Bacterial Infection and Antibiotic Resistance Seen in the Laboratory of the University Hospital of Befelatanana
Fidiniaina Mamy Randriatsarafara1, Zafindrasoa Domoina Rakotovao-Ravahatra2, Miora Koloina Ranaivosoa3, Andriamiadana Luc Rakotovao4
1Public Health Specialist. Public Health Department of the Faculty of Medicine Antananarivo, Madagascar
2Biologist doctor, Laboratory of Joseph Raseta Befelatanana University Hospital Antananarivo, Madagascar
3Biologist doctor. Laboratory of Biochemistry of Joseph Ravoahangy Andrianavalona University Hospital Antananarivo, Madagascar
4Professor in biological hematology, Medical Biology Department of the Faculty of Medicine Antananarivo, Madagascar
*Corresponding author: Zafindrasoa Domoina Rakotovao-Ravahatra, Laboratory of Joseph Raseta Befelatanana University Hospital Antananarivo, Madagascar.
Received: 18 October 2021; Accepted: 26 October 2021; Published: 13 January 2022
Article Information
Citation: Fidiniaina Mamy Randriatsarafara, Zafindrasoa Domoina Rakotovao-Ravahatra, Miora Koloina Ranaivosoa, Andriamiadana Luc Rakotovao. Bacterial Infection and Antibiotic Resistance Seen In the Laboratory of the University Hospital of Befelatanana. Archives of Microbiology and Immunology 6 (2022): 7-19
View / Download Pdf Share at FacebookAbstract
Background: Bacterial infections are common in hospitals. The objectives of the present study are to describe the bacteriological profile of bacterial infections and to assess the antibiotic resistance of bacteria at the Befelatananana University Hospital. Methods: This is a retrospective, cross-sectional and descriptive study from July 01 to December 31, 2019 at the laboratory of the University Hospital of Befelatanana. Results: Among the 1150 patients, 397 were affected by bacterial infections, for a prevalence of 34.5%. Enterobacteriaceae were the most frequently isolated with 164 (40%) bacteria identified. Women (40.8%; p = 10-6), subjects with suppurations and sores (70.7%; p = 10-6), subjects in intensive care (57.7%; p = 10- 6) and patients with pus swabs (80%; p = 10-6) were most affected by bacterial infections. The majority of bacteria were resistant to penicillins and cotrimoxazole (40.6% to 100% resistance). Staphylococci and streptococci were all susceptible to vancomycin. All Gram-negative bacilli were susceptible to amikacin and imipenem except the genus Acinetobacter which showed 58.3% resistance to amikacin and 75% resistance to imipenem. Conclusion: Empirical treatments for infectious diseases and self-medication should be avoided in order to improve the management of patients with bacterial infections.
Keywords
<p>Antibiotic resistance, enterobacteria, self-medication, resuscitation</p>
Article Details
1. Introduction
In Madagascar, infectious pathologies constitute a major public health problem. They are responsible for 17 million deaths worldwide, including 43% in developing countries against 1% in industrialized countries [1]. Among these infectious pathologies, bacterial infections are starting to grow in importance today. Invasive bacterial infections are the cause of a number of serious pathologies including meningitis, bronchopneumopathies, septicemia, peritonitis, typhoid fever and especially diarrhea which still remains a real public health problem for the child population. in developing countries [2]. The development and use of antibiotics over the past 70 years has led to a major decline in terms of mortality and morbidity associated with infectious bacterial diseases around the world. However, although these molecules have saved millions of patients, their use has led to strong antibiotic resistance in a growing number of species and a growing number of antibiotics. In developing countries, poverty, malnutrition, poor hygienic conditions, insufficient access to medicines, the absence of efficient healthcare systems, government crises, civil wars and frequent population displacements have contributed considerably to the emergence and spread of antibiotic resistance in these regions of the world [3]. The growing increase in these antibiotic resistance has prompted us to carry out this study, the objectives of which are to describe the bacteriological profile of bacterial infections and to assess the antibiotic resistance of these bacteria isolated in the biological samples seen in the laboratory of the Joseph Raseta University Hospital Center, Befelatananana.
