Cassava Cultivation under Abiotic Stress: Emphasis on Waterlogging Tolerance Using Arbuscular Mycorrhizal Fungi
Branly Wilfrid Effa Effa1*, Dick Sadler Demikoyo Khangou1, Stéphane Mibemu Guibinga1, François Ndjelassili2, Mesmin N’dong Biyo’o1, Abdala Gamby Diedhiou3,4,5,6
1Laboratoire de Biotechnologies Végétales, Institut de Recherches Agronomiques et Forestières (IRAF), Centre National de la Recherche Scientifique et Technologique (CENAREST) BP 2246.
2Laboratoire de Pédologie, Institut de Recherches Agronomiques et Forestières (IRAF), Centre National de la Recherche Scientifique et Technologique (CENAREST) BP 2246.
3Département de Biologie Végétale, Faculté des Sciences et Techniques, Université Cheikh Anta Diop (UCAD), Dakar, Sénégal.
4Laboratoire Mixte International Adaptation des Plantes et Microorganismes associés aux Stress Environnementaux (LAPSE), Centre de recherche de Bel-Air, Dakar, Sénégal.
5Laboratoire Commun de Microbiologie (LCM), Centre de Recherche de Bel-Air, Dakar, Sénégal.
6Centre d’Excellence Africain en Agriculture pour la Sécurité Alimentaire et Nutritionnelle (CEA-AGRISAN), UCAD, Dakar, Sénégal.
*Corresponding Author: Branly Wilfrid Effa Effa, Laboratoire de Biotechnologies Végétales, Institut de Recherches Agronomiques et Forestières (IRAF), Centre National de la Recherche Scientifique et Technologique (CENAREST) BP 2246. Orcid number: 0000-0001-9287-5805
Received: 05 April 2024; Accepted: 12 April 2024; Published: 23 May 2024.
Article Information
Citation: Branly Wilfrid Effa Effa, Dick Sadler Demikoyo Khangou, Stéphane Mibemu Guibinga, François Ndjelassili, Mesmin N’dong Biyo’o, Abdala Gamby Diedhiou. Cassava Cultivation under Abiotic Stress: Emphasis on Waterlogging Tolerance Using Arbuscular Mycorrhizal Fungi. Journal of Environmental Science and Public Health. 8 (2024): 86-100.
View / Download Pdf Share at FacebookAbstract
Cassava is a staple food for over 750 million people worldwide mainly in Africa, thus is closely associated with food security. However, in Africa the Congo Basin region receives a high level of rainfall, leading to more or less prolonged waterlogging of the soil. Waterlogging one of the major abiotic causes of constraints in crop production worldwide, could be involved in the reduction of cassava production in Congo Basin. Nevertheless, there are aerobic microbes such as arbuscular mycorrhiza fungi (AMF), which establish endomycorrhizal symbioses, mutualistic symbiosis between plants and arbuscular mycorrhizal (AM) fungi. They may enhance host plants nutrient acquisition and protect them also from abiotic stress. AMF diversity in wetland rice roots and in roots of Littorella uniflora submerged in lakes have been postponed. Furthermore, molecular techniques reveal that both terrestrial and aquatic plants can harbor a rich diversity of AMF species in their roots. Cassava is highly mycotrophic. Our review describes the main abiotic stresses faced by cassava, with particular emphasis on waterlogging stress when cassava is associated with AMF. The review suggests possible solutions to enable farmers to maintain acceptable yields despite the occurrence of these various abiotic stresses.
Keywords
<p>Cassava, Abiotic stress, Waterlogging, AMF, Endomycorrhizal symbiosis, Acceptable yield.</p>
Article Details
1. Introduction
Domestication results in morphological and physiological changes in the domesticated organism, known as domestication syndrome [1]. These changes help to differentiate the domesticated species from its wild ancestor [2]. Hunter-gatherers over 10,000 years ago (in the Neolithic period) are thought to be the originators of domestication, and are considered to be the pioneers of agriculture [3]. Cultivated plants clearly show significant differences at morphological and physiological levels (domestication syndrome), and respond better to the needs of human beings [4]. For cassava, whose cultivated species was published by Crantz in 1766, Manihot esculenta Crantz subsp. esculenta, and its wild ancestor is Manihot esculenta subsp. Flabellifolia [5]. Studies have been carried out to improve certain traits observable in the ancestor M. esculenta subsp. flabellifolia [4]. Cassava (Manihot esculenta Crantz) is a highly perennial crop belonging to the Euphorbiaceae family and native to tropical America [6]. It is an herbaceous plant with tuberous, starchy roots and a gnarled stem [7]. Its origins probably lie in northeastern and central Brazil [5,8,9], where it was domesticated 7,000 years ago [8], with a second center of diversity and domestication in Central America [10]. Cassava was introduced to Africa in the 16th century by the Portuguese, Barre and Thevet were the first to mention cassava in their writings in 1558 [11]. Cassava was mainly grown in the Portuguese colonies of the Gulf of Benin, Sao Tomé and Principe and the mouth of the Congo, before its cultivation progressed in the Congolese basin (Zaire, among the Bushongos of Kassaï, in 1650) in the 17th century [11]. Cultivation and consumption of cassava increased in Africa at the end of the 19th century [11]. In Asia, in addition to introductions in Ceylon and Calcutta at the end of the 18th century [11,12,13], introductions were made by the Portuguese in Goa, India, in the 16th century [11]. Today, it is found in all tropical and subtropical regions. Cassava is the most important food crop in the humid tropics, thanks to its plasticity and the volume of its production and consumption [14]. It is the staple food of over 800 million people in tropical zones, including 500 million in Africa, and its production is constantly increasing at a higher rate than that of cereals [15]. Cassava is prized for its starch-rich tuberized roots and protein-rich leaves [16], which is borne out not only by the extent of its cultivated area but also by the diversity of its uses in both human and animal nutrition [16]. It ranks fifth among the world's food crops, behind maize, rice, wheat and potato (FAO, 2008). Cassava ranks second (32%) in global production of food roots and tubers, with annual production of over 268 million tons of fresh roots harvested in 2014 behind potatoes, which contribute 45% of the total (FAOSTAT, 2016). According to [17], Africa is the world's leading producer with an annual output of 110 million tons, followed by Asia with 55 million tons and Latin America and the Caribbean (37 million tons). With production in Africa estimated at 134 million tons in 2010, this makes the crop the continent's leading food resource [18]. Among the 6 CEMAC countries, Gabon ranks 4th for both production and yield in 2017 (FAO STAT, 2021). Among the countries where cassava is consumed fresh or processed the most, i.e. more than 50 kilo calories per person per day, African countries are the most numerous; there are around 23 African countries out of a total of 34. Cassava consumption in Central Africa has increased over the years, and has done so for the last fifty years, making the people of Central Africa the leading consumers of cassava. The color of the pulp of tuberous cassava roots is one of the parameters used to distinguish cassava varieties, and these colors are due to the action of certain molecules. For example, the yellow or orange color observed in tuberous cassava roots would indicate the presence of carotenoids [19]. Carotenoids are lipid molecules belonging to the terpene family, which are compounds with a wide variety of functions. These molecules are essential for humans, serving as precursors to vitamin A, retinol [20,21]. Carotenoids are also thought to be involved in the prevention of certain diseases thanks to their antioxidant properties (Bast et al., 1998), including eye diseases such as Age-related Macular Degeneration (AMD), cardiovascular disease, certain cancers and light-induced erythema [22]. Carotenoids are also thought to help regulate the immune system and stabilize the genome [23]. However, despite its strengths, cassava cultivation is faced with constraints such as heat, drought, cold, flooding, viral and bacterial diseases, pest attacks, agronomic, edaphic and socioeconomic factors leading to insufficient propagation materials [7]. Viral diseases are on the increase across the African continent. This poses a major threat to food security, particularly for small-scale subsistence farmers [24]. The latter unwillingly contribute to the spread of viruses through the exchange of infected cuttings, leading to a constant reduction in yields [25]. Plant resilience to abiotic and biotic stresses is only made possible by the biochemical and molecular processes that these changes trigger in plants to adapt [26]. Cassava is grown in intertropical zones with one or two season rainfall patterns ranging from 600 to over 4,000 mm [27], to drier regions (up to 500 to 600 mm rainfall) where its production is progressing [15]. In situations where cassava becomes waterlogged due to the environment or heavy rainfall, this favors root rot and thus lower yields. Our review aims to present work carried out on the production of cassava under conditions of abiotic stress, mainly by proposing possible solutions for cassava production in waterlogged conditions. The mechanisms put in place by cassava to tolerate waterlogging under endomycorrhizal or non-endomycorrhizal symbiosis will be described.
