SETD2 Deficiency Promotes Inflammatory Bowel Disease via Oxidative Stress and FasL-Mediated Apoptosis
Yueduo Wang*, 1, Shenghai Shen2, Haorui Zhu2
1SDU-ANU Joint Science College, Shandong University, Weihai 264209, China
2Division of Life Science, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR 999077, China
*Corresponding author: Yueduo Wang, SDU-ANU Joint Science College, Shandong University, Weihai 264209, China.
Received: 14 September 2023; Accepted: 22 September 2023; Published: 25 October 2023
Article Information
Citation: Yueduo Wang, Shenghai Shen, Haorui Zhu. SETD2 Deficiency Promotes Inflammatory Bowel Disease via Oxidative Stress and FasLMediated Apoptosis. Fortune Journal of Health Sciences. 6 (2023): 397-402.
View / Download Pdf Share at FacebookAbstract
Background: SET-domain-containing 2 (SETD2) is known as the only trimethyltransferase for regulating histone H3 lysine 36 (H3K36) methylation, being involved in the generation and development of many diseases. Although SETD2 has been found to modulate oxidative stress in the intestinal epithelial tissue of inflammatory bowel disease (IBD) samples, the specific functions of SETD2 in IBD development are yet to be fully understood.
Methods: Setd2-flox (Setd2F/F) mice were crossed with Villin-cre transgenic (Setd2Vli-Cre) mice to generate intestinal epithelium-specific deletion of Setd2 (Setd2Vli-KO) mice, followed by genotyping with PCR. RNA-seq expression profiles were gathered from the GEO database for differential expression analysis. According to gene ontology (GO) analysis, four genes were selected as indicators for further expression validation. Total RNA from IBD-induced Setd2Vil-KO mice intestinal epithelium tissue was extracted for RT-qPCR. TUNEL assay was conducted for detecting the apoptotic signaling level, and immunofluorescence was utilized for detecting the FasL protein expression in IBD-induced Setd2Vil-KO mice intestinal epithelium tissue.
Results: Four genes (Cybb, Lpo, FasL, Tnfrsf8) related to oxidation-reduction and apoptotic process-promotion were identified as indicators. Compared with wild-type mice, down-regulation of Cybb and Lpo was detected, as well as the up-regulation of FasL and Tnfrsf8 were up-regulated in the intestinal epithelial tissue of Setd2Vli-KO mice via RT-qPCR validation. Furthermore, TUNEL assay and immunofluorescence revealed significant enhancement in apoptotic signaling and FasL protein expression.
Conclusions: SETD2 deficiency promotes FasL-mediated apoptosis and oxidative stress in IBD intestinal epithelium tissue and hence plays an essential role in IBD pathogenesis. Altogether, our research provided new insights into IBD development.
Keywords
Inflammatory bowel disease; SET-domain-containing 2; Oxidative stress; Apoptosis
Inflammatory bowel disease articles; SET-domain-containing 2 articles; Oxidative stress articles; Apoptosis articles
Article Details
1. Introduction
Intestinal epithelial tissue is one of the most active self-renewing tissues in mammals, which is essential for maintaining mucosal barrier homeostasis, generally affected by multiple factors [1, 2]. Among them, oxidative stress is a significant factor that initiated by excessive accumulation of reactive oxygen species (ROS), resulting in promoting cell apoptosis and inflammation, and hence promotes intestinal epithelium homeostasis disorder, such as IBD [2, 3]. As a chronic and recurrent inflammatory disorder in mucosal barriers, IBD is typically sub-classified as Crohn's disease (CD) and ulcerative colitis (UC) [1, 4]. Since long-term IBD has been proven to be more likely to generate colorectal cancer (CRC) [2], it is necessary to prevent IBD deterioration.