2. Materials and Methods
2.1 Type of Study
This is a retrospective, descriptive and cross-sectional study from July 01, 2019 to December 31, 2019 at the laboratory of the Joseph Raseta Befelatanana University Hospital Center (CHUJRB) in Antananarivo Madagascar. All bacteriological and antibiogram results from patients during the study period who met the inclusion criteria were included in the presented study. Thus, this study included the results of bacteriological analysis and antibiogram of 1150 patients.
2.2 Inclusion criteria
The inclusion criteria were constituted by all samples for bacteriological examinations with completed analysis request forms and compliant samples.
2.3 Exclusion criteria
The exclusion criteria were constituted by the results of bacteriological analysis including commensal bacteria of the skin or mucous membranes. Likewise, the bacteriological results of bacterial colonies whose quantity was below the positivity threshold were rendered negative. In addition, bacteriological examinations carried out during the study period for other research or projects were excluded from the study.
2.4 Study parameters
The study parameters were represented by age, gender, clinical information, services, types of samples (blood culture, stool culture, pus, sputum, throat, materials, effusion fluid, cerebrospinal fluid ( CSF), urethral smear, cervico-vaginal smear, cytobacteriological examination of urine and other samples), results of bacteriological analysis and results of antibiograms.
2.5 Data collection
Data were collected from analysis request sheets, benchtop sheets and anti biogram result sheets.
2.6 Statistical analysis
Data entry and processing were carried out using the Epi-info 3.5.2 software. The comparison of the percentages used the Chi-square tests of Mantel Haenszel or the corrected Chi-square of Yates in the event of small numbers. The statistical significance level used was p = 0.05.
2.7 Ethical considerations
The present study respected the notion of volunteering, anonymity and confidentiality. It was only implemented after obtaining the authorization of the Director of the CHUJRB Establishment and the Head of Department of the CHUJRB laboratory.
3. Results
3.1 Prevalence of Bacterial Infections
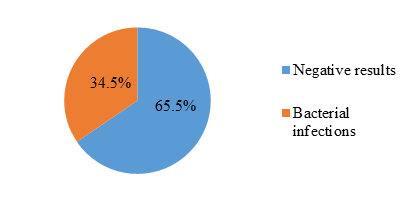
Among 1150 patients, 397 presented with bacterial infections showing a prevalence of 34.5% (figure 1).
3.2 Prevalence of Bacteria Responsible for Bacterial Infections
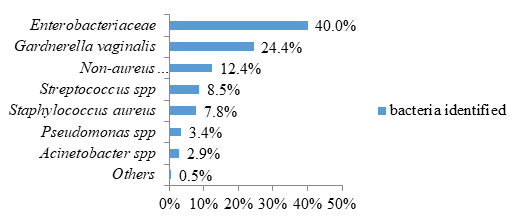
Regarding bacterial infections, the most frequent bacteria were represented by Enterobacteriaceae concerning 164 patients (40.0%) and the species Gargnerella vaginalis concerning 100 patients (24.4%) (figure 2).
3.3 Associated Factors with Bacterial Infections
Women were the most affected by bacterial infections with a highly significant difference (40.8%; p = 10-6). The subjects in the 2 extreme ages of life are the most affected by bacterial infections representing respectively 93 cases (35.8%) and 71 cases (37.8%) but without significant difference (p = 0.7; NS) . The subjects with bacterial infections presented with suppurations and wounds (41 cases or 70.7%) or fever (93 cases or 52.2%) in the majority of cases with a highly significant difference (p = 10- 6). Patients hospitalized in intensive care and surgery were the most likely to present bacterial infections representing respectively 82 cases (57.7%) and 25 cases (55.6%) with a highly significant difference (p = 10- 6). Pus swabs and genitourinary swabs were the most likely to show positive bacteriological results. Among these bacterial infections, 52 cases (80%) involved pus samples, 115 cases (69.3%) genital samples and 115 cases (31.9%) urine samples with a highly significant difference (table 1).
