Relation of Schizophrenia to Psychosis with Respect to Immune Dysregulation and Its Impact on Memory and Learning
Yumna Matanat, Ifrah Amjad Naseer *, Arooba Murtaza, Noor Fatima Tareen, Ayesha Khalid, Wareesha Nabeel, Iman Afzal, Samreen Riaz
Institute of Microbiology and Molecular genetics, University of the Punjab, Lahore, Pakistan.
*Corresponding author: Ifrah Amjad Naseer, Institute of Microbiology and Molecular genetics, University of the Punjab, Lahore, Pakistan
Received: 24 February 2022; Accepted: 08 March 2022; Published: 14 March 2022
Article Information
Citation: Yumna Matanat, Ifrah Amjad Naseer, Arooba Murtaza, Noor Fatima Tareen, Ayesha Khalid, Wareesha Nabeel, Iman Afzal, Samreen Riaz. Relation of Schizophrenia to Psychosis with Respect to Immune Dysregulation and Its Impact on Memory and Learning. Archives of Microbiology and Immunology 6 (2022): 123-140
View / Download Pdf Share at FacebookAbstract
Since decades, immune dysfunction and the involvement of infectious agents in the pathophysiology of schizophrenia are under greater importance. Schizophrenia is a long lasting state of mental uncertainty that leads to unreliable perception, not suitable actions and feelings, and a sense of mental fragmentation. Patients with schizophrenia show different characteristics of immunological diseases, such as previous infections, anti-inflammatory cytokines or other inflammatory proteins in blood-co-existence of other autoimmune diseases. Its diagnosis is done over a large period of time showing continuous signs of the disturbance that persists for at least six months. Once detected, the psychiatrist diagnosis is made through a series of psychic tests, to avoid the diagnosis of other mental states or diseases. Currently, hundreds of genes across many chromosomes have been identified for schizophrenia, including some genes from the Major Histocompatibility Complex (MHC). Genetic studies offer the hope of gaining new insight into the mechanisms that increase a person’s susceptibility to develop schizophrenia, in particular by identifying potential new targets for treatment. But the genetic basis of schizophrenia has proven exceptionally difficult to unravel. Given the rapid evolution of molecular genetic research technologies, breakthrough progress is imminent in the near future. In this paper we will look on the major role of cytokines in schizophrenia, neuro-inflammation and its effects on memory and learning through cytokines, genetic aspects, and correlation between schizophrenia and (autoimmune and atopic diseases) and lastly its treatment with respect to the immune system.
Keywords
<p>Psychotic effects, microglial activation, cytokines, neuroinflammation, memory impairment, brain activity and dopamine levels, Interferons and interleukins, anti-psychotic drugs</p>
Article Details
1. Introduction
Schizophrenia is a chronic psychiatric disorder that affects almost 1% of the world’s population. Schizophrenic patients experience behavioral and intellectual problems such as inability to express one’s emotions, detachment from reality, hallucinations, and difficulties in social interactions. Schizophrenic patients often encounter hardships in work and studies, i.e. their memory and learning is negatively affected.
Schizophrenia is a medically identified mental illness, with roots in genetics, immune system, and environmental factors. It presents in late teenage to early adolescence and is diagnosed through a variety of brain and blood tests, and physical examination. Typical symptoms include disorganized speech, delusion, hallucinations and catatonia. Here, we discuss the immune-genetic aspects of this disorder, its root causes, manifestations, and treatment in an attempt to review the underlying reasons behind the origin of schizophrenia and its influence on daily life activities such as learning and memory development [1, 2, 3]
2. Cytokines profile in Schizophrenia
Cytokines are small proteins that lead to the communication among different cells of the immune system. They exist in two forms i.e. soluble forms or membrane-bounded forms. Cytokines alert an immune cell to elevate or decline the enzymatic action and hence provide the signals to the cells whether to die or persist. Cytokines are produced by T-helper cells, macrophages or dendritic cells. The major functions of cytokines are the activation of humoral or cellular immunity, the pro-inflammatory or anti-inflammatory responses, the settlement of hematopoiesis, and the healing of wounds [4]
Cytokines are particularly associated with Schizophrenia. One of the aspects of Schizophrenia is such that the levels of cytokines are altered. They are considered as a potent leader of Schizophrenia development.
2.1-Categories of cytokines in Schizophrenia
|
Category |
Example |
|
Enhanced cytokines |
Interleukin-6, TNF-α, Interleukin-12, IL-1β |
|
Cytokines that are not altered |
IL-2, IL-4, IL-17 |
|
Enhanced and non-altered |
IL-8, INF-γ (Interferon) |
|
Can be enhanced or declined |
IL-10 [5] |
3. Meta-analysis on cytokines in Schizophrenic patients
Cytokines concentration is modified by different factors like age, gender, obesity, hormone therapy and trauma, etc. Studies have been done on Africans as they demonstrated higher inflammatory rates as compared to whites. This was due to poor socio-economic status (SES). After study conduction, it was demonstrated that Schizophrenic patients have usually poor SES. Insomnia, a common feature of Schizophrenic patients, is also due to abnormal levels of cytokines. [6]
Smith and Maes study said that macrophages and T-helper cells produce Tumor Necrosis Factor and Interleukin that play a key role in abnormal levels of cytokines and Schizophrenia patients. Data from 60 studies was gathered that demonstrated the presence of IFN- γ, IL-2, IL-4, IL1RA, TNF- α and IL-10 in Schizophrenic patients. This is characterized by inflammatory syndrome in Schizophrenia [7].