2. Overview of abiotic stress
In order to meet current and future global food demand, and in view of demographic trends, increases in food crop production levels are necessary, but these increases must be balanced against climate change, which is having a negative impact on yields, and contrasting levels of technology between countries [28, 29]. Studies carried out on meteorological factors such as temperature and rainfall have shown that temperature has an impact on plant growth while rainfall has an impact on plant production [30]. Given the importance of temperature and rainfall factors on agricultural activities because of their strong correlation [30]. It seems appropriate to increase studies on the interactions of these two meteorological factors, the results of which will be made available to farmers [30,31,32]. However, certain agricultural practices also contribute to environmental degradation [28]. But there are many stresses that can be either plant-specific or location-specific [33]. Studies have shown that temperatures between 25°C and 35°C enable cassava plants to photosynthesise and grow well (Mabrouk et al 1992). [34] showed that temperatures below 17°C or above 37°C delayed the appearance of shoots and leaves on cassava cuttings. Environmental factors such as temperature, drought, light, salinity and flooding cause abiotic stresses in plants, leading to major losses in world production [35, 36] have shown that plant growth, flowering, fruiting and ripening are affected by abiotic stresses, considerably reducing yields and leading to a reduction in crop quality, nutritional composition and market value. If the trend is not reversed, increasingly severe climate change will lead to lower crop yields and even the disappearance of certain plant species that are not very resilient due to the abiotic stresses caused by certain meteorological factors. During abiotic stress, plants are more exposed to attacks from pests and pathogens because of their fragility. However, they always seek to protect themselves from abiotic stress by responding physiologically through adaptive biochemical and morphological changes. These adaptive changes in plants in the face of abiotic stresses are only possible if the duration of the stress is relatively short; these responses often vary from one plant to another and depend on the abiotic stress in question. Plants subjected to abiotic stress for a relatively long period begin a withering process that is often irreversible, which has a negative impact on their yields. Plants mainly respond to abiotic stress by accumulating reactive oxygen species (ROS) [37], reactive nitrogen species (RNS) (Wani et al., 2021) and reactive sulphur species (RSS) [38]. This results in the activation of several signalling pathways whose importance in cell biology no longer needs to be demonstrated [39]. However, in plants, abiotic stress disrupts the transport of nutrients and also provokes inadequate cellular responses [39]. It should be noted that plants sometimes react simultaneously to several abiotic stresses, for example: flooding, excessive solar radiation and heat, which occur at the same time in the field, particularly in equatorial zones.
3. Heat
Studies have shown that high temperatures reduce crop production [26, 40]. High temperatures lead to significant heat stresses, the responses of which in plants often result in adaptation difficulties induced by these temperature increases [30]. A model based on meteorological variables has shown that an increase in temperature leads to a drop in crop yields [41]. According to [42], maize and soybean yields fall by around 17% for each degree rise in temperature during the vegetative stage. Heat shock induces a response at transcriptional level [26], [43] have shown that conserved cis-regulatory elements (HSEs) are at the origin of this response and are binding sites for heat shock factors (HSFs). The gene spotted leaf 7 (spl7) encodes a HSF [44] and spl7 may be involved in controlling cell death caused by high temperature [26]. High temperatures induce heat shock proteins (HSPS) and late embryogenesis abundant (LEA) proteins in plants [26]. High temperatures also cause oxidative stress in plants, which has the effect of denaturing functional and structural proteins [33]. Extreme temperatures cause a considerable reduction in agricultural yields [45]. Existing studies on the heat tolerance of cassava have mainly been carried out in West Africa and the Sahelian zone [46]. In cassava, stomata can open at temperatures of up to 30°C [47,48].
4. Cold
Cassava is known for its sensitivity to low temperatures [49, 50, 51, 52, 53]. The temperature of an area is a key factor in deciding whether or not to plant a cassava plot, given the harmful impact of low temperatures on cassava [51]. Factors such as the internal water status of the plant and the water vapor pressure deficit between the leaf and the surrounding air have a considerable impact on the stomatal response when the plant is subjected to given temperatures [54]. Stomatal closure is observed in cassava at temperatures below 20°C [55,56]. When plants are subjected to stress, transcription factors (TFs) cascade down target genes to prevent potential damage to plant cells during transcriptional regulation [50]. Plants are subject to cold stress when they are either in the presence of temperatures below 0°C (frost) or in the presence of temperatures between 0°C and 15°C (chill); the manifestations of this are physiological and biochemical changes and abnormal plant development [57]. When plants are subjected to low temperatures (freezing or chilling), there are immediate effects on the fluidity of cell membranes and on enzymatic activities, which have an impact on various cellular processes [57]. These low temperatures also affect the quantity of RNA molecules during DNA transcription and, consequently, the quantity of proteins during translation [58]. The ecology of cassava plants in tropical regions does not allow them to survive in freezing conditions [53,59] showed that young cassava plants subjected to temperatures between 0°C and 15°C stopped developing and their leaves dried. Plants exposed to stress for several days lose vigor; young stems soften, petioles turn downwards and the chlorophyll content of the leaves decreases [51], which reduces the absorption of solar radiation [52]. However, cold enables cassava plants to produce more proline, malondialdehyde (MDA), soluble sugars and reactive oxygen species (ROS) [51].