Table 1: Associated Factors with Bacterial Infections
|
Associated factors |
Negative results |
Bacterial infections |
Total (N) |
p-value |
||
|
n |
% |
n |
% |
|||
|
Gender |
||||||
|
Female |
374 |
59.2 |
258 |
40.8 |
632 |
10-6 |
|
Male |
379 |
73.2 |
139 |
26.8 |
518 |
|
|
Age (years) |
||||||
|
≤19 |
167 |
64.2 |
93 |
35.8 |
260 |
0,7 |
|
20-39 |
263 |
66.9 |
130 |
33.1 |
393 |
|
|
40-59 |
206 |
66.7 |
103 |
33.3 |
309 |
|
|
≥60 |
117 |
62.2 |
71 |
37.8 |
188 |
|
|
Clinical information |
||||||
|
suppurations and wounds |
17 |
29.3 |
41 |
70.7 |
58 |
10-6 |
|
Fever |
85 |
47.8 |
93 |
52.2 |
178 |
|
|
Joint disorders |
11 |
91.7 |
1 |
8.3 |
12 |
|
|
Pneumonia |
13 |
50 |
13 |
50 |
26 |
|
|
Effusion fluid |
149 |
93.7 |
10 |
6.3 |
159 |
|
|
Digestive disorders |
28 |
87.5 |
4 |
12.5 |
32 |
|
|
Genitourinarydisorders |
99 |
50 |
99 |
50 |
198 |
|
|
Neurologicaldisorders |
99 |
98 |
2 |
2 |
101 |
|
|
Others |
252 |
65.3 |
134 |
34.7 |
386 |
|
|
Departments |
||||||
|
Surgery |
20 |
44.4 |
25 |
55.6 |
45 |
10-6 |
|
Out-patients |
185 |
58 |
134 |
42 |
319 |
|
|
InternalMedicine |
401 |
76.1 |
126 |
23.9 |
527 |
|
|
Pediatrics |
87 |
74.4 |
30 |
25.6 |
117 |
|
|
Intesive care units |
60 |
42.3 |
82 |
57.7 |
142 |
|
|
Types of samples |
||||||
|
Urine |
245 |
68.1 |
115 |
31.9 |
360 |
10-6 |
|
Cerebrospinalfluid |
204 |
98.6 |
3 |
1.4 |
207 |
|
|
Other |
94 |
62.7 |
56 |
37.3 |
150 |
|
|
Pus |
13 |
20 |
52 |
80 |
65 |
|
|
Pap smear and urethral smear |
51 |
30.7 |
115 |
69.3 |
166 |
|
|
Effusion fluid |
185 |
91.6 |
17 |
8.4 |
202 |
|
3.4 Antibiotic Resistance
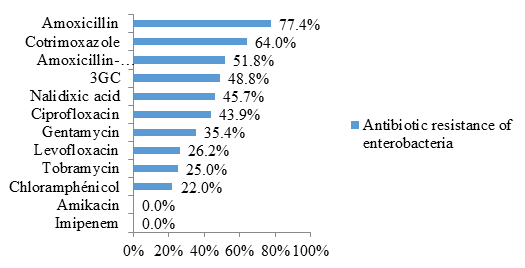
Enterobacteriaceae were resistant to amoxicillin (77.4% resistance) and cotrimoxazole (64% resistance) in the majority of cases. Likewise, 51.8% were resistant to the amoxicillin-clavulanic acid combination and 48.8% to 3rd generation cephalosporins (3GC) (figure 3).
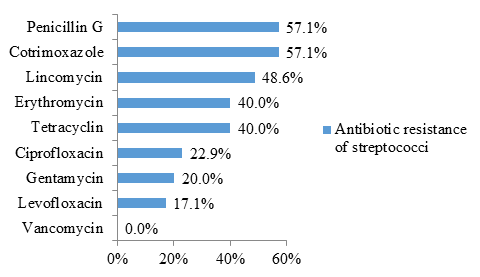
The figure 4 shows that streptococci were resistant to penicillin G (57.1% resistance) and cotrimoxazole (57.1% resistance) in more than half of the cases.