Epigenetic regulation, such as histone modification and nucleosome remodeling, plays a key role in many biological processes and disease pathogenesis, including IBD [2, 5]. As an important way of epigenetic regulation, histone methylation has been proven to be related to mucosal barrier disorder and IBD generation. For example, EZH2 inactivates the trimethylation of histone H3 lysine 27, which overexpression promotes inflammatory response and cell apoptosis in intestinal epithelial tissues [1, 6]. Intriguingly, SETD2 was found to have a high mutation rate (17%) in IBD samples, while acting as the only known factor for altering the trimethylation states of H3K36 in mammalian cells [2, 6]. It has been proven that SETD2 plays an essential role in maintaining genomic stability functions, such as transcriptional regulation, DNA damage repair, chromosome segregation, and alternative splicing [7, 8]. Therefore, SETD2 has recently been extensively investigated in a variety of tumors, including prostate cancer metastasis [6], hepatocarcinoma [9], and pancreatic cancer [2]. Although SETD2 has been found to reduce oxidative stress in the intestinal epithelial tissue of IBD samples, the specific functions of SETD2 in IBD development are yet to be fully understood. In this study, RNA-seq expression datasets were gathered to investigate the possible role of SETD2, and Setd2Vil-KO mice were generated as IBD pathological models to validate the differential expression results. Accordingly, our findings provided theoretical support for the pathogenesis mechanism of IBD.
2. Materials and Methods:
2.1 RNA-seq datasets collection and differential expression analysis
Two RNA-seq microarray datasets (GSE112366, GSE151968) were collected from the NCBI Gene Expression Omnibus (GEO) database (http://www.ncbi.nih.gov/geo) as MINiML files. Differential expression analysis was conducted using R package limma. Wilcoxon t-tests were used to calculate inter-group differences.
2.2 IBD mice model establishment and genotyping
Setd2F/F and Villin-Cre transgenic mice (C57BL6J; Shanghai Biomodel Organism Co.,) were bred and maintained under pathogen-free conditions in an accredited animal facility. The IBD mice pathological model was established as follows:
1) Setd2F/F and Villin-Cre mice were crossed to generate Setd2Vil-KO mice.
2) For the first 7 days, wild-type mice and Setd2Vil-KO mice were given drinking water containing 3% dextran sodium sulfate (DSS) daily, respectively.
3) For the subsequent 5 days, wild-type mice and Setd2Vil-KO mice were given drinking water without DSS daily.
4) Then wild-type mice and Setd2Vil-KO mice were both given drinking water containing 2% DSS for 5 days daily, respectively.
5) For the subsequent 5 days, wild-type mice and Setd2Vil-KO mice were given drinking water without DSS daily.
6) The tail tips of mice were gathered, adding 100 μL solution of 25 mM NaOH and 0.2 mM EDTA as alkaline lysis reagent, followed by boiling at 95°C for 1 hour. Subsequently, 100 μL of 40mM Tris HCl (pH=5.5) was added, followed by centrifuging at 12000 g for 5 minutes. The supernatant sample was collected for PCR and genotyping.
2.3 RNA extraction and Real-Time PCR
Total RNA of the intestinal epithelium tissues of Setd2Vil-KO mice was extracted with a Trizol kit (Invitrogen) according to the manufacturer's instructions. 2 g total RNA and SuperScript II (Invitrogen) were applied to synthesize first-strand cDNA. Then, SYBR Green Universal Master Mix reagent (Roche) was used to perform the reaction. β-actin was selected as a reference gene, and all samples were exposed to three parallel experimental replicates. A p < 0.05 was considered significant.
2.4 TUNEL assay and immunofluorescence
The intestinal epithelium tissues of Setd2Vil-KO mice were handled according to Swiss roll technique and 4% paraformaldehyde was then applied as a fixative overnight. Treated tissues were embedded in paraffin, and cut into 5-mm pieces, and paraffin sections were rehydrated and heated to induce antigen retrieval. Apoptotic signaling in the intestinal epithelial tissues of Setd2Vil-KO mice was detected by the TUNEL staining assay kit (Fluorescence, 488 nm #25879 CST). Primary antibodies applied for immunofluorescence were anti-FasL (sc-19681,200ug/ml). A fluorescence microscope was applied to observe both TUNEL assay and immunofluorescence.