According to a meta-analysis conducted by Miller, Buckley et al, on the basis of cytokines, patients were divided into three groups: Drug-naïve first episode psychosis, acute-relapse phase of psychosis and medicated outpatients of Schizophrenia. Results proclaimed that patients with first episode psychosis and acute-relapse psychosis were having greater concentrations of IL-2, IL-6, TNF- α and IFN- γ. These concentrations ultimately constructed a relationship between psychosis and Schizophrenia. Those Schizophrenic patients that had undergone anti-psychotic drugs were having declined levels of these cytokines. So, the conclusion was made, saying that, “Schizophrenia status and cytokine profile highly depend on anti-psychotic treatment and the clinical position of patient.” [8]
4. Insomnia in Schizophrenia in light of cytokines
Cytokines also play a part in sleep regulation. Interleukin 1 (IL-1) and TNF- α regulate the sleep behavior. Alterations in these cytokines lead to insomnia which is a greatest hallmark of Schizophrenic patients. Loss of sleep is accompanied by many different symptoms in Schizophrenia like depression, memory impairment and fatigue. Insomnia in Schizophrenia leads to the development of chronic inflammation which is mediated by cytokines. There is an inhibitor of IL-1 i.e. the IL1-receptor antagonist (IL1RA) that actually declines the sleep levels in such patients. Tumor Necrosis factor soluble receptor (sTNFR) inhibits the sleep levels [9].
5. Psychotic aspects and cytokines
Cytokines play a very important role in inflammatory responses. Several mood disorders are linked to pro-inflammatory status of cytokines in Schizophrenic patients. Psychological stress in Schizophrenia is directly linked to immune system and nervous system. Cytokines signal the brain to undergo neuro-immune, neurochemical and behavioral changes leading to change in mood. This change in thinking and mood leads to psychiatric disorder like Schizophrenia ultimately causing psychological and physiological stress.
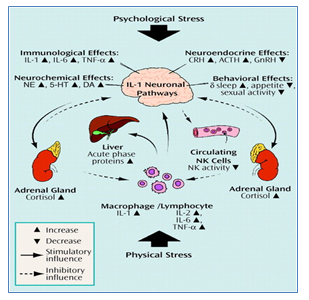
Figure 1: Stress in Schizophrenia by cytokine action in communication with different neuronal pathways: Both psychological stress (e.g., academic stress, depression) and physical stress (e.g., infection, trauma) can activate interleukin-1 (IL-1). IL-1 activates other cytokines and acute phase proteins to elevate the stress and psychiatric disorders. IL-1 activates neurochemical and neuroendocrine factors that stimulate psychological stress in Schizophrenia patients. IL=interleukin, TNF-α=tumor necrosis factor-α, CRH= corticotrophin-releasing hormone, GnRH=gonadotropin-releasing hormone, NE=norepinephrine, 5-HT=serotonin, DA=dopamine, NK=natural killer. [10]
6. Microglial activation in Schizophrenic patients
Microglia is derived from mesoderm like blood and immune system cells. They play a role in innate immunity and leads to the formation of cytokines and free radicals as well. Microglial activation in the case of Schizophrenic patients leads to unnecessary synapse pruning which cause neuro-inflammation in brain and has prolonged impact on learning behavior and memory. Due to the production of radicals, there is an oxidative stress that renders its part in pathogenesis of Schizophrenia. [11]
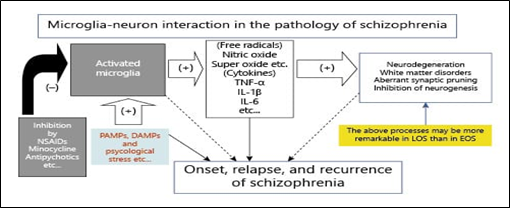
Figure 2: Role of microglia in Schizophrenia. Activated microglia leads to the formation of cytokines that cause disorders in white and gray matter of brain by neurodegeneration. As a result, synapse formation is inhibited somehow that causes the onset of Schizophrenia. Antipsychotic drugs like non-steroidal anti-inflammatory drugs (NSAIDs) can inhibit the microglial activity. PAMPs: Pathogen associated molecular pattern molecules. DAMPs: Damage-associated molecular patterns. Microglia can become activated through PAMP and DAMP. [12]
7. Immunological causes of schizophrenia
As we know, schizophrenia is a brain disorder, in which the structure and function of certain brain activity is altered. In this disease the part of the brain that effect the working of memory and learning activity of the patient, mainly effecting the prefrontal and medial temporal region of the brain. We are more interested in finding the immunological aspects of schizophrenia. The immunity is developed because of cytokines. So, when cytokines are affected the adaptive immunity is weakened. The human is unable to produce antibodies that help them to defend against these neurological damages.
In order to find the immunological causes of schizophrenia, the scientists have to draw an experiment that include patient suffering from different neurological disorder i.e., patients suffering from schizophrenia, patients suffering from bipolar disorder and some were healthy people that were used as control whether to see if the experiment being held is right or wrong. For the immunological aspect to be involved the blood plasma was taken from each group and it was being examined under MRI to see what immunological factor is involved.
8. Experimental evidence of cytokines
The experiment involved individual in an approximate ration or 2:1 of effected and unaffected individuals. The experiments showed that their cytokine profile was affected. The immune response that is mediated by cytokines was affected and there were total of 5 mediators that were actually affected.
- Interleukins; they act as pro inflammatory cytokines. Without them our body can’t differentiate.
- Helper T cytokine 1; they act as the creator of pro inflammatory response
- Helper T cytokine 2; they balance the effect if type 1 T-cell.
- Helper T cytokines 17; act as defense mechanism against pathogens
- Helper T regulator.