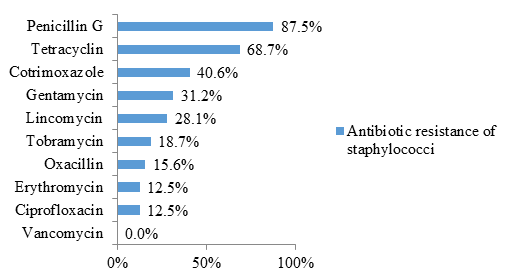
Staphylococci were also resistant to penicillin G (87.5% resistance) and cotrimoxazole (40.6% resistance) in the majority of cases. Likewise, 68.7% of the isolates were resistant to tetracycline (figure 5).
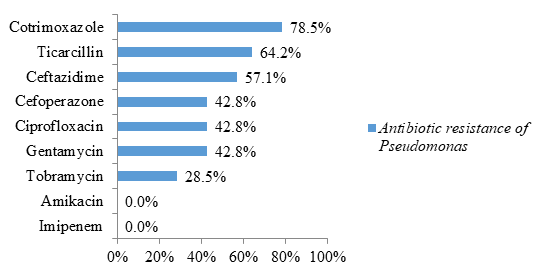
Regarding non-fermenting Gram-negative bacilli, bacteria of the genus Pseudomonas were highly resistant to cotrimoxazole (78.5% resistance) and ticarcillin (64.2% resistance) (figure 6).
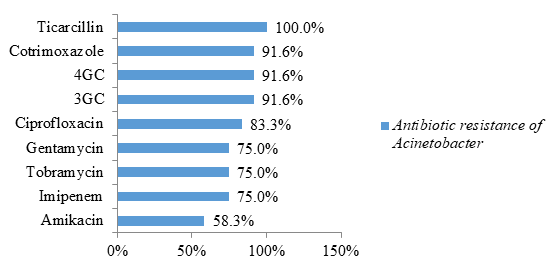
The bacteria of the genus Acinetobacter were all resistant to ticarcillin (100% resistance). Likewise, 91.6% were resistant to cotrimoxazole, 3rd generation cephalosporins (3GC) and 4th generation cephalosporins (4GC). These bacteria of the genus Acinetobacter have been the most formidable in terms of antibiotic resistance. Indeed, 75% of these bacteria have a carbapenemase (75% resistance to imipenem) and were resistant to all classes of antibiotics tested. Likewise, 58.3% were resistant to amikacin while the other previous bacteria were all sensitive to this antibiotic (figure 7).
Other bacteria were identified but they were rare and were susceptible to all the antibiotics tested in the vast majority of cases.
4. Discussion
4.1 Prevalence of Bacterial Infections
This study found a prevalence of bacterial infections of 34.5%. In the literature, this prevalence varies according to the types of studies and the germs studied. Thus, a study in Congo also found a prevalence of 34.5% of nosocomial bacterial infections [4]. Another study in a maternity hospital in Congo found a 15.5% incidence of urinary tract infections and surgical site infections [5]. On the other hand, a study carried out in 27 hospitals in the Mediterranean region showed a lower prevalence of nosocomial bacterial infections, of the order of 10.5% [6].This study found a higher prevalence of bacterial infections compared to other studies because we considered all bacterial infections in all types of specimen, age, gender, clinical information and departments combined, allowing us to have the overall prevalence of infections. Bacterial Based on this result, a third of the results of bacteriological examinations carried out at the CHUJRB laboratory are all positive. This high prevalence prompts us to take adequate measures to fight against these infections. In hospitals, hygiene measures will have to be strict to limit the spread and to kill the germs responsible for nosocomial infections.