3. Results
3.1 SETD2 deficiency altered antioxidative and apoptosis gene expression in IBD
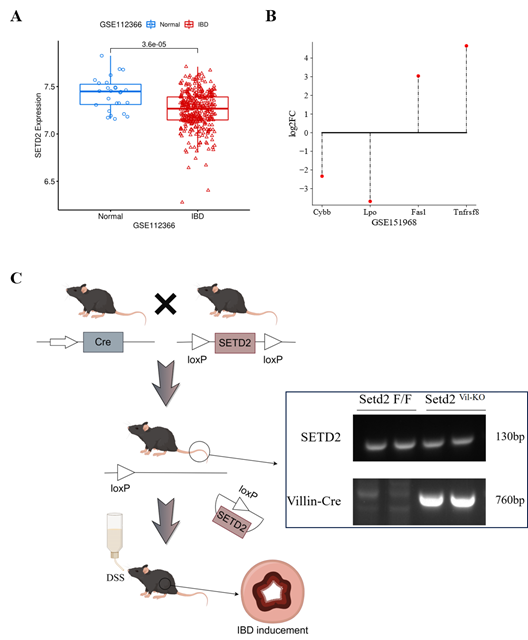
To explore the possible role of SETD2 in IBD, we collected two microarray expression datasets from the GEO database. Among the differential expression results in dataset GSE112366, SETD2 was found significantly down-regulated in IBD samples (Figure 1A), which indicates that SETD2 might be a protective factor in IBD generation and deterioration. Since Li et al. have found that SETD2 modulates oxidative stress in IBD [2], we conducted differential expression analysis for dataset GSE151968. According to Li et al.’s conclusion, differential genes related to apoptosis regulation, inflammatory response, and antioxidation were significantly enriched in GO analysis. Thus, we selected four genes as the indicators of these biological process, based on the maximum absolute value of log2FC, as shown in Figure 1B. Among them, antioxidative genes cytochrome b-245 heavy chain (Cybb) and lactoperoxidase (Lpo) presented down-regulated. while, the expression of two other cell apoptosis-related genes Fas ligand (FasL) and tumor necrosis factor receptor superfamily member 8 (Tnfrsf8) were enhanced compared with wild-type mice This further suggests that SETD2 might be a protective factor for IBD development.
(A) SETD2 expression level was significantly down-regulated in IBD samples.
(B) The expression differences of four indicator genes.
(C) An overview of the establishment of Setd2Vli-KO IBD mice in this study, followed by genotyping.
3.2 SETD2 deficiency altered antioxidative and apoptosis gene expression in IBD
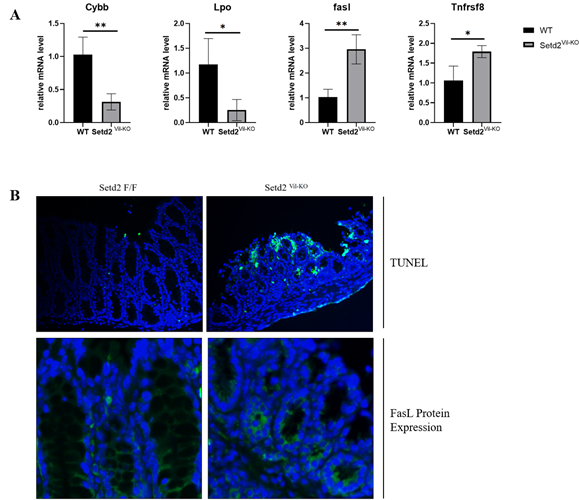
To validate these results, we generated and genotyped IBD mice, as shown in Figure 1C. The expression of four indicator genes was detected via RT-qPCR, wherein the results were consistent with Li et al.’s, as shown in Figure 2A. The relative expression levels of Cybb and Lpo were decreased about 3 and 4 times, while FasL and Tnfrsf8 were enhanced about 3 and 0.7 times, respectively. These results indicated that SETD2 loss was indeed responsible for the down-regulation of Cybb and Lpo, al well as the up-regulation of FasL and Tnfrsf8.
(A) Relative expression level the four indicator genes in Setd2Vil-KO mice, validated via RT-qPCR analysis. Student's t-test was applied for the determination of the statistical significance of mean ± S.E.M. (, (*) p < 0.05, (**) p < 0.01.)
(B) The TUNEL assay (Scale Bars: 50 μm.) and immunofluorescence (Scale Bars: 100 μm.) of the Setd2Vil-KO mice intestinal epithelial tissue. Blue fluorescence represents DAPI dye, and green fluorescence represents target signaling.