The first meta-investigation of plasma test displayed there was increment level of interleukin (IL) - 1 receptor enemy, dissolvable IL-2 receptor, and IL-6 in people with schizophrenia. In a moment meta-examination, included expanded fiery cytokines IL-1β and IL-6, and the administrative cytokine changing development factor-β (TGF-β). Significantly, these cytokine levels were standardized after treatment. Potential characteristic markers that stayed raised after antipsychotic treatment like raised IL-12, interferon-γ (IFN-γ), and cancer rot factor-α (TNF-α) were likewise recognized. Taken together, the two investigations obviously show that cytokine levels are modified in the plasma/sera of people with schizophrenia.
9. Relation of schizophrenia and bipolar cytokines
Sometimes there are people who have both of these diseases. This is because the cytokines of these 2 overlaps. A little study of bipolar syndrome specially their cytokine reveals there is a similarity between bipolar and schizophrenia especially in their cytokines. When a study was conducted in a ration of approximately 1:1 of people having bipolar and people having no disorder concluded in their meta- analysis that their plasma have increase the level of similar mediators such IL-6 receptor, TNF-α, soluble IL-2, IL-4, IL-10, IL- 1 receptor antagonist, and soluble TNF-1 receptor.
On a serious note, there was high level of IL-4 and IL-10, but in schizophrenia patient their level was not that high.
On the other hand, the patients of bipolar have less level of IFN-γ which was seen high in schizophrenia patient. But the study of similarity between these two was on some candid cytokines.
10. Genetics involved in schizophrenia
Schizophrenia is developed by changing factors in genes. There is a chance of increasing the risk of these diseases by few changes in the gene even a very small change put the chance at risk. There is not much knowledge about its genetic fate and not many people have studied in detail as it is a rare disease and only first stage of this disease is curable. There are chances of its relationship with the environment. For instances, if a new born is exposed to some pathogen before he\ she is born or if the delivery is done in stressful condition.
If one chromosome out of many gets multiplied and gets missed, is the main cause of having this disorder as it put other genes at risk too. If we talk about which chromosome gets multiplied or deleted so a chromosome called 22Q11 gets a little change in the 22 region. This happens only in rare cases. If a someone has schizophrenia by deletion of this chromosome so there are chances of having other diseases as well e.g., weak immunity, heart diseases and babies born with open mouth pallet caused by deletion syndrome 22Q11.2. The chances of running this disease in families but we know that it doesn’t run in families.
11. Neuro inflammation In Schizophrenia
Schizophrenia is an intense brain related illness that disrupts the normal functioning of the mind and affects roughly 1% of the population in the whole world. Schizophrenia patients normally suffer from positive symptoms including delusions, hallucinations and disorganized speech, and negative symptoms such as decreased response [19].
The immune system includes a complex mechanism of cells and balancing substances that has developed largely to protect human beings from infection and malignancy. It may be widely idea about as which includes an innate reaction, performing as a rapid, non-particular first line of defense, and an adaptive reaction this is slower and antigen particular. The innate response is balanced through neutrophils and macrophages that sense them and kill invading organisms. Inflammatory cytokines, secreted through macrophages and other cells, assist this process. The adaptive reaction includes immunological reminiscence, and consists of T (thymic) lymphocytes that sense antigens and cause breakdown of inflamed cells, and B lymphocytes that release antibodies as a part of the humoral reaction [19].
Help for an immune-mediated reason in schizophrenia comes from genome-wide association studies that file big relations between schizophrenia and markers close to the most important histocompatibility complicated (MHC) location on chromosome 6. This region performs incorporation of many immune-related genes, together with those related to antigen presentation and inflammatory mediators. A 2014 genome-extensive association study identified 108 genetic loci (83 formerly undetected) that are related to schizophrenia. Broadly, these represent genes expressed within the mind and immune cells concerned in adaptive immunity (CD19 and CD20B lymphocytes), further to the MHC. Moreover, associations with the immune-associated genes remained good sized after the MHC area becomes excluded, suggesting that those findings have been now not pushed by using the sturdy association at the MHC. Any other genome-huge related study suggested considerable genetic overlap related to the MHC vicinity among schizophrenia and multiple sclerosis, a situation characterized by immune dysfunction. [20]
12. Inflammatory Cytokines
[21]Meta-analyses of many studies show that schizophrenia is related with disturbance of the cytokine milieu and the propensity for the production of pro anti-inflammatory cytokines. Longitudinal research of anti- inflammatory markers and next disorders are scarce. Findings from the ALSPAC birth cohort suggested that multiplied serum awareness of the pro anti-inflammatory cytokine interleukin 6 at age 9 years is related with twofold increased possibility of betterment of a psychotic ailment at age 18 years. The study also proves a sturdy dose-reaction affiliation between increased interleukin 6 concentrations in formative years and subsequent chance of subclinical psychotic reviews in young maturity, which persists after several capability confounders are taken under deliberation, careless of gender, weight of the body, and psychological and behavioral problems previous the size of youth interleukin 6.