4.2 Prevalence of Bacteria Responsible for Bacterial Infections
Regarding the bacteria responsible for bacterial infections in the present study, Enterobacteriaceae are the most common. Indeed, the literature emphasizes that Enterobacteriaceae constitute the main source of community and hospital infections [7]. These Enterobacteriaceae were found in almost all biological samples. These Enterobacteriaceae are common because they include a large number of bacterial species such as Escherichia coli, Klebsiellapneumoniae, Enterobacter cloacae, Serratiamarcescens, Proteus mirabilis, Morganellamorganii, Citrobacterfreundii and many more. These Enterobacteriaceae are normally present in the human intestine. But they can happen accidentally in other parts of the body where they are responsible for sometimes very severe bacterial infections that are life-threatening to the patient in the absence of early treatment. After Enterobacteriaceae, Gardnerella vaginalis is the second bacterium identified. This bacterium is isolated in FCVs and is responsible for bacterial vaginosis in women. A study carried out in Togo also showed that Gardnerella vaginalis is the bacteria most frequently identified in FCV with a prevalence of 55.1% [8]. Bacterial vaginosis is an imbalance of the microbial flora in the vagina. It is characterized by the disappearance of lactobacilli and the multiplication of anaerobic germs such as Gardnerella vaginalis. It is not a sexually transmitted infection. It is due to an imbalance of the vaginal flora with disappearance of the protective effect of the Döderlein bacillus. It is the most common specific cause of vaginal infection. The prevalence is higher in sexually active women and reaches almost a third [9].
This study also found gram-positive cooci such as streptococci and staphylococci. Streptococci have been isolated in the various biological samples but especially in throat samples (strep throat (streptococcus pyogenes) and in FCV (Streptococcus agalactiae vaginitis). In the absence of prompt management, strep throat A (Streptococcus pyogenes) can lead to complications such as rheumatic fever (AAR) or peritoneal abscess [10]. Likewise, streptococcal B vaginitis (Streptococcus agalactiae) is serious in pregnant women and must be treated quickly to avoid neonatal infections, especially meningitis in term newborns [11-12]. Concerning the staphylococci, they were also isolated in the various biological samples but especially in the samples of pus at the level of the skin. The most common species was Staphylococcus aureus. The literature also claims that these bacteria can cause skin and soft tissue infections [13]. Indeed, satphylococci are skin germs and cause secondary infections in the skin in the event of a wound or injury.
4.3 Associated factors with bacterial infections
By gender, women were the most affected by bacterial infections compared to men with a highly significant difference. In fact, from a morphological point of view, women are more fragile than men and are more vulnerable to diseases. Moreover, women are always second to men in all circumstances of daily life [14]. A woman's immune system can also be reduced by certain circumstances such as menstruation or pregnancy which are specific to women.
Depending on age, this study did not find a significant relationship between age range and bacterial infections. Thus, these infections can affect all patients regardless of age. Regarding clinical information, patients with suppurations of the skin, wounds, fever and genitourinary disorders were most affected by bacterial infections with a highly significant difference. Indeed, there are many bacterial flora which line the skin and mucous membranes, in particular staphylococci, responsible for the high frequency of bacterial infections in the damaged skin. Likewise, fever is the first sign presented by the patient when there is external aggression on the body by foreign bodies or microorganisms, especially bacteria, which explains its high frequency [15].
Regarding services, patients hospitalized in the surgical and intensive care units were the most affected by bacterial infections with a highly significant difference. Indeed, according to other studies, 20 to 25% of all nosocomial infections are acquired in intensive care units [16]. These bacterial infections are mainly due to the invasive procedures performed on the patient in the surgical and intensive care units. Among these invasive procedures, we can cite bladder catheterization, tracheal intubation, chest and abdominal drains, catheterization and many more [17].
In this study, pus, pap smear and urethral smear showed positive bacteriological results in the majority of cases. In fact, suppuration is always linked to any infection because the pus is a sign of the body's defense against external aggression. Likewise, bacterial infections in the genitourinary systems are frequent because of various situations including the proximity between the digestive tract and the genitourinary system.
4.4 Antibiotic resistance
Regarding antibiotic resistance, this study showed that Enterobacteriaceae are highly resistant to cotrimoxazole. Indeed, this antibiotic is abused by the population since it is sold in small grocery stores and everyone can get it. The misuse of this antibiotic as well as self-medication is at the origin of this high resistance. Likewise, these germs are also highly resistant to beta-lactams such as amoxicillin, amoxicillin-clavulanic acid and third generation cephalosporins. The high consumption of these antibiotics by the population is at the origin of this resistance [18]. Furthermore, the present study has shown that Enterobacteriaceae are beginning to become increasingly resistant to other classes of antibiotics such as quinolones (nalidixic acid, ciprofloxacin, levofloxacin), aminoglycosides (gentamycin) and phenicols (chloramphenicol). Fortunately, all identified Enterobacteriaceae were susceptible to imipenem and amikacin. A study in Doula also found that imipenem (1.3% resistance) and amikacin (12.9% resistance) are still effective against Enterobacteriaceae in the majority of cases [19]. These results confirm that penems and amikacin are the antibiotics of choice for treating Enterobacteriaceae infections.