5. Drought
The increase in demand for water due to population growth and economic activity, mainly urbanization and industrialization, is having a major impact on the global reduction in freshwater reserves, with the strongest impacts observed in Africa [60]. Between now and 2050, the combined effects of demographic growth (an increase in the world's population of between 1 and 1.3 billion) and climate change could mean that almost 1.8 billion people (80% of them in developing countries) will have to live with quantities of water that are insufficient to meet their needs [60]. Water scarcity thus remains the most critical threat to food security [61, 62]. Water deficit leads to alterations in metabolic functions in plants, one of the most important of which is the reduction or cessation of photosynthetic pigment synthesis (among which chlorophyll), reducing biomass production and yield [63]. Chlorophylls a and b, which are generally found in higher plants, are sensitive to water deficit, and a reduction in their content leads to an increase in lipid peroxidation and free radicals, and degradation of the chloroplast membrane [63]. However, the extent and impact of drought depend on a number of factors, including the water retention capacity of the soil, rainfall and evaporative demand [64]. The so-called resurrection plant, Craterostigma plantagineum, is one of the rare angiosperms able to survive a period of severe drought, with a relative water content in the leaves of less than 2% [65]. In fact, in mature C. plantagineum subjected to drought, changes have been observed in mRNAs and proteins [65]. These changes enable C. plantagineum to continue or even complete its cycle while minimizing the effects of drought, these mechanisms put in place by C. plantagineum make it a drought-tolerant plant. Cassava is more sensitive to water deficit up to 4 months after planting [66, 67], at which stage plant losses can be close to 90% [67]. Cassava is a drought-tolerant plant [68], but drought has a negative impact on tuberous root yield [69] and on growth and physiological parameters [70, 71, 72].
5.1 Genes and signaling pathways induced in response to water deficit
In cassava, understanding the genetic determinism of the response to drought is essential for choosing cultivars for use in water-deficient areas. Like other plants, cassava reacts to drought either by avoiding it, escaping it or tolerating it. The main mechanism on which cassava relies is avoidance, which it achieves by closing its stomata and forming several roots [73]. This is only possible thanks to the involvement of several genes that are either up-regulated or down-regulated for an optimal response of cassava to drought [68]. As the cassava genome sequences are known [74], the putative drought response genes can be identified. These genes have several functions: osmotic protection, heat shock and other responses to oxidative stress, as well as signal transduction proteins [68,75] showed that the MeALDH, MeZFP, MeMSD and MeRD28 genes are up-regulated only in drought-tolerant cassava varieties. The MeMSD and MeALDH genes being known to be reactive oxygen species (ROS) reducers, and the MeZFP and MeRD28 genes involved in osmotic adjustments [75].
ABA concentration increases dramatically in response to water deficit [76, 77, 78]. The increase in ABA induces stomatal closure and the accumulation of metabolites and stress proteins [76,79,80,81,82,83]. The perception of water deficit signals is transmitted from the plasma membrane to the nucleus [84, 85] via receptor kinases (RLK) and Mitogen Activated Protein Kinases (MAPK) capable of inducing the phosphorylation of other proteins [86, 87]. Numerous transcription factors (TFs) regulating this signaling under water deficit conditions have been identified in rice. These TFs bind to cis-regulatory elements of genes encoding proteins belonging to the bZIP, AP2/ERF, MYB, NAC, WRKY, NF-Y, CAMTA, and bHLH families [88, 89]. Molecular analysis of ABA-dependent pathway response gene promoters led to the identification of the ABRE motif, which has ACGTGG/T as its main sequence [90]. This motif can be recognized by bZIP FTs [91]. Overexpression of some of these FTs can induce tolerance to water deficit. For example, overexpression of the ONAC022 gene in rice improves water deficit tolerance through the ABA-dependent pathway [92].
In the ABA-independent pathway, rice DEHYDRATION RESPONSIVE ELEMENT BINDING PROTEIN (DREB) transcription factors such as DREB, DREB1/CBF and DREB2 play a key role in water deficit tolerance [93, 94]. LATE EMBRYONIC ABUNDANT (LEA) proteins, which are involved in water deficit tolerance, can accumulate in vegetative tissues under water stress as well as in seeds during dehydration [95]. For example, overexpression of the OsEM1 and OsLEA3-1 genes that code for LEA proteins induces water deficit tolerance in rice by improving organ integrity [96, 97]. Auxin, a phytohormone that is particularly important for root development [98], also plays a role in water deficit tolerance in rice [99]. Indeed, overexpression of the OsPIN3t [100], OsGH3.2 [101] and OsGH3.13 [102] genes and the auxin/IAA OsIAA6 gene [103], which targets the TIR1 auxin receptor, induces better tolerance to water deficit [99]. In addition, overexpression of the ethylene response factor (JERF1) increases proline content, reduces water loss, increases ABA synthesis and regulates the expression of ABA synthesis genes, thus improving water deficit tolerance in rice [104].
5.2 Physiological mechanisms for adapting to water deficit
In the presence of low soil moisture, cassava develops adaptation mechanisms such as: escape mechanisms, avoidance mechanisms, and tolerance mechanisms, through morpho physiological changes [105, 106, 107, 108].
Escape mechanisms
The water deficit escape mechanism is defined as the ability of a plant to complete its development cycle before the onset of the water deficit. It essentially comprises two mechanisms, rapid phenological development and developmental plasticity [106]. Rapid phenological development enables plants, under conditions of limited water supply, to produce flowers and then seeds with a minimum of vegetative growth [106]. Developmental plasticity, on the other hand, allows plants, during a period of abundant rainfall, to have good vegetative growth and produce flowers and then seeds abundantly [106].
Avoidance mechanisms
The main mechanism on which cassava relies is avoidance [73]. The water deficit avoidance mechanism is defined as the ability of a plant to maintain relatively high tissue water potential despite low soil moisture. Rice genotypes that avoid water deficit generally have deep, thick roots with a high branching and soil penetration capacity, a high root to aerial part ratio, leaf curling elasticity, early stomatal closure and high cuticular resistance [109, 110]. Stomatal closure reduces water loss from leaves and decreases gas exchange between the plant and the atmosphere, which impacts the rate of photosynthesis and ultimately reduces crop yield, but allows plants to survive short-term water deficits [111]. Regulation of stomatal conductance during water stress results from signals from the root system, which are still poorly understood [112]. Leaf rolling reduces water loss where stomatal closure is incomplete and reduces the heat load on the leaf by reducing the exposed surface [113]. There are complex interdependencies between transpiration, assimilation and leaf temperature, so leaf curl is of adaptive importance. From an agronomic point of view, leaf curl is generally considered to be an indication of susceptibility to water deficit in rice [113]. The cuticle is organized as a continuous layer of hydrophobic lipids that covers the aerial organs of plants, forming a protective barrier against the external environment [114]. It is synthesized by epidermal cells and is composed of cutin and waxes [114]. Cuticular waxes constitute the primary structure of the cuticle and enable plants to limit non-stomatal water loss [115]. In rice subjected to water deficit, there is overexpression of the OsGL1-6 gene, which is involved in the biosynthesis of cuticular wax (in the leaves), the accumulation of which induces tolerance to water deficit [114]. In roots, a marked reduction in the diameter of coronal roots, leading to relatively thinner roots to save water resources, has been observed during a water deficit [116]. In addition, the formation of lateral roots increases, which increases their surface area for absorbing water [99]. The reduced risk of cavitation of xylem vessels under water deficit is attributed to the reduction in the diameter or number of these vessels [116]. The diameter of sclerenchyma cells increases while their suberisation and the formation of aerenchyma cells decrease [116].