No links between serum C-reactive protein concentrations at baseline and upcoming psychiatric issues were observed, but another longitudinal study suggested improved risk of late, or very late, onset schizophrenia for accelerated serum C-reactive protein concentrations at baseline. Moreover longitudinal studies are needed to confirm whether the increase in serum concentrations of pro anti-inflammatory cytokines in schizophrenia and linked psychosis is the cause or effect of contamination, even though these findings suggest causal mechanisms. [21]
[22] Antipsychotic-naive first-episode psychosis and acute psychotic relapse are also related with increased serum concentrations of interleukin 6 and other pro anti-inflammatory cytokines, together with tumor necrosis component α (TNFα), interleukin 1β, interferon γ, and decreased serum concentrations of anti-inflammatory cytokine interleukin 10, which can be normalized after remission of symptoms with antipsychotic treatment. Decreased interleukin 2 which is manufacturing in vitro by way of T cells accumulated from sufferers with schizophrenia become thought to be pointing towards the direction of autoimmune reasons of psychosis. However, acute psychosis is not associated with changes in serum interleukin 2 concentrations. The concentration of soluble interleukin 2 receptor will increase in schizophrenia that is probably to be a compensatory mechanism that inhibits interleukin 2 manufacturing. For that reason, the statistics are constant with an increase in pro anti-inflammatory cytokines in acute psychosis. But few studies have adjusted for critical immune-modulatory factors consisting of body mass or smoking, or examined cytokines in cerebrospinal fluid, in which an increase in interleukin 6 concentration has been said in schizophrenia.
One study suggested increased serum interleukin 6 concentrations in humans with an at-risk intellectual country for psychosis compared with controls. some statistics endorse that serum cytokine concentrations, including interleukin 6, are related with contamination severity, period, and antipsychotic therapy, but little is thought regarding the institutions between pressure, cortisol and cytokine concentrations in one of a kind ranges of schizophrenia. Consequently, greater studies are had to apprehend the associations between cytokine concentrations, disease prodrome, progression, and remedy reaction [22].
Longitudinal findings of first-episode psychosis, persons at medical high hazard for development of psychosis, and people with remedy refractory infection could be beneficial to have a look at these issues. rather than merely reporting group variations in cytokine serum or cerebrospinal fluid concentrations, future studies have to study associations between cytokines, cognitive and social functioning, comorbid physical illness, and structural and practical brain indices in people with psychosis and healthy controls.[23]
13. Effect of Neuro inflammation on Memory and Learning Through Cytokines
Latest studies have made some big advancement in arranging the outcomes of precise cytokines within the brain on learning and memory. But, these studies have also exposed important roles of cytokines in modulation of memory. Given the complexity of inflammatory signaling in the mind, it is suggested that transferring the point of interest from man or woman cytokines to networked activation of cytokines may be a constructive method to acknowledge the impact of inflammatory signaling on memory and cognitive properties.
Interleukin 1β (IL-1β), Interleukin 6 (IL-6) and tumor necrosis thing α (TNFα) are a number of the most typical and normally studied cytokines in brain. These proteins are strongly upregulated inside the bloodstream after systemic inflammatory activities which include LPS injection, sepsis model, surgical procedure, and different peripheral accidents. Similarly, IL-1β, IL-6 and TNFα are strongly expressed inside the hippocampus after manipulations within the periphery or brain and are consequently properly positioned to modulate memory [24].
There may be a fewer evidence for involvement of IL-1β, TNFα, and IL-6 in precise memory processes such as acquisition, consolidation, or retrieval. As an example, peripheral IL-6 stages correlate with reminiscence retrieval and submit-schooling injection of LPS disrupts consolidation of context fear conditioning through IL-1 .Maximum research. But, have used transgenic models, persistent injection, or acute injection of cytokine or inflammatory stimulus previous to training, demonstrating roles in modulation of learning and memory, but obscuring their role in unique memory methods [24].
14. The Genetic Aspect of Schizophrenia – The Genes Involved
Schizophrenia, like many other biological disorders, is not the outcome of mutation in a single gene, rather it is a polygenic trait i.e. a cumulative effect produced by a number of different mutated genes [25]Currently, hundreds of genes across many chromosomes have been identified as ‘candidate genes’ for schizophrenia, including some genes from the Major Histocompatibility Complex (MHC). Some of the important genes include dysbindin (DTNBP1), neuregulin (NRG1), catechol-O-methyltransferase (COMT), as well as some alleles of C4 gene encoding the complement system [26].
14.1- DNTNBP 1
Dystrobrevin-binding protein 1, or DTNBP1, is a gene located on the short arm of chromosome 6, and its encoded protein, dysbindin, is present in high amounts in the CNS. It is a component of Biogenesis of Lysosome-related Organelle Complex 1(BLOC-1). In the brain and spinal cord, BLOC-1 subunits are derived from the synaptic membranes, leading to the hypothesis that these subunits play a part in the control and delivery of post-synaptic receptors. High normal amounts of dysbindin are found in hippocampus, neocortex and cerebellum, which are some of the important areas affected in schizophrenia.
Although highly conserved, SNPs of DTNBP1 that contribute to schizophrenic symptoms have been identified. Important SNPs include rs1997679 and rs9370822, which cause visual hallucinations; rs4236167 causes auditory ones; rs9370822 and rs9370822 are associated with olfactory hallucinations. Furthermore, a reduced expression of mRNA and protein product of DTNBP1 gene has been proven to be associated with the cognitive deficits that schizophrenic patient’s exhibit. [27]
14.2-NRG1
Neuregulin, like dysbindin, is abundantly present in cerebellum, midbrain and frontal cortex, and is involved in myelination, neuronal migration, and function of neurotransmitter receptors. Neuregulin is cleaved by the protease β-secretase 1, also called BACE 1, to perform its role by activating the ErbB signaling pathway. Disruption in the amount of NRG1, and improper or no cleavage by BACE 1 can lead to a number of different endphenotypes associated with schizophrenia, including PPI and eye movement deficits, and the neuropathology of schizophrenia. [28]
14.3-PPI or pre-pulse inhibition, is a phenomenon in which a weak pulse can lower the startle response that a subsequent stronger stimulus would produce. [29] It allows the organism to adapt to strong environmental stimuli. Schizophrenic patients or healthy people who carry the risk genotype of NRG, exhibited reduced or impaired pre-pulse inhibition. [28]
Eye Movement Deficits in schizophrenia patients have been widely investigated, though there is no enough proof of involvement of NRG1 genotypes in impaired eye movement [28]. SPEM, which stands for Smooth Pursuit Eye Movement, refers to smooth eye movements required to stabilize the image of a moving object [30]. Some studies found the SNP8NRG243177 genotype to be related to impaired SPEM in healthy individuals, while others demonstrated a significantly higher percentage of impaired SPEM in schizophrenic patients as compared to that in healthy controls.