Furthermore, streptococci were highly resistant to penicillin G and cotrimoxazole. These 2 antibiotics are abused by the population because cotrimoxazole is sold in small grocery stores and can be bought without a prescription in pharmacies. The literature asserts that self-medication antibiotic arises with seriousness in developing countries where these drugs are readily available often without a medical prescription [20]. In addition, streptococci are starting to become increasingly resistant to other classes of antibiotics such as macrolides (erythromycin), lincosamides (lincomycin) and cyclins (tetracycline). Fortunately, resistance to quinolones (ciprofloxacin, levofloxacin) and aminoglycosides (gentamycin) are rare. Likewise, all streptococcal isolates were sensitive to vancomycin. Other studies have also found the same result [21-22]. These results confirm that glycopeptides (vancomycin, teicoplanin) are the antibiotics of choice for treating streptococcal infections.
In this study, staphylococci were highly resistant to penicillin, cotrimoxazole, and tetracycline. As stated in the previous paragraphs, these 3 classes of antibiotics are abused by the population. Thus, self-medication increases the resistance of germs to these 3 classes of antibiotics. Other classes of antibiotics like aminoglycosides (gentamycin, tobramycin) and lincosamides (lincomycin) start to become ineffective. Fortunately, isolates of methicillin-resistant Staphylococcus aureus (MRSA) were rare in the present study (15.6% resistance to oxacillin). Likewise, all isolates were sensitive to vancomycin. These results confirm that glycopeptides (vancomycin, teicoplanin) are the antibiotics of choice for treating staphylococcal infections.
In this study, non-fermenting gram negative bacilli were represented by Acinetobacter sppand Pseudomonas spp. Strains of Acinetobacter spp were highly resistant to cotrimoxazole and beta-lactams (ticarcillin, C3G and C4G). Resistance to other classes of antibiotics (quinolones represented by ciprofloxacin and aminoglycosides represented by gentamycin and tobramycin) were also higher compared to those of bacteria reported in the previous paragraphs Similarly, 75% had a carbapenemase (resistance to imipenem) and were resistant to all antibiotics tested. A similar study carried out in Saudi Arabia identified multi-resistant strains of Acinetobacter baumannii (94%) [23]. The study carried out by Guckan et al also demonstrated the multi-resistant nature of Acinetobacter baumannii strains [60]. These strains were resistant to ciprofloxacin (97.3%), gentamicin (77.2%), trimethoprim-sulfamethoxazole (68.9%), imipenem (89.1%), meropenem (90 , 3%) [24]. However, colistin (5.5%) and netilmicin (19.5%) were effective on these strains [24]. Another recent study in Iran showed resistance to Acinetobacter baumannii with cefixime (99%), ceftazidime (99%), ciprofloxacin (98%), meropenem (99%), trimethoprim-sulfamethoxazole (99%) %), imipenem (91.5%), ceftriaxone (99%), levofloxacin (96.5%), amikacin (70%) and gentamycin (85%) [61]. In contrast, tigecycline (4%) and colistin (0%) are effective [25]. According to these 2 studies, colistin remains the molecule of choice for the treatment of infections caused by Acinetobacter baumannii [24-25].
