immunol 101 (1998): 439-444.
- Matricardi PM, et al. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ 320 (2000): 412-417.
- Walker MM, N Powell and NJ Talley. Atopy and the gastrointestinal tract--a review of a common association in unexplained gastrointestinal disease. Expert Rev Gastroenterol Hepatol 8 (2014): 289-299.
- Bukowski JA and RJ Lewis. Is the hygiene hypothesis an example of hormesis? Nonlinearity Biol Toxicol Med 1 (2003): 155-166.
- McIntire JJ, et al. Immunology: hepatitis A virus link to atopic disease. Nature 425 (2003): 576.
- Shaheen SO, et al. Measles and atopy in Guinea-Bissau. Lancet 347 (1996): 1792-1796.
- von Hertzen LC. Puzzling associations between childhood infections and the later occurrence of asthma and atopy. Ann Med 32 (2000): 397-400.
- Paunio M, et al. Measles history and atopic diseases: a population-based cross-sectional study. Jama 283 (2000): 343-346.
- Paunio M, et al. Acute infections, infection pressure, and atopy. Clin Exp Allergy 36 (2006): 634-639.
- Obihara CC, et al. Mycobacterial infection and atopy in childhood: a systematic review. Pediatr Allergy Immunol 18 (2007): 551-559.
- Strannegard IL, et al. Prevalence of allergy in children in relation to prior BCG vaccination and infection with atypical mycobacteria. Allergy 53 (1998): 249-254.
- Soysal A, et al. Lack of an inverse association between tuberculosis infection and atopy: by T-cell-based immune assay (RD1-ELISpot). Pediatr Allergy Immunol 19 (2008): 709-715.
- Wennergren G and S Kristjansson. Relationship between respiratory syncytial virus bronchiolitis and future obstructive airway diseases. Eur Respir J 18 (2001): 1044-1058.
- Lell B, et al. Atopy and malaria. Wien Klin Wochenschr 113 (2001): 927-929.
- Barnes KC, AV Grant and P Gao. A review of the genetic epidemiology of resistance to parasitic disease and atopic asthma: common variants for common phenotypes? Curr Opin Allergy Clin Immunol 5 (2005): 379-385.
- Matricardi PM, et al. Cross sectional retrospective study of prevalence of atopy among Italian military students with antibodies against hepatitis A virus. BMJ 314 (1997): 999-1003.
- Martinez FD and PG Holt. Role of microbial burden in aetiology of allergy and asthma. Lancet 354 (1999): SII12-II15.
- Martinez FD. The coming-of-age of the hygiene hypothesis. Respir Res 2 (2001): 129-132.
- Matricardi PM and S Bonini. High microbial turnover rate preventing atopy: a solution to inconsistencies impinging on the Hygiene hypothesis? Clin Exp Allergy 30 (2000): 1506-1510.
- Simonsen L, et al. Infectious Disease Surveillance in the Big Data Era: Towards Faster and Locally Relevant Systems. J Infect Dis 214(2016): S380-S385.
- Petri WA, et al. Enteric infections, diarrhea, and their impact on function and development. The Journal of Clinical Investigation 118 (2008): 1277-1290.
- Bolton DL, et al. Priming T-cell responses with recombinant measles vaccine vector in a heterologous prime-boost setting in non-human primates. Vaccine 30 (2012): 5991-5998.
- Whelan J, et al. Evaluation of Hepatitis A Vaccine in Post-Exposure Prophylaxis, The Netherlands, 2004-2012. PLoS ONE 8 (2013): e78914.
- Glaziou P, et al. Global Epidemiology of Tuberculosis. Cold Spring Harbor Perspectives in Medicine 5 (2015): a017798.
- Kaakoush NO, et al. Global Epidemiology of Campylobacter Infection. Clin Microbiol Rev 28 (2015): 687-720.
- Zaas AK, et al. The current epidemiology and clinical decisions surrounding acute respiratory infections. Trends Mol Med 20 (2014): 579-588.
- Pickup J. Trends in home and consumer hygiene, in Are we too clean? ? a question of immunity balance. RIPH Symposium Report, R. Stanwell-Smith, Editor. Royal Institute of Public Health: London 2003: 6-7.
- Beasley R, et al. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 351 (1998): 1225-1232.
- Tarlo S, P Cullinan and B Nemery. Occupational and Environmental Lung Diseases: Diseases from Work, Home, Outdoor and Other Exposures, ed. S Tarlo, P Cullinan and B Nemery 2010.
- Greene VW. Cleanliness and the health revolution. 1984, New York, N.Y. (475 Park Ave. South, New York 10016): Soap and Detergent Association.