Different studies have also shown that the NRG1 SNP rs3924999 is linked to the functioning of ASEM in healthy individuals, which proposes a potential role of different NRG1 genotypes in the eye movement deficits of schizophrenia patients [28].
Neuropathology of schizophrenia includes the characteristics such as brain atrophy, reduction in brain volume, and reduction in the white and gray matter in many areas of the brain. Since NRG1 is responsible for myelination of neurons, these characters can be attributed to variation in the NRG1 gene structure in schizophrenia. However, more studies are required to link NRG SNPs to neuropathology of schizophrenia [28].
14.4-Catechol-O-Methyltransferase:
The COMT gene is one of the most important candidate genes for schizophrenia because of two well-known reasons: its involvement in the metabolism of catecholamine neurotransmitters including dopamine, and its presence in the region of a common microdeletion of chromosome 22 (Digeorge syndrome) which results in several different psychiatric manifestations, of which schizophrenia is relatively important [31].
14.5-Dopamine Metabolism and Val/Met SNP of COMT:
COMT is particularly involved in catabolic clearance of dopamine in the prefrontal cortex (PFC), a major site affected in schizophrenia. The most recent hypothesis for dopamine utilization presents an “inverted U shaped curve” to demonstrate the amount of dopamine required for healthy functioning of the PFC. The model states that there is only a narrow range in which dopamine levels will produce optimal brain activity [32].
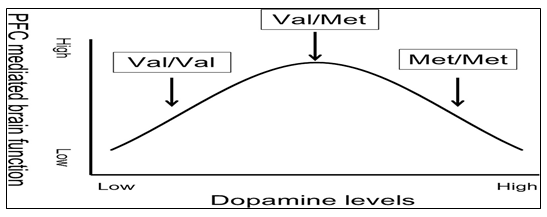
Figure 5: The Inverted U shaped Curve for Dopamine Utilization: Optimal PFC activity is seen at intermediate levels of dopamine found in Val/Met individuals. Individuals on either side of this curve have abnormally high or low levels of COMT which accounts for various disorders such as schizophrenia and Parkinson’s disease [33].
An SNP rs4680 in the codon 108 of the soluble cytoplasmic COMT (S-COMT), or at the codon 158 of membrane bound COMT (MB-COMT) results in a Valine-to-Methionine polymorphism, with the Valine and Methionine allele conferring high and low COMT activity, respectively. No significant association has been found between Val/Met substitution and schizophrenia. However, if factors other than the SNP rs4680 are considered, including environmental effects, effects of other SNPs in the COMT gene, population differences, and allele and phenotypic frequencies that may vary in and among populations, the Val/Met variation can be attributed to schizophrenia. According to the inverted-U model then, an individual to the left of the curve who has low normal levels of dopamine in brain would benefit more with the Methionine allele (low activity), and for an individual with higher normal levels of dopamine, the Valine allele (high activity) would be more beneficial. The high and low normal levels of dopamine are, of course, determined by factors other than the Val/Met SNP, and must be taken into account when studying the association of rs4680 with schizophrenia [32].
15 Digeorge Syndrome:
Digeorge syndrome refers to a microdeletion of about 3 Mb, which spans more than forty genes. Commonly known as 22q11.2 deletion syndrome and velocardiofacial syndrome (VCFS) [34], this microdeletion includes the COMT gene. People with VCFS have a higher rate of developing different kinds of psychiatric disorders such as schizophrenia, attention deficit disorder (ADD), obsessive compulsive disorder (OCD) [32], autism, and learning and intellectual disabilities [34]. In addition, schizophrenic patients have shown increased rates of the 22q11.2 deletion as compared to healthy individuals. These findings make COMT a strong positional candidate for schizophrenia among other genes in the 3 Mb deletion regions [31].
16 Complement Component 4 (C4):
The C4 gene is the strongest candidate for schizophrenia out of the >100 genes that have been linked to the disorder so far. C4 not only explains the neurological symptoms of schizophrenia, but also provides an association of the disorder to the immune system.
C4 has two functional forms i.e. C4A and C4B, and is present in great variation among individuals, both with respect to DNA sequence and copy numbers. According to a study, the structural and copy number variations that produced an increased expression of C4A were directly associated with high risks of schizophrenia development.
In addition to its role in the body’s immunity, C4 also plays a major part in synaptic pruning. It tags a weak synapse with another protein of the complement system called C3 so that it can be identified for pruning. Excessive expression of C4 thus leads to excessive synaptic pruning [35], ultimately resulting in fewer synapses, which is a characteristic feature of the brains of schizophrenic patients [36]
17 Schizophrenia as a consequence of Intrauterine or early childhood infections
Several intrauterine and early childhood infections were the first reasoning considered in the etiology of schizophrenia. Environmental studies have revealed that the more children that were born in winter and spring developed schizophrenia. This was considered as a link between early infections and schizophrenia. Scientists made an effort to find the correlation between Schizophrenia and inflammatory processes in the early 20th century.