Tolerance mechanisms
The mechanism of tolerance to water deficit is the ability of plants to survive despite low water content in their tissues [117]. Genetically, water deficit tolerance in rice is a complex and polygenic trait, involving morpho-physiological mechanisms [118] such as maintenance of turgidity by osmotic adjustment, increased cell elasticity, reduction in cell size and conservation of the integrity of protoplasmic structures [119]. Tolerance mechanisms group together a set of physiological processes that enable the plant to adapt to the reduction in water resources by maintaining metabolic activity up to a certain point drought adaptation mechanisms involve molecular responses that have an impact on the physiology of the entire plant and incorporate modifications to the growth of aerial parts, root architecture, water uptake and transpiration [120].
6. Waterlogging
6.1 Waterlogging tolerance without Arbuscular Mycorrhizal Fungi (AMF)
The disruptions observed in the occurrence of rainfall could have a more negative impact than the global warming so decried by people around the world [121,122,123]. Studies have shown that soil waterlogging affects more than 17 million km2 of the Earth's land surface [124,125]. Frequent waterlogging of soils is thought to be responsible for a 40-80% reduction in yields [124,126,127,128], which can be explained by the lower level of gas diffusion in water compared with air [125]. This leads to a reduction in the amount of oxygen in the rhizosphere, having a direct and negative impact on the mitochondria in terms of their role in supplying the energy needed to transport and adsorb nutrients [129,130]. However, if anaerobic conditions are transient and last less than 48 hours, the damage caused to the mitochondrial membrane remains reversible and oxidative phosphorylation resumes as soon as aerobic conditions return [129]. Thus, flooding induces reduction of photosynthesis and respiration, limits stomatal conductance and hydraulic conductance, and obviously decreases plant growth [126]. The plant's underground organs, such as roots and rhizomes, are the first to be exposed to soil waterlogging. These organs preferentially function in aerobic conditions. Studies have shown that the oxygen atom is quantitatively the most important element in the earth's crust and that its presence in molecular form (O2) in the biosphere helps to keep aerobic organisms alive [131]. Plants are photosynthetic and autotrophic organisms, and photosynthesis consists of the synthesis, by chlorophyll plants, of organic matter using carbon dioxide (CO2), light energy and water. This organic matter (carbohydrates) contributes to the development and growth of the whole plant and is involved in the production of energy through respiration by the mitochondria. This requires an optimum supply of oxygen (O2). Oxygenases enzymes are the main oxygen fixers in organic molecules [131].
Among terrestrial plants, few are tolerant of persistent waterlogging of soils. According to [132], cassava would not survive in persistent waterlogged soil conditions. Cassava is preferentially grown in localities with a minimum rainfall of 700 mm per year [48]. Cassava belongs to the group of roots and tuber crops [132, 133] is a tuberous-rooted plant [7, 16]. Both sweet potato and cassava are drought-resistant root and tuber crops, so it would be interesting to draw on the results of studies conducted on sweet potato to gain a better understanding of cassava's responses to various stresses, particularly abiotic stresses. Crops produce various antioxidant compounds to adapt to oxidative stress [134]. Sweet potato, crop belonging to the group of root and tuber crops, is the model crop used to study the effects of soil waterlogging on the growth and development of crops in this group [135]. In sweet potatoes, an increase in antioxidants and antioxidant enzymes has been observed in the leaves when grown in waterlogged soil [134]. These antioxidants and antioxidant enzymes produced are the manifestation of tolerance to waterlogging. Antioxidant enzymes play an important role in preventing or reducing the damage caused by toxic oxygenated substances on plant cells. These oxidant enzymes are mainly glutathione reductase (GR), catalase (CAT), superoxide dismutase (SOD) and ascorbate peroxidase (APX); they are also involved in the elimination of these oxygenated substances [136]. It has been observed that the activities of the antioxidant enzymes glutathione reductase, catalase, superoxide dismutase and ascorbate peroxidase are increased in Citrange de Carrizo (Poncirus trifoliata L. Raf. Citrus sinensis L. Osb.) and Citrumelo CPB 4475 (Poncirus trifoliata L. Raf. Citrus paradisi L. Macf.) when grown in waterlogged soils; these enzymatic activities are thought to be associated with a delay in the onset of oxidative damage thanks to the trapping of activated oxygen species [130]. Ascorbate peroxidase is also involved in protecting plants against oxidative damage by eliminating hydrogen peroxide [134,137] have shown that when sweet potatoes are grown in waterlogged soil, the leaves produce ROS which are subsequently eliminated by antioxidant enzymes including GR [134,135] observed that an increase in the activities of superoxide dismutase (SOD) and catalase (CAT) in the sweet potato variety Taoyuan1 helped detoxify reactive oxygen species when the sweet potato was grown in waterlogged soil. A member of the triazole family, paclobutrazol (PBZ) ((2RS, 3RS)-1-4 (-chlorophenyl)- 4, 4 dimethyl-2-1, 2, 4-triazol-1-yl-penten-3-ol) [134], is thought to boost the antioxidant activities of certain cereals, which could increase their tolerance to oxidative stress [138]. The most relevant indicators of plant responses to adaptation to waterlogging are elevated ethylene levels and/or decreased O2 concentrations [124]. During photosynthesis, the peroxisome is involved in the photorespiration process and contributes to tolerance of oxidative stress [131]. The peroxisome is an intracellular organelle that performs essential metabolic functions despite lacking DNA.