3.3 SETD2 deficiency promotes FasL-mediated apoptosis in Setd2Vil-KO mice
Since the significant up-regulation of FasL and Tnfrsf8 expression was detected in RT-qPCR, we further evaluated the relevance between cell apoptosis and Setd2 deficiency in IBD samples. Through the TUNEL assays and immunofluorescence, the apoptotic signaling and FasL protein expression levels in the Setd2Vil-KO mice intestinal epithelium tissue were detected. In line with our previous findings, both apoptotic signaling and FasL protein expression were found significantly up-regulated, as shown in Figure 2B. Altogether, these results showed that SETD2 deficiency leads to the enhancement of FasL-mediated apoptosis in the intestinal epithelial tissues of IBD Setd2Vil-KO mice.
4. Discussion
IBD is a chronic inflammatory disorder in mucosal barriers with multiple triggers, among which epigenetic regulation plays a key role in IBD development. As an essential epigenetic regulator, SETD2 is known as the unique histone H3K36 trimethyltransferase, which has been found frequently mutated in IBD patients. However, the specific functions of SETD2 in IBD development remain unclear. In this study, we revealed that SETD2 deficiency in intestinal epithelium tissues was responsible for the increase of oxidative stress as well as the enhancement of FasL-mediated apoptosis in IBD samples. These results supported the fact that SETD2 was frequently mutated in IBD patients.
ROS contributes to the development of many diseases, such as cardiovascular disease, inflammation, and cancer [10, 11]. Significant down-regulation of Cybb and Lpo were found via RT-qPCR, CYBB is a primary component of the phagocyte NADPH oxidase (NOX) system, which disorder is highly likely leads to cause granulomatous disease (GCD), and about one-third of GCD patients develop IBD [12, 13]. Meanwhile, lactoperoxidase (Lpo) is also responsible for ROS scavenging thus the down-regulation of Cybb and Lpo indicates the reduction of antioxidant capacity promoting oxidative stress levels in the intestinal epithelium of Setd2Vil-KO mice [14]. These findings were also consistent with the study of Min et al., among which a total of 906 antioxidative genes, including Prdx3, Prdx6, Gclm, and Srxn1, were significantly down-regulated after SETD2 knockout in the intestinal epithelium tissue [2]. Therefore, these results demonstrated that SETD2 deficiency was mainly responsible for the enhancement of oxidative stress levels.
Cell apoptosis is critical for the elimination of damaged, senescent, or useless cells without causing microenvironment damage and inflammation [15], which has been found to accelerate in IBD patients [16]. The up-regulation of FasL and Tnfrsf8 was also detected via RT-qPCR, wherein FasL is a crucial death factor belonging to the tumor necrosis factor (TNF) family, responsible for the receptor-triggered programmed apoptosis process [17]. Typically, the FasL gene is low expressed in IECs [18]. However, the overexpression of FasL was detected in UC patients, while maintaining at a normal level in CD patients [19, 20]. Furthermore, both apoptotic signaling and FasL protein expression were found enhanced in the Setd2Vil-KO mice intestinal epithelium tissue. Therefore, our findings revealed for the first time that SETD2 deficiency resulted in FasL-mediated apoptosis and uncovered part of the potential association of epigenetics regulation with apoptosis in IBD pathogenesis.
It is worth mentioning that the tumor necrosis factor (TNF) family appears to play an essential role in SETD2 loss IBD mice. Noteworthy, both Tnfrsf8 and FasL belong to the TNF superfamily [17, 21], and Tnfrsf8 signaling has been found to up-regulate the expression of the Fas gene [22]. Nevertheless, little is known about the specific functions of TNF family members in SETD2 deficiency IBD patients. Considering that apoptosis is a complicated biological process with multiple types and triggers, it therefore remains unclear whether SETD2 loss would lead to other types of cell apoptosis in IBD development. Thus, the mechanism of epigenetic regulation triggered by SETD2 loss in IBD has also not been fully understood yet. Overall, this study first determined the relationship between Setd2 mutation and cell apoptosis in the context of IBD. Considering that SETD2 mutation accounted for a higher risk of development into CRC in IBD patients (about 17%), our findings provided theoretical support and a basis at the molecular level for the mechanism, therapy, and prevention of such diseases.