Meninger has listed 20 cases of psychosis survivors in the epidemic of influenza. About one third of the patients were identified as ‘domentia preecox’( the term previously used for schizophrenia). Scientists began investigating each possible infection that was likely to show same results after the initial idea of influenza as a possible cause of schizophrenia. Some other intrauterine infectious diseases were suggested as culprits other than influenza infection which includes: birth canal infections, juvenile paralysis, and parasitic infection with Toxoplamsa gondii, rubella, herpes virus type and upper repiratory tract inflammations [37].
|
Study |
Psychotic disorder |
Type of childhood CNS infection |
Risk estimate (95% CI) |
Measure of risk |
|
Non-affective psychosisa |
Cytomegalovirus |
16.6 (4.30–65.10) |
Risk ratio |
|
|
Mumps virus |
2.7 (1.20–6.20) |
|||
|
Schizophrenia |
CBV-5 meningitis |
12.5% (− 4.90%–29.90%) |
Cumulative incidence |
|
|
Schizophrenia |
Tuberculosis |
15 (2.00–120.00) |
Odds Ratio |
|
|
Affective psychosis |
Tuberculosis |
12 (1.60–91.00) |
||
|
Chicken pox |
0.33 (0.20–0.70) |
Infections were thought to be agents that might change the development of fetal brain, or were capable to change material predisposed to schizophrenia which will be phenotypically expressed. Some scientists have suggested that an increase in inflammatory protein in pregnancy is sufficient to cause schizophrenia in a child without a real infection. C- reactive protein (CRP) and tumor necrosis alpha were considered as the most adverted causative agent. Except during pregnancy, infection might have a role in the schizophrenia in the postpartum period as well. A meta-analysis of 2424 cases revealed that early childhood CNS infection nearly double the chances of developing schizophrenia comparing with healthy controls [38].
18 Correlation between schizophrenia and autoimmune diseases
The notion of immunological pathways that play a role in the etiology of the subsystem of psychiatric disorders has grown in interest in recent decades. One of the findings that have aroused interest here is a clear link between autoimmune diseases and psychiatric disorders. This is supported by genetic findings that link immune-related genetic markers to schizophrenia, and clinical studies look for increased levels of inflammation in patients with psychosis. Several large-scale epidemiological studies have found a positive link between autoimmune diseases and psychiatry.
Patients with schizophrenia show different characteristics of immunological diseases, such as previous infections, anti-inflammatory cytokines or other inflammatory proteins in blood-co-existence of other autoimmune diseases. The main function of immune system is to differentiate self and non-self-antigen. Whenever antigen enters into the body, it is recognized by the immune system and a series of responses are activated for the elimination of intruder. Failure of differentiation between self and non-self- antigen i.e., the failure of natural tolerance results in the development of autoimmune diseases. Autoimmune diseases are more common i about 3.6% schizophrenia patients, and a maximum of 3.1% of autoimmune patients are related to schizophrenia. Many studies have covered the comparison between autoimmune diseases and schizophrenia. These studies have revealed that 6 independent mutant loci were present in genomes of patients suffering from schizophrenia and that of Crohn's disease, Multiple Sclerosis, psoriasis, rheumatoid arthritis and Ulcerous colitis. It was found that the proteins involved in the immunological processes are encoded by 6 of 108 loci of their genome [40].
Anti-NMDA receptor auto antibodies were reported to be found in the cerebrospinal fluid of patients of schizophrenia. In comparison to healthy patients, these patients are three times as likely to have elevated titer of -NMDA-receptor auto-antibodies. This is also the same case in bipolar affective disorder and depression. Anti-NMDA receptor causes autoimmune processes in encephalitis, a progressive disease that manifests initially with psychiatric symptoms. Patient can recover by the removal of autoantibodies and immune therapy. Opposition to NMDAR and glutamate hypo-function has long been suggested as a process for causing psychological symptoms and cognitive impairment in Schizophrenia. Injecting NMDAR anti-ketamine into healthy volunteers causes positive psychological symptoms such as delusions and hallucinations as well as false symptoms [41].
19 Correlation between Schizophrenia and Atopic diseases
Atopic diseases in children, especially asthma, are more common in patients who later develop schizophrenia. Other atopic diseases besides asthma are more common in patients with schizophrenia, such as atopic dermatitis, urticarial and atopic rhinitis showed that asthma patients have a higher risk of developing schizophrenia. The study included 25023 asthma patients. After 6 years of follow-up, 100 of them developed schizophrenia. On the other hand, schizophrenia developed in only 138 out of 50046 healthy control subjects. One should also keep in mind that asthma sufferers often take corticosteroids regularly, which are often thought to be the cause of psychiatric disorders [42].
20 Treatment of schizophrenia in the view of immune system
Studies have shown an association of the imbalance in type 1 i.e., the cell mediated immune response and type 2 i.e., the humoral immune response, in the occurrence of Schizophrenia. Hence, the treatment options for schizophrenia include the involvement of such antipsychotic drugs that target to improve the balance of the pathways and the production of immune elements that help to appease the inflammatory responses.
20.1 How antipsychotic drugs work?
The patients of schizophrenia have altered type 1 immune response due to low levels of IL-2 (interleukin-2) and IFN γ (interferon), which in turn decrease the cytokine type-1 production. This leads to a disruption in the pathways involved in the immune responses [43]. The main area of work for anti-psychotic drugs lies in correcting this disruption caused in the type-1 and type-2 immune responses. When treated with neuroleptic agents, the problem of low levels of IFN γ is solved because of the elevated levels of memory cells; which play an important role in producing IFN γ (44). Also, the results of a study on patients of schizophrenia treated with soluble receptors of interleukins-2 (i.e., sIL-2R) showed there was an increase in IL-2, hence regulating the response of type-1 immunity [45]. In some cases of schizophrenia, the tumor necrosis factor TNF-α and its receptor tend to decrease resulting in low immunity. When they were subjected to clozapine, the TNF-α and its receptor increased in production [46].