6.2 Arbuscular Mycorrhizal Fungi (AMF) one key to improve cassava production under waterlogging
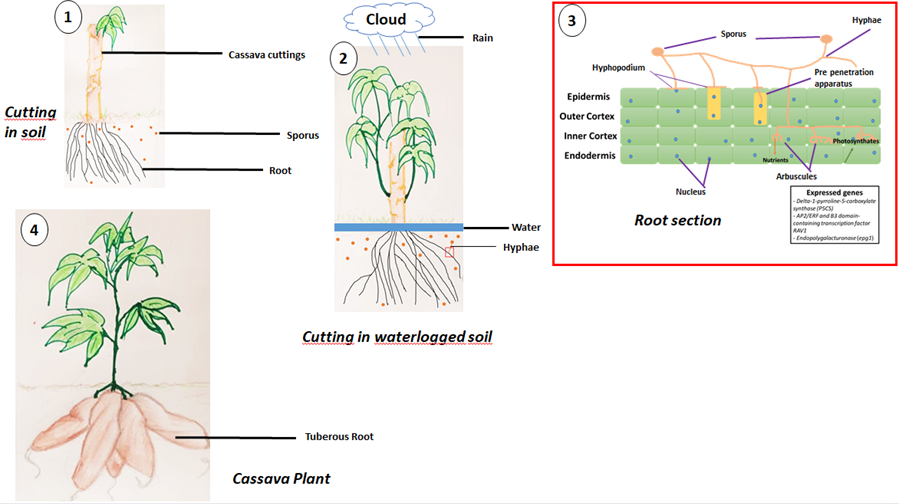
At a conference in Strasbourg in 1879, Anton de Bary describes an ecological relationship between two organisms of different species that are in direct contact [139]. Fungi are among the most numerous microorganisms living in the soil, with millions of species of fungi, some of which have not yet been clearly identified. Fungi are very active in the ecosystem, particularly in regulating the physiological processes that take place in the soil [140,141] have shown that AMFs play an important role in biological regulation by improving plant growth and development, but also by protecting them against pathogens. In addition, thanks to its extra-root hyphae, the AMF takes nutrients in forms that cannot be assimilated by the host plant and from areas that are not easily accessible to the host plant in exchange for photosynthates provided by the host plant, in addition to helping it to withstand unfavorable environmental and soil conditions (Figure; [142]). Mycorrhizae, thanks to their extra-radicular hyphae (physical extension of the root system) of small diameters, explore a larger surface area of soil and are also capable of penetrating into soil microsites inaccessible to plant roots [143]. The association of roots and arbuscular mycorrhiza fungi (AMF) will make it possible to define a new zone called mycorrhizosphere, this term “mycorrhizosphere” makes it possible to characterize the volume of soil under the influence of mycorrhizas [144,145]. The word endomycorrhiza comes from the Greek words ενδον, endon meaning "in", mycos meaning "fungus", and rhiza meaning "root". During endomycorrhizal symbiosis, the fungus penetrates inside the roots [146]. This association is mutualistic [147,148,149], i.e. mutually beneficial. It can be observed in almost all terrestrial plants (almost 90%), with the exception of plants belonging to the families Brassicaceae, Caryophyllaceae, Cyperaceae, Juncaceae, Chenopodiaceae and Amaranthaceae, which have very few mycorrhiza associations [150]. The fungus takes advantage of the carbon resources synthesized by the plant via photosynthesis, which are essential for its metabolism and multiplication. In return, the fungal hyphae improve the water and mineral nutrition of the host plant by increasing the volume of soil explored and producing various extracellular enzymes (proteinases, phosphatases, etc.) capable of mobilizing nutrients from complex soil compounds [151]. The main types of mycorrhizae encountered are: ectomycorrhizae, arbuscular endomycorrhizae, ericoid endomycorrhizae, arbutoid endomycorrhizae, orchidaceae mycorrhizae, ectendomycorrhizae and sebacinoid mycorrhizae [150]. The establishment of endomycorrhizal symbiosis depends on the host plant species, the fungal species and soil conditions [152]. Eighty percent of terrestrial plants form endomycorrhizal symbioses with AMFs, however plants belonging to the Brassicaceae, Caryophyllaceae, Cyperaceae, Juncaceae, Chenopodiaceae and Amaranthaceae families do not form mycorrhizal associations [153]. This is the case for the model plant Arabidopsis thaliana, which belongs to the Brassicaceae family [154]. For plants with the ability to establish symbioses with AMFs, there is no host specificity [148]. Optimizing plant-AMF partnerships is a challenging task given the dependence of these interactions on soil conditions [155]. Indeed, the relative availability of nutrients in the soil has an impact on the establishment of the symbiosis between the plant and the AMF [156]. Soils containing limiting amounts of P and N for plant growth are generally favorable for symbiosis establishment [148,157,158]. Studies on fungi forming endomycorrhizae with plant roots have mainly focused on the terrestrial environments where they are found preferentially, and on the roles they play when plants are subjected to abiotic and/or biotic stresses. Fungi forming endomycorrhizae with plant roots have been classified as obligate aerobes [158,159]. This is because past studies have shown that soils with a persistent water table are anoxic [20], and that plants surviving there do not establish mycorrhizal symbiosis with arbuscular mycorrhiza fungi (AMF) [160]. The positive importance of arbuscular mycorrhiza fungi (AMF) in the development and growth of the plants with which they are associated has been recognized for decades. However, there are few studies on the mycorrhizal status of aquatic plants (from rivers, ponds, streams and marshes, etc.). AMFs were first observed in the roots of aquatic plants with a water level between 0.3m and 0.8m depth [161]. A well-developed aerenchyma is thought to be responsible for the tolerance of AMFs to waterlogging [162]. More recently, endomycorrhizae have been observed in the roots of plants living in waterlogged soil conditions; mangrove and mangrove-associated species [163,164], the aquatic plants Littorella uniflora and Lobelia dortmanna [165] and the submerged aquatic macrophytes Isoëtes lacustris and I. echinospora [166]. Furthermore, no dysfunction was observed in the establishment of endomycorrhizal symbiosis in soils subject to short-term waterlogging [167,168,169]. Molecular analyses have also shown that aquatic and terrestrial plants can harbor the same diversity of mycorrhiza fungi in their roots [164], even though studies have shown that there may be differences between the fungal communities of AMFs found in waterlogged soils and soils subjected to other abiotic stresses, which is evidence of the existence of environmental selection enabling these communities to be differentiated [170,171,172,173].
Cassava, a terrestrial plant belonging to the root crop group, has difficulty surviving in waterlogged soils, especially if the waterlogging lasts for a relatively long time [174] have shown that when cassava cuttings are planted in waterlogged soil, regenerative growth of the cuttings is inhibited, and that even at harvest, waterlogged soil reduces the starch content of tuberous roots. AMFs form mutualistic associations with nearly 80% of plant species, including cassava [175], which is a plant of recognized importance in Africa, where it is grown mainly for its tuberous roots [176]. There is little research on AMFs that form mutualistic associations with cultivated species, including cassava, even though agricultural practices can have a negative impact on the diversity of AMFs in soils [169,177] showed that mycorrhized plants contained significantly more proline than non-mycorrhized plants in waterlogged and non- waterlogged conditions, and that the pyrroline-5-carboxylate synthase (P5CS) enzyme was up regulated in waterlogged soil in mycorrhized plants. Accumulation of proline would enable mycorrhized plants to tolerate waterlogging [169]. Proline is the only proteinogenic amino acid with a secondary rather than a primary amine, which gives it a special geometry and exceptional conformational rigidity, essential for primary metabolism [178,179] were among the first to study the accumulation of proline in plants, using Lolium perenne as their model plant. Environmental stresses are thought to be responsible for the increase in proline in plants [178], given that nearly 80% of land plants establish endomycorrhizal symbioses. Proline is involved in proper seed germination, flowering and other phases of plant development. This suggests that the P5CS homologous gene delta-1-pyrroline-5-carboxylate synthase identified in the Manihot esculenta cultivar AM560-2 on chromosome 7 at locus LOC110619320 and on chromosome 10 at locus LOC110625066 [180, 181] may be involved in the tolerance of mycorrhizal cassava to waterlogging. Insufficient oxygen supplies to submerged plant tissue has a negative impact on yields [182]. However, there are root tissues that enable plants to reduce the negative impact of hypoxia or even anoxia; these are called aerenchyma. An aerenchyma is a spongy lacunous plant tissue that allows air to circulate, also known as an aeriferous parenchyma. The aerenchyma is thus an adaptive response of plants grown in waterlogged soils [183]. It can be observed in the roots of aquatic or semi-aquatic species [184]. In sugarcane, the rav1 (RELATED TO ABSCISIC ACID INSENSITIVE3 (ABI3) AND VIVIPAROUS1 (VP1)) and epg1 (endopolygalacturonase) loci are thought to contain genes involved in the formation of aerenchyma in roots [185]. The study of homologous genes preferentially expressed in mycorrhized cassava roots will provide a better understanding of waterlogging tolerance mechanisms. These genes are: gene AP2/ERF and B3 domain-containing transcription factor RAV1 identified in the Manihot esculenta cultivar AM560-2 on chromosome 14 at locus LOC110600122 [186,187,188,189], and epg1 in mycorrhizal cassava subjected to waterlogging would enable further investigations into the strategies put in place by the AMF - cassava pair to tolerate waterlogged soils.