- Beumer R, et al. The infection potential in the domestic setting and the role of hygiene practice in reducing infection. 2002, International Scientific Forum on Home Hygiene.
- Exner M, et al. Household cleaning and surface disinfection: new insights and strategies. J Hosp Infect 56 (2004): S70-S75.
- Barker J, IB Vipond and SF Bloomfield. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J Hosp Infect 58 (2004): 42-49.
- Cogan TA, SF Bloomfield and TJ Humphrey. The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcases in the domestic kitchen. Lett Appl Microbiol 29 (1999): 354-358.
- Scott E, SF Bloomfield and CG Barlow. Evaluation of disinfectants in the domestic environment under 'in use' conditions. J Hyg (Lond) 92 (1984): 193-203.
- Josephson KL, JR Rubino and IL Pepper. Characterization and quantification of bacterial pathogens and indicator organisms in household kitchens with and without the use of a disinfectant cleaner. J Appl Microbiol 83 (1997): 737-750.
- Larson E and C Gomez Duarte. Home hygiene practices and infectious disease symptoms among household members. Public Health Nurs 18 (2001): 116-127.
- Curtis V, et al. Hygiene in the home: relating bugs and behaviour. Soc Sci Med 57 (2003): 657-672.
- Aiello AE and EL Larson. What is the evidence for a causal link between hygiene and infections? Lancet Infect Dis 2 (2002): 103-110.
- Rona RJ. Asthma and poverty. Thorax 55 (2000): 239-244.
- Kramer JM, et al. Campylobacter contamination of raw meat and poultry at retail sale: identification of multiple types and comparison with isolates from human infection. J Food Prot 63 (2000): 1654-1659.
- Miller JE. Predictors of asthma in young children: does reporting source affect our conclusions? Am J Epidemiol 154 (2001): 245-250.
- McConnell R, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet 359 (2002): 386-391.
- Camargo CA, et al. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 159 (1999): 2582-2588.
- Hijazi N, B Abalkhail and A Seaton. Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax 55 (2000): 775-779.
- Fogarty A, et al. Dietary vitamin E, IgE concentrations, and atopy. The Lancet 356 (2000): 1573-1574.
- Bodner C, et al. Antioxidant intake and adult-onset wheeze: a case-control study. Aberdeen WHEASE Study Group. Eur Respir J 13 (1999): 22-30.
- Fujimura KE, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 22 (2016): 1187-1191.
- Tanaka M and J Nakayama. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int 66 (2017): 515-522.
- Stiemsma LT, et al. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci (Lond) 130 (2016): 2199-2207.
- Zheng, H, et al, Altered Gut Microbiota Composition Associated with Eczema in Infants. PLoS One 11 (2016): e0166026.
- Bunning BJ, RH DeKruyff and KC Nadeau. Epigenetic Changes During Food-Specific Immunotherapy. Curr Allergy Asthma Rep 16 (2016): 87.
- Yang IV, CA Lozupone and DA Schwartz. The environment, epigenome, and asthma. J Allergy Clin Immunol 140 (2017): 14-23.
- Samanta S, et al. Epigenetic dysfunctional diseases and therapy for infection and inflammation. Biochim Biophys Acta 1863 (2017): 518-528.
- Sommer MOA, et al. Prediction of antibiotic resistance: time for a new preclinical paradigm? Nat Rev Microbiol 2017.
- Crofts TS, AJ Gasparrini and G Dantas. Next-generation approaches to understand and combat the antibiotic resistome. Nat Rev Microbiol 15 (2017): 422-434.
- Dickey SW, GYC Cheung and M Otto. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov 16 (2017): 457-471.
- Otter JA, et al. Controversies in guidelines for the control of multidrug-resistant Gram-negative bacteria in EU countries. Clin Microbiol Infect 21 (2015): 1057-1066.
- Lambrecht, B.N. and H. Hammad, The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol 18 (2017): 1076-1083.
- Shanson DC. Infections of the gastrointestinal tract, in Microbiology in clinical practice, D.C. Shanson, Editor. 1999, Butterworth-Heinemann: Oxford ; Boston. p. 251-258.
- Skirrow MB. Campylobacteriosis, in Zoonoses : biology, clinical practice, and public health control, S.R. Palmer, E.J.L. Soulsby, and D.I.H. Simpson, Editors. 1998, Oxford University Press: Oxford. p. 37-46.
- Forman, D, Helicobacter pylori infection and cancer. Br Med Bull 54 (1998): 71-78.
- Anon. Official Journal of European Communications C195 (1999): 3.
- Schmitz FJ, et al. Impact of hygienic measures on the development of methicillin resistance among staphylococci between 1991 and 1996 in a university hospital. J Hosp Infect 38 (1998): 237-240.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