Moreover, there is protein molecule residing on the lymphocytes and macrophages called ICAM-1 i.e., soluble intercellular adhesion molecule-1 [43]. In schizophrenic cases the level of mentioned protein deviates which becomes a hindrance in the activation of type-1 immune response. This hindrance can also be eliminated by the use of antipsychotic drugs of short-term therapy, in which the levels of ICAM-1 are increased [47]. There is a link in the IL-6 mechanism and the humoral response [43]. The antipsychotic drug treatment provides to enhance the levels of IL-6, thus improving the immune response by triggering the macrophages and monocytes [45]. Some of the antipsychotic drugs include Aripiprazole, Asenapine, Brexpiprazole, Cariprazine ,Clozapine Some drugs that are used in the form of injections are Haloperidol decanoate, Paliperidone, Risperidone [48]
In addition to antipsychotics, if the patients are treated with anti-inflammatory drugs, then they both have a combined effect to treat the negative and positive symptoms of schizophrenia. Some of the anti-inflammatory drugs are Celecoscib, Minocycline, and aspirin [52]
20.2: Relation of tryptophan degradation pathway with Schizophrenia
The tryptophan metabolism is done by the reactions of kynurein pathway KP. This pathway produces a metabolite called kynurenic acid KYNA, which further is a source of QUIN i.e., quinolinic acid. These two products are antagonists of an important molecule that functions in most of the brain activities as it plays a role in synapses, this molecule is called NDMA receptor (N methyl, d- aspartate). The major enzymes in this pathway that slightly affect this pathway are IDO indoleamine 2, 3 dioxygenase and tryptophan 2, 3 dioxygenase TDO. The alteration in the levels of these enzymes can alter the metabolism of tryptophan. According to a hypothesis, in schizophrenia, the KYNA levels in the body increase which disturb the brain activities and motor functions [49, 51].
20.3 COX-2 inhibition to treat schizophrenia
To treat the altered levels of KYNA and QUIN in the patients of schizophrenia, various approaches are met, one of which is cyclo-oxygenase COX inhibition. The COX -2 inhibitory treatment worked to reduce the levels of KYNA. The treatment study included the administration of cox-2 inhibitor; celecoxib along with an additional risperidone. The results of this application proved to increase the activity of type-1 immune response. Additional studies provided that the effect of these inhibitors is not the same all the time [50].
20.4 Effect of risperidone
The role of risperidone in treating schizophrenia is vital as it has shown positive results in minimizing the harmful factors. It works to stabilize the cytokine levels in the schizophrenic patients, the levels are brought back to normal values and it is effective in the patients with preliminary onset of disease. It reduces the production of nitric oxide which can toxify the environment for neurons [52]. In addition to medication, schizophrenia also needs to be dealt with the intervention of some psychological ways; they include rehabilitation, psychosocial therapy, family support and skill training.
21 Conclusions
Hence, Schizophrenia leads to psychotic disorders. Being characterized by changes in thinking, cognition, insomnia and emotions make a path for hallucinations and a prominent impact on memory and learning. According to meta-analysis, Schizophrenia is marked by 15% suicides. Because of immune dysregulation, neuro-inflammation takes place because of abnormal levels of cytokines. Schizophrenia is such a mental disorder that is not inclined to be made fun of as it is common in people who are homeless or who have poor socio-economic status. Having an effect on everyday life, it should be treated properly before it can damage the neuronal action due to microglial activation and enhanced neuro-inflammation due to cytokines. There should be more research done for the proper cure of Schizophrenia as the antipsychotics may not solve the issues regarding the deep impact on insomnia and memory loss. A schizophrenic patient should be turned back to a disillusioned person by the complete immune therapy and proper counseling regarding personality modification and control over unnecessary and uncontrolled thoughts.
References
- Wang H, Xu J, Lazarovici P & Zheng W. Dysbindin-1 Involvement in the Etiology of Schizophrenia. International journal of molecular sciences, 18 (2017): 2044.
- Reviewed by Smitha Bhandari. Retrieved from https://www.webmd.com/schizophrenia/mental-health-schizophrenia (2021).
- Carroll MC, Stevens B, McCarroll SA. Schizophrenia risk from complex variation of complement component.
- Judith A. Owen, Jenny Punt, Sharon A.Stranford, Kuby Immunology, The journal of experimental medicine. W.H Freeman and Company 7 (1992).
- Sara Momtazmanesh, Ameneh Zare-Shahabadi, Nima Rezaei (2019), Cytokines alterations in Schizophrenia: An updated review. Frontiers in psychiatry (2019).
- Maju Mathew Koola, Cytokines in Schizophrenia: a hope or a hype. Indian Journal of Psychology medicine 38 (2016): 2.
- Potvin S, Sti E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in Schizophrenia: A systematic quantitative review (2008).
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick. Metanalysis of cytokine alterations in Schizophrenia; Clinical status and anti-psychotic effects. Psychiatry (2011).
- James M. Krueger. The role of cytokines in sleep regulation. Current pharmacological design 14 (2008): 32.
- Ziad Kronfol MD and Daniel G, Remick. Cytokines and the Brain: Implications for Clinical psychiatry.
- Meiyan Wang, Lei Zhang and Fred H. Gage. Microglia, complement and Schizophrenia. Nature Neuroscience 22 (2019): 333-334.
- Monji A, Mizoguchi Y. Neuroinflammation in Late onset Schizophrenia: The standpoint of microglial hypothesis (2007).