Regarding the isolates of Pseudomonas spp, they are less resistant than the strains of Acinetobacter spp. Likewise, all isolates were susceptible to imipenem (absence of carbapenemase) and amikacin. Like Enterobacteriaceae, the antibiotics of choice for the treatment of Pseudomonas spp infections are penemes (imipenem, meropenem) and amikacin. According to these results in non-fermenting gram-negative bacilli, bacteria of the genus Acinetobacter, which produce carbapenemases, are among the highly resistant emerging bacteria [7]. Infection by these microorganisms is responsible for therapeutic difficulties or even dead ends. In addition, these germs represent a potential danger as sources, reservoirs and vehicles of carbapenemases as well as a risk of hospital epidemics which can quickly spiral out of control [7]. Fortunately, amikacin has been effective against the majority of isolates of Acinetobacter spp. In addition to colistin which has been reported in the literature, amikacin can be considered as the molecule of choice to be included in the treatment regimen for infections caused by carbapenemase-producing bacteria [7].
Regarding the antibiotic resistance of the other bacteria identified in this study, they are rare since these other bacteria are not frequent in hospitals and in the community and they are sensitive to all antibiotics in the majority of cases. For example, even though Neisseria meningitidis meningitis is very serious, it can be easily treated with beta-lactams because these bacteria are sensitive to all the antibiotics tested.
5. Conclusion
This study identified the bacteria most frequently responsible for bacterial infections in hospitals and in the community. It also identified the factors associated with these infections and assessed the antibiotic resistance of the bacteria isolated. Enterobacteriaceae were the most frequent. Likewise, women were often affected by Gardnerella vaginalis vaginosis. Women, people hospitalized in intensive care and surgery as well as patients with suppurations and wounds, were the most affected by bacterial infections. Regarding antibiotic resistance, penicillins and cotrimoxazole are no longer effective against most germs. Fortunately, penemes (imipenem), broad-spectrum aminoglycosides (amikacin) and glycopeptides (vancomycin) are still effective today and represent the antibiotics of choice. However, some bacteria of the genus Acinetobacter have been resistant to imipenem and amikacin. In the literature, colistin is the drug of choice against these bacteria of the genus Acinetobacter. Thus, this study encourages clinicians to avoid empirical treatments for bacterial infections before bacteriological analysis. If possible, wait for the results of the antibiogram to know the antibiotic of choice for the patient. If the treatment cannot wait, it is recommended that the sample be taken before the antibiotic treatment. Then the probabilistic treatment can be carried out and will be adjusted according to the result of the antibiogram. This attitude will help reduce the antibiotic resistance of germs. Likewise, it is necessary to inform and educate the population about the danger of self-medication.
Acknowledgements
We would like to thank all the staff of the laboratory of University Hospital of Befelatanana and all the laboratory technicians. Similarly, we would like to express our gratitude to the director of establishment for authorizing us to carry out this study.
Conflicts of Interest
The authors do not declare any conflict of interest
References
- Coulibaly F. Invasive bacterial infections in the pediatric department of CHU-Gabriel Toure (2007): 103.
- Schreiber JR, Jacobs MR. Antibiotic- resistant au pneumococci. Pediatric Clinics of North America 42 (1995):519-537.
- Laxminarayan RA, Duse C, Wattal AK, Zaidi HF, Wertheim N, et al. Antibiotic resistance-the need for global solutions. Lancet Infectious Diseases 13(2013):1057-1098.
- Kakupa DK, Muenze PK, Byl B, Wilmet MD. Study of the prevalence of nosocomial infections and associated factors in the two university hospitals of Lubumbashi, Democratic Republic of Congo. Pan AfricanMedical Journal 24(2016): 275.
- Lukuke HM, Kasamba E, Mahuridi A, Ngatu NR, Narufumi S, et al. Nosocomial urinary tract and surgical site infection rates in the Maternity Ward at the General Referral Hospital inNosocomial urinary tract and surgical site infection rates in the Maternity Ward at the General Referral Hospital in Katuba, Lubumbashi, Democratic Republic of the CongoKatuba, Lubumbashi, Democratic Republic of Congo. Pan African Medical Journal 28(2017):57.
- Amazian K, Rossello J, Castella A, Sekkat S, Terzaki S, et al. Prevalence of nosocomial infections in 27 hospitals in the Mediterranean region. Eastern Mediterranean Health Journal 16(2010): 1070-1078.
- Maamar B, Abdelmalek R, Messadi AA, Thabet L. Clinical and epidemiological survey of infections due to carbapenemase producing Enterobacteriaceae in burns. Annals of Burns and Fire Disasters 32 (2019):10-16.