Figure: Colonization of cassava roots by AMF during a waterlogging event and probable genes expressed. (1) Cassava cuttings planted in soil without abiotic stress. (2) Cassava cuttings in waterlogged soil due to heavy rainfall. The cutting exudes strigolactones as a request for help, and the AMF show that they have received these signals by secreting Myc factors which are perceived by the roots; this is the start of the symbiosis. (3) When reaching the root epidermal surface, hyphal tips develop into a hyphopodium that represent the first AM colonization event. In cells located directly under the hyphopodium, intense cytoplasm reorganization result in the formation of a pre-penetration apparatus that guides hyphal penetration through the inner cortex where arbuscules are initiated. Arbuscule development relies on the delivery of plant photosynthates at the periarbuscular membrane. In exchange, AMF transfer nutrients. Delta-1-pyrroline-5-carboxylate synthase (P5CS), AP2/ERF and B3 domain-containing transcription factor RAV1, and Endopolygalacturonase (epg1) are expressed genes enabling cassava to tolerate short-term waterlogging. (4) Cassava plant with tuberised roots ready for harvesting,
7. Conclusion
Thanks to their field capacity, their maximum soil water retention capacity, soils can retain a certain level of water during irrigation and rainfall. However, with the changes observed in recent years in the equatorial African region, rainfall is becoming increasingly intense and the soil's capacity is being exceeded very quickly, resulting in stagnant water. This stagnant water can be visible for several days in certain localities, mainly in low-lying areas, which is devastating for so-called terrestrial plants, including cassava, a vitally important plant for the people of Central Africa, for whom it is a staple food. The solution would be to move towards flood-tolerant plants. Since the creation of flood-tolerant species is expensive and the technology is beyond the reach of small-scale growers, the mutualistic associations formed by AMFs and cassava roots could be an interesting short-term solution. This mutualization will enable yields to be maintained at an acceptable level in waterlogged conditions, although the waterlogging need not last for very long.
Conflict of interest statement:
The authors declare no conflict of interest.References
- Harlan JR. Geographic patterns of variation in some cultivated plants. J Heredity 4 (1975): 182-191.
- Hancock JF. Contributions of Domesticated Plant Studies to Our Understanding of Plant Evolution. Ann Bot 96 (2005): 953-963.
- Diamond J. Evolution, Consequences and Future of Plant and Animal Domestication. Nat 418 (2002): 700-707.
- Carvalho LJCB, Anderson JV, Chen S, et al. Domestication syndrome in cassava (Manihot esculenta crantz): assessing morphological traits and differentially expressed genes associated with genetic diversity of storage root (2018): 10-5772.
- Allem AC. The Origin of Manihot Esculenta Crantz (Euphorbiaceae). Genetic Res Crop Evol 41 (1994): 133-150.
- Jorge MAB. Regeneration guidelines: cassava (2008): 1- 9.
- Cacaï GHT, Ahanhanzo C, Dangou JS, et al. Effets de différentes combinaisons hormonales sur l’organogenèse in vitro de quelques cultivars locaux et variétés améliorées de Manihot esculenta Crantz (manioc-Euphorbiaceae) cultivées au Bénin. Int J Biol Chem Sci 6 (2012): 1593-1607.
- Allem AC. The origins and taxonomy of cassava. Cassava: biology, production and utilization (2001): 1-16.
- Olsen KM, Schaal BA. Microsatellite variation in cassava (Manihot esculenta, Euphorbiaceae) and its wild relatives: further evidence for a southern Amazonian origin of domestication. Am J Bot 88 (2001): 131-142.
- Nassar NM. Conservation of the genetic resources of cassava (Manihot esculenta) determination of wild species localities with emphasis on probable origin n. Econ Bot 32 (1978): 311–320.
- Silvestre P, Arraudeau M, et al. Paris: G.P. Maisonneuve et Larose; Agence de Coopération Culturelle et Technique. Techniques Agricoles et Productions Tropicales 32 (1983): 262.
- Hillocks RJ, Thresh JM, Bellotti A. Cassava: biology, production and utilization. Cabi (2002).
- Onwueme IC. Cassava in Asia and the Pacific. In: Hillocks RJ, Thresh JM, Bellotti AC, editors. Cassava: Biology, Production and Utilization. CABI Publishing, Wallingford, UK (2002): 55–66.
- N'zué B, Zouhouri PG, Sangaré A. Performances Agronomiques De Quelques Varietes De Manioc (Manihot esculenta Crantz) Dans Trois Zones Agroclimatiques De La Cote D'ivoire. Agronomie africaine 16 (2004): 1-7.
- Vernier P, N’Zué B, Zakhia-Rozis N. Le manioc, entre culture alimentaire et filière agro-industrielle (2018): 235.
- Gnonlonfin GJB, Koudande OD, Sanni A, et al. Farmers’ perceptions on characteristics of cassava (Manihot esculenta Crantz) varieties used for chips production in rural areas in Benin, West Africa. Int J Biol Chem Sci 5 (2011): 870-879.
- Tetchi FA, Rolland-Sabaté A, N’Guessan AG, et al. Molecular and physicochemical characterisation of starches from yam, cocoyam, cassava, sweet potato and ginger produced in the Ivory Coast. J Sci Food Agriculture 87 (2007): 1906-1916.
- Von Grebmer K, Headey D, Béné C, et al. Global Hunger Index: the challenge of hunger: building resilience to achieve food and nutrition security 79 (2013): 66.
- Britton G, Liaasen-Jensen S, Pfander H. Carotenoids. Volume 1B: spectroscopy (1995):360.
- Armstrong GA. Carotenoid genetics and biochemistry (1999): 321-352.
- Bauernfeind JC. Carotenoids as colorants and vitamin A precursors. Academic Press (1981): 938.
- Eisenreich W, Rohdich F, Bacher A. Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci 6 (2001): 78-84.
- Fraser PD & Bramley PM. The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43 (2004): 228-265.
- Segnou S. Vegetative growth and yield potential in cassava (2002): 161-164.