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4235761/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6915198/#:~:text=Based%20on%20meta%2Danalyses%2C%20we,4%2C%20and%20IL%2D17%2C
- https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-018-1197-2#:~:text=The%20pro%2Dinflammatory%20cytokines%20IL,%2Danalytic%20studies%20%5B14%5D
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6915198/#:~:text=Based%20on%20meta%2Danalyses%2C%20we,4%2C%20and%20IL%2D17%2C
- https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-018-1197-2#:~:text=The%20pro-inflammatory%20cytokines%20IL,-analytic%20studies%20%5B14%5D
- https://medlineplus.gov/genetics/condition/schizophrenia/#inheritance
- https://www.tandfonline.com/doi/abs/10.5455/bcp.20161123044657
- Bergdolt L, and Dunaevsky A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Neurobiol.175 (2019): 1–19.
- Boerrigter D, Weickert TW, Lenroot R, Galletly C, Liu D, Burgess, M, et al. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. Neuroinflammation14 (2017): 188.
- https://pubmed.ncbi.nlm.nih.gov/31267432/
- https://jnm.snmjournals.org/content/jnumed/50/11/1801.full.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4250287
- Salleh MR. The genetics of schizophrenia. The Malaysian journal of medical sciences : MJMS11 (2004): 3–11.
- Henriksen Mads G, Nordgaard Julie, Jansson Lennart B. Genetics of Schizophrenia: Overview of Methods, Findings and Limitations. Frontiers in Human Neuroscience 11 (2017).
- Wang H, Xu J, Lazarovici P & Zheng W. Dysbindin-1 Involvement in the Etiology of Schizophrenia. International journal of molecular sciences, 18 (2017): 2044.
- Zhang Z, Huang J, Shen Y, Li R. BACE1-Dependent Neuregulin-1 Signaling: An Implication for Schizophrenia. Frontiers in Molecular Neuroscience 10 (2017).
- Retrieved from: https://med.stanford.edu/sbfnl/services/bm/ep/pre-pulse.html
- Roussos P, Chemerinski E, Siever LJ. Schizoid and Schizotypal Personality Disorder. EncyclOpedia of Human Behavior 2 (2012).
- Shifman S, Bronstein M, Sternfeld M, Pisanté-Shalom A, Lev-Lehman E, Weizman A, et al. A Highly Significant Association between a COMT Haplotype and Schizophrenia.The American Journal of Human Genetics 71 (2002).
- Williams HJ, Owen MJ & O'Donovan MC. Is COMT a susceptibility gene for schizophrenia?. Schizophrenia bulletin, 33 (2007): 635–641.
- Watanabe K, Kakeda S, Yoshimura R, Ide S, Hayashi K, Katsuki A et al. Variation in the Catechol-O-Methyl Transferase Val108/158Met Is Linked to the Caudate and Posterior Cingulate Cortex Volume in Healthy Subjects: Voxel-Based Morphometry Analysis of Brain Magnetic Resonance Imaging, PLOS ONE, 10 (11).
- Medline Plus [Internet]. Bethesda (MD): National Library of Medicine (US); 22q11.2 deletion syndrome;
- Carroll MC, Stevens B, McCarroll SA. Schizophrenia risk from complex variation of complement component 4.
- Sekar A, Bialas A, de Rivera H. et al.Schizophrenia risk from complex variation of complement component 4. Nature 530 (2016): 177–183.
- https://www.frontiersin.org/files/Articles/439798/fpsyt-10-00131-HTML/image_m/fpsyt-10-00131-g001.jpg
- https://ars.els-cdn.com/content/image/1-s2.0-S0889159120300799-gr1.jpg
- https://www.sciencedirect.com/science/article/pii/S0920996412003167
- https://www.sciencedirect.com/science/article/pii/S0920996412003167
- https://www.managedhealthcareexecutive.com/view/mental-health-condition-linked-autoimmune-disease
- Müller N, Myint AM & Schwarz MJ. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders--relation to drug treatment. Dialogues in clinical neuroscience11 (2009).
- Müller N, Riedel M, Schwarz MJ, et al. Immunomodulatory effects of neuroleptics to the cytokine system and the cellular immune system in schizophrenia. In Wieselmann G, ed. Current Update in Psychoimmunology. Vienna, Austria; New York, NY: Springer;(1997).
- Müller N, Empl M, Riedel M, Schwarz M, Ackenheil M. Neuroleptic treatment increases soluble IL-2 receptors and decreases soluble IL-6 receptors in schizophrenia. Eur Arch Psychiatry Clin Neurosci(1997).
- Pollmächer T, Schuld A, Kraus T, Haack M, Hinze-Selch D. [On the clinical relevance of clozapine-triggered release of cytokines and soluble cytokine-receptors]. Fortschr Neurol Psychiatr(2001).
- Schwarz MJ, Riedel M, Ackenheil M, Müller N. Decreased levels of soluble intercellular adhesion molecule-1 (slCAM-1) in unmedicated and medicated schizophrenic patients.
- https://www.mayoclinic.org/diseases-conditions/schizophrenia/diagnosis-treatment/drc-20354449
- Schwarcz R., Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther.
- Riedel M, Strassnig M, Schwarz MJ, Müller N. COX-2 inhibitors as adjunctive therapy in schizophrenia: rationale for use and evidence to date. CNS Drugs 19 (2005): 805-19.
- Okusaga et al. Kynurenine and Tryptophan Levels in Patients with Schizophrenia and Elevated Antigliadin Immunoglobulin G Antibodies, Psychosomatic Medicine: 78 (2016): 931-939.
- http://www.psychiatriadanubina.com/UserDocsImages/pdf/dnb_vol30_noSuppl%204/dnb_vol30_noSuppl%204_180.pdfdy-98971


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