- Tchelougou D, Karou DS, Kpotsra A, Balaka A, Assih M, et al.Vaginal infections in pregnant women at the Sokodé regional hospital (Togo) between 2010 and 2011. Médecine et Santé Tropicales 23(2013):49-54.
- Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstetrics and Gynecology 109 (2007):114-120.
- Hofmann Y, Berger H, Wingeier B, Huber B, Boggian K, et al. Treatment of strept throat. Forum Medical Suisse 19(2019):481-488.
- Gibbs RS, Schrag S, Schuchat A. Perinatal infections due to group B streptococci. Obstetrics and Gynecology 104(2004):1062–1076.
- Jaureguy F, Carton M, Teboul J, Butel MJ, Panel P, et al. Risk factors and screening strategy for group B streptococcus colonization in pregnant women: results of a prospective study Journal de GynecologieObstetriqueetBiologie de la Reproduction 32 (2003):132–138.
- Ould Salem ML, Ghaber SM, Ould Baba SEW, Ould Maouloud MM. Antibiotic susceptibility of community-acquired strains of Staphylococcus aureus in Nouakchott Region (Mauritania). Pan African Medical Journal 24(2016): 276.
- Kebe M, Charbit Y. Gender and Vulnerability among Senegalese Women Heads of Households. Revue Européenne de Migrations Internationales 23 (2007): 51-55.
- Bourrillon A. Infectious pathologies. Pediatrics (2011): 427–539.
- Ducel G, Fabry J, Nicolle L, Girard R, Perraud M, et al. Practical guide: Prevention of nosocomial infections. 2nd edition. Geneva: World Health Organization(2008): 71.
- Merzougui L,Barhoumi T,Guizani T, Barhoumi H,Hannachi H,et al. Nosocomial infections in the Intensive Care Unit: annual incidence rate and clinical aspects, Kairouan, Tunisia. Pan African Medical Journal 30 (2018): 143.
- Randriatsarafara FM, Ralamboson J, Rakotoarivelo R, Raherinandrasana A, Andrianasolo R. Antibiotic consumption at Antananarivo University Hospital: prevalence and strategic challenges. Santé Publique 27 (2015): 249- 255.
- Ebongue CO, Tsiazok MD, Nda Mefo'o JP, Ngaba GP,Beyiha G, et al. Evolution of antibiotic resistance of Enterobacteriaceae isolated at the Douala General Hospital from 2005 to 2012. Pan African Medical Journal 20(2015): 227.
- Hounsa A, Kouadio L, De Mol P. Self-medication with antibiotics from private pharmacies in the city of Abidjan in Ivory Coast. Médecine et Maladies Infectieuses 4 (2010):333-340.
- Camara M, Dieng A, BouhBoye CS. Antibiotic Susceptibility of Streptococcus Pyogenes Isolated from Respiratory Tract Infections in Dakar, Senegal. Microbial Insights 6(2013):71–75.
- SENTRY Surveillance Program. Comparative in-vitro activity of daptomycin against Grampositive microorganisms: SENTRY surveillance Program, Spain (2002–2006). EnfermedadesInfecciosas yMicrobioogialClinica 26 (2008):489–494.
- Alyamani EJ, Khiyami MA, Booq RY, Alnafjan BM, Altammami MA, et al. Molecular characterization of extended-spectrum beta-lactamases (ESBLs) produced by clinical isolates of Acinetobacter baumannii in Saudi Arabia. Annals of Clinical Microbiology and Antimicrobials 14(2015):38.
- Guckan R, Kilinc C, Demir AD, Capraz A, Yanik K. Antimicrobial susceptibility of Acinetobacter baumanniicomplex isolated from different clinical samples in a tertiary care hospital. Journal of Antibiotics Research 1(2015):103.
- Goudarzi H, Douraghi M, Ghalavand Z, Goudarzi M. Assessment of antibiotic resistance pattern in Acinetobacter baumannii carrying bla oxA type genes isolated from hospitalized patients. Novelty in Biomedicine 1(2013):54-61.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