- Zinga I, Nguimalet CR, Lakouetene DP, et al. The impacts of African cassava mosaic in Central African Republic (2008): 47-60.
- Wang W, Vinocur B, Altman A. Plant Responses to Drought, Salinity and Extreme Temperatures: Towards Genetic Engineering for Stress Tolerance. Planta 218 (2003): 1-14.
- Sardos J, Rodier-Goud M, Dambier D, et al. Evidence for spontaneous polyploidization in cassava Manihot esculenta Crantz. Plant System Evol 283 (2009): 203-209.
- Conway G & Toenniessen G. In the Twenty-First Century (1999): 402.
- Cassman KG. Ecological Intensification of Cereal Production Systems: Yield Potential, Soil Quality, and Precision Agriculture. Proc National Acad Sci 96 (1999): 5952-5959.
- Siloko IU, Ukhurebor KE, Siloko EA, et al. Effects of Some Meteorological Variables on Cassava Production in Edo State, Nigeria via Density Estimation. Sci African 13 (2021): e00852.
- Cantelaube P & Terres JM. Seasonal Weather Forecasts for Crop Yield Modelling in Europe. Tellus A: Dynamic Meteorol Oceanography 57 (2005): 476.
- Cong RG & Brady M. The Interdependence between Rainfall and Temperature: Copula Analyses. The Sci World J (2012): 1-11.
- Smirnoff N. Drought Tolerance in Higher Plants. Genetical, Physiological and Molecular Biological Analysis. New Phytologist 138 (1998): 563-566.
- Keating BA & Evenson JP. Effect of Soil Temperature on Sprouting and Sprout Elongation of Stem Cuttings of Cassava (Manihot Esculenta Crantz.). Field Crops Res 2 (1979): 241-251.
- Kopecká R, Kameniarová M, Cerný M, et al. Abiotic stress in crop production. Int J Mol Sci 24 (2023): e6603.
- Oshunsanya SO, Nwosu NJ, Li Y. Abiotic stress in agricultural crops under climatic conditions. In: Jhariya MK, Banerjee A, Meena RS, Yadav DK, eds. Sustainable agriculture, forest and environmental manage-ment. Singapore: Springer Singapore (2019): 71–100.
- Nadarajah KK. ROS homeostasis in abiotic stress tolerance in plants. Int J Mol Sci 21 (2020): e5208
- Alvi AF, Iqbal N, Albaqami M, et al. The emerging key role of reactive sulfur species in abiotic stress tolerance in plants. Physiologia Plantarum 175 (2023): e13945.
- Aroca A, García-Díaz I, García-Calderón M, et al. Photorespiration: regulation and new insights on the potential role of persulfidation. J Exp Bot 74 (2023): 6023–6039.
- Feng S, Krueger AB, Oppenheimer M. Linkages among Climate Change, Crop Yields and Mexico–US Cross-Border Migration. Proc Nat Acad Sci 107 (2010): 14257-14262.
- Schlenker W & Lobell DB. Robust Negative Impacts of Climate Change on African Agriculture. Environ Res Lett 5 (2010): 014010.
- Lobell DB & Asner GP. Climate and Management Contributions to Recent Trends in U.S. Agricultural Yields. Sci 299 (2003): 1032-1032.
- Schöffl F, Prändl R, Reindl A. Regulation of the heat-shock response. Plant Physiol 117 (1998):1135–1141.
- Yamanouchi U, Yano M, Lin H, et al. A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc Natl Acad Sci USA 99 (2002): 7530–7535.
- Bray EA, Bailey-Serres J, Weretilnyk E. Responses to abiotic stresses. In: Gruissem W, Buchannan B, Jones R (eds) Biochemistry and molecular biology of plants. Am Soci Plant Physiol (2000): 1158–1249.
- Jarvis A, Ramirez-Villegas J, Campo BVH, et al. Is Cassava the Answer to African Climate Change Adaptation? Tropical Plant Biol 5 (2012): 9-29.
- Bueno A. Numero adequado de ambientes para avaliar cultivares de mandioca. Adequate number of environments to evaluate cassava cultivars. Rev Bras Mandioca 5 (1986): 83-93.
- El-Sharkawy MA & Cock JH. Photosynthesis of cassava (Manihot esculenta). Exp Agric 26 (1990): 325–340.
- Luo X & Huang Q. Studies on the cold resistance of cassava. J Agricultural Sci 4 (2012): 104.
- An D, Ma Q, Wang H, et al. Cassava C-repeat binding factor 1 gene responds to low temperature and enhances cold tolerance when overexpressed in Arabidopsis and cassava. Plant Mol Biol 94 (2017): 109–124.
- An D, Yang J, Zhang P. Transcriptome profiling of low temperature-treated cassava apical shoots showed dynamic responses of tropical plant to cold stress. BMC Genomics 13(2012): 64.
- An F, Li G, Li QX, et al. The Comparatively Proteomic Analysis in Response to Cold Stress in Cassava Plantlets. Plant Mol Biol Rep 34 (2016): 1095−1110.
- Li S, Cheng Z, Dong S, et al. Global identification of full-length cassava lncRNAs unveils the role of cold-responsive intergenic lncRNA 1 in cold stress response. Plant, Cell Environ 45 (2022): 412−426.
- Berry J, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. – Annu. Rev Plant Physiol 31 (1980): 491-543.
- Ekanayake IJ, Osiru DSO, Porto MCM. Physiology of cassava. IITA Research Guide 60. Training Program, IITA, Ibadan, Nigeria (1997): 22.
- Akparobi SO, Togun AO, Ekanayake IJ, et al. Effect of low temperatures on dry matter partitioning and yield of cassava clones Tropical Sci 42 (2002): 22-29.
- Zhu J. Abiotic stress signaling and responses in plants Cell 167 (2016): 313−324.
- Avila LM, Obeidat W, Earl H, et al. Shared and genetically distinct Zea mays transcriptome responses to ongoing and past low temperature exposure. BMC Genomics 19 (2018): 761.
- El-Sharkawy MA. International research on cassava photosynthesis, productivity, eco physiology, and responses to environmental stresses in the tropics. Photosynthetica 44 (2006): 481−512.
- Schlosser CA, Strzepek K, Gao X, et al. The Future of Global Water Stress: An Integrated Assessment ». Earth’s Future 2 (2014): 341-61.
- Bouman BAM, Lampayan RM, Tuong TP. Water Management in Irrigated Rice: Coping with Water Scarcity. Los Baños, Philippines: Int Rice Res Instit (2007): 59.
- Farooq M, Wahid A, Kobayashi N, et al. Plant Drought Stress: Effects, Mechanisms and Management. Agronomy for Sustainable Development 29 (2009): 185-212.
- Sahebi M, Hanafi MM, Rafii MY, et al. Improvement of Drought Tolerance in Rice (Oryza Sativa L.): Genetics, Genomic Tools, and the WRKY Gene Family. BioMed Res Int (2018): 1-20.
- Wery J, Silim SN, Knights EJ, et al. Screening techniques and sources and tolerance to extremes of moisture and air temperature in cool season food legumes, Euphytica 73 (1994): 73–83.