Author's contribution
Conceptualization YW (leading). and S.S(support).; Formal analysis, S.S.; Investigation, Y.W., and S.S; Methodology, Y.W.; Project administration, Y.W.; Resources, Y.W.; Supervision, Y.W.; Validation, Y.W.; Visualization, Y.W. and S.S; Writing—original draft, Y.W.; Writing—review & editing, Y.W., S.S. and H.Z. All authors have read and agreed to the published version of the manuscript.
Ethical statement
All the procedure has been approved by the Ethics Review Board of Renji Hospital.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to Dr. Li of Shanghai Jiao Tong University for the support of this work.
References
- Liu Y, Peng J, Sun T, et al. Epithelial EZH2 serves as an epigenetic determinant in experimental colitis by inhibiting TNFα-mediated inflammation and apoptosis. Proc Natl Acad Sci U S A 114 (2017): E3796-E3805.
- Liu M, Rao H, Liu J, et al. The histone methyltransferase SETD2 modulates oxidative stress to attenuate experimental colitis. Redox Biol 43 (2021): 102004.
- Liu M, Sun T, Li N, et al. BRG1 attenuates colonic inflammation and tumorigenesis through autophagy-dependent oxidative stress sequestration. Nat Commun 10 (2019): 4614.
- Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol 20 (2014): 91-99.
- Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell 150 (2012): 12-27.
- Yuan H, Han Y, Wang X, et al. SETD2 Restricts Prostate Cancer Metastasis by Integrating EZH2 and AMPK Signaling Pathways. Cancer Cell 38 (2020): 350-365.
- Yuan H, Li N, Fu D, et al. Histone methyltransferase SETD2 modulates alternative splicing to inhibit intestinal tumorigenesis. Journal of Clinical Investigation 127 (2017): 3375-3391.
- Lu M, Zhao B, Liu M, et al. Pan-cancer analysis of SETD2 mutation and its association with the efficacy of immunotherapy. npj Precis Onc 5 (2021): 51.
- Li X, Li Q, Ju L, et al. Deficiency of Histone Methyltransferase SET Domain-Containing 2 in Liver Leads to Abnormal Lipid Metabolism and HCC. Hepatology 73 (2021): 1797-1815.
- Tong L, Chuang CC, Wu S, Zuo L. Reactive oxygen species in redox cancer therapy. Cancer Lett 367 (2015): 18-25.
- Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev 61 (2009): 290-302.
- Chen H, Sun Q, Zhang C, et al. Identification and Validation of CYBB, CD86, and C3AR1 as the Key Genes Related to Macrophage Infiltration of Gastric Cancer. Front Mol Biosci 8 (2021): 756085.
- Singel KL, Segal BH. NOX2-dependent regulation of inflammation. Clin Sci (Lond) 130 (2016): 479-490.
- Conner GE, Salathe M, Forteza R. Lactoperoxidase and Hydrogen Peroxide Metabolism in the Airway. Am J Respir Crit Care Med 166 (2002): S57-S61.
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 267 (1995): 1456-1462.
- Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol 180 (1996): 152-159.
- Lettau M, Paulsen M, Schmidt H, Janssen O. Insights into the molecular regulation of FasL (CD178) biology. Eur J Cell Biol 90 (2011): 456-466.
- Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev 193 (2003): 70-81.
- Merger M, Viney JL, Borojevic R, et al. Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine. Gut 51 (2002): 155-163.
- Suzuki A, Sugimura K, Ohtsuka K, et al. Fas/Fas ligand expression and characteristics of primed CD45RO+ T cells in the inflamed mucosa of ulcerative colitis. Scand J Gastroenterol 35 (2000): 1278-1283.
- Xu ML, Gabali A, Hsi ED, et al. Practical Approaches on CD30 Detection and Reporting in Lymphoma Diagnosis. Am J Surg Pathol 44 (2020): e1-e14.
- Muta H, Boise LH, Fang L, Podack ER. CD30 signals integrate expression of cytotoxic effector molecules, lymphocyte trafficking signals, and signals for proliferation and apoptosis. J Immunol 165 (2000): 5105-5111.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 