- Bartels D, Schneider K, Terstappen G, et al. Molecular cloning of abscisic acid-modulated genes which are induced during desiccation of the resurrection plant Craterostigma plantagineum. Planta 181 (1990): 27-34.
- George J, Mohankumara CR, Nair GM, et al. Cassava agronomy research and adaptation of improved practices—Major achievements during past 30 years. In: Howeler R, & Tan SL (eds.) Cassava’s Potential in Asia in the 21st Century: Present Situation and Future Research and Development Needs. Proc Sixth Regional Workshop (2001): 279–299.
- Turyagyenda LF, Kizito EB, Baguma Y, Osiru D. Evaluation of Ugandan cassava germplasm for drought tolerance. Int J Agriculture Crop Sci 5 (2013a): 212-226.
- Muiruri SK, Ntui VO, Tripathi L, et al. Mechanisms and approaches towards enhanced drought tolerance in cassava (Manihot esculenta). Curr Plant Biol 28 (2021): 1-7.
- Koundinya AVV, Hegde V, Sheela MN, et al. Evaluation of Cassava Varieties for Tolerance to Water Deficit Stress conditions. J Root Crops 44 (2018): 70-75.
- Helal NAS, Eisa SS, Attia A. Morphological and Chemical Studies on Influence of Water Deficit on Cassava. World J Agricultural Sci 9 (2013): 369-376.
- Carvalho LMD, Carvalho HWLD, Oliveira IRD, et al. Produtividade e tolerância à deficiência hídrica de cultivares de mandioca nos Tabuleiros Costeiros do Nordeste. Ciência Rural 46 (2016): 796-801.
- Oliveira EJ, Morgante CV, Chaves ARM, et al. Evaluation of cassava germplasm for drought tolerance under ûeld conditions. Euphytica 213 (2017): 188.
- Orek C, Gruissem W, Ferguson M, et al. Morpho-physiological and molecular evaluation of drought tolerance in cassava (Manihot esculenta Crantz). Field Crops Res 255 (2020): 107861.
- Nuñez-Muñoz L, Vargas-Hernández B, Hinojosa-Moya J, et al. Plant drought tolerance provided through genome editing of the trehalase gene. Plant Signaling & Behavior 16 (2021): 1877005.
- Turyagyenda LF, Kizito EB, Ferguson M, et al. Physiological and molecular characterization of drought responses and identification of candidate tolerance genes in cassava. AoB Plants 5 (2013b): plt007.
- Alves AAC. Cassava botany and physiology. In Cassava: biology, production and utilization (2001): 67-89.
- Alves AA & Setter TL. Abscisic acid accumulation and osmotic adjustment in cassava under water deficit. Environ Exp Bot 51 (2004): 259-271.
- Kumar M. Recent Advances in the Drought Stress Tolerance in Rice (2019).
- Kwak JM. NADPH Oxidase AtrbohD and AtrbohF Genes Function in ROS Dependent ABA Signaling in Arabidopsis. EMBO J 22 (2003): 2623-2633.
- Lenis JI, Calle F, Jaramillo G, et al. Leaf retention and cassava productivity. Field Crops Res 95 (2006): 126–134.
- El-Sharkawy MA. Physiological characteristics of cassava tolerance to prolonged drought in the tropics: implications for breeding cultivars adapted to seasonally dry and semiarid environments. Brazil J Plant Physiol 19 (2007): 257-286.
- Wang P, Song CP. Guard-Cell Signalling for Hydrogen Peroxide and Abscisic Acid. New Phytologist 178 (2008): 703-18.
- Okogbenin E, Setter TL, Ferguson M, et al. Phenotypic approaches to drought in cassava. Front Physiol 4 (2013): 93.
- Sanders PM, Anhthu Q, Bui AQ, et al. Anther Developmental Defects in Arabidopsis Thaliana Male-Sterile Mutants. Sexual Plant Reproduction 11 (1999): 297-322.
- Ramanjulu S & Bartels D. Drought- and Desiccation-Induced Modulation of Gene Expression in Plants: Dehydration-Induced Gene Expression. Plant, Cell & Environ 25 (2002): 141-51.
- Das R & Pandey GK. Expressional Analysis and Role of Calcium Regulated Kinases in Abiotic Stress Signaling. Curr Genomics 11 (2010): 2-13.
- Seybold H, Trempel F, Ranf S, et al. Ca2+ signalling in plant immune response: from pattern recognition receptors to Ca2+ decoding mechanisms. New Phytologist 204 (2014): 782-790.
- Licausi F, Ohme-Takagi M, Perata P. APETALA 2/Ethylene Responsive Factor (AP 2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytologist 199 (2013): 639-649.
- Castilhos G, Lazzarotto F, Spagnolo-Fonini L, et al. Possible roles of basic helix-loop-helix transcription factors in adaptation to drought. Plant Sci 223 (2014): 1-7.
- Marcotte JWR, Russell SH, Quatrano RS. Abscisic acid-responsive sequences from the em gene of wheat. The Plant Cell 1 (1989): 969-976.
- Hobo T, Asada M, Kowyama Y, et al. ACGT-containing abscisic acid response element (ABRE) and coupling element 3 (CE3) are functionally equivalent. Plant J 19 (1999): 679-689.
- Hong Y, Zhang H, Huang L, et al. Overexpression of a stress-responsive NAC transcription factor gene ONAC022 improves drought and salt tolerance in rice. Front Plant Sci 7 (2016): 166009.
- Srivasta A, Mehta S, Lindlof A, et al. Over-represented promoter motifs in abiotic stress-induced DREB genes of rice and sorghum and their probable role in regulation of gene expression. Plant Signaling Behavior 5 (2010): 775-784.
- Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci 5 (2014): 85756.
- Goyal K, Walton LJ, Tunnacliffe A. LEA proteins prevent protein aggregation due to water stress. Biochem J 388 (2005): 151-157.
- Xiao B, Huang Y, Tang N, et al. Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theoretical and Applied Genet 115 (2007): 35-46.
- Yu J, Lai Y, Wu X, et al. Overexpression of OsEm1 encoding a group I LEA protein confers enhanced drought tolerance in rice. Biochem Biophysical Res Commun 478 (2016): 703-709.
- Ren D, Rao Y, Wu L, et al. The pleiotropic ABNORMAL FLOWER AND DWARF1 affects plant height, floral development and grain yield in rice. J Int Plant Biol 58 (2016): 529-539.
- Kim Y, Chung YS, Lee E, et al. Root response to drought stress in rice (Oryza sativa L.). Int J Mol Sci 21 (2020): 1513.
- Zhang Q, Li J, Zhang W, et al. The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J 72 (2012): 805-816.
- Du H, Wu N, Fu J, et al. A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J Exp Bot 63 (2012): 6467-6480.
- Zhang SW, Li CH, Cao J, et al. Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3. 13 activation. Plant Physiol 151 (2009): 1889-1901.
- Ljung K. Auxin metabolism and homeostasis during plant development. Development 140 (2013): 943-950.
- Zhang Z, Li F, Li D, et al. Expression of ethylene response factor JERF1 in rice improves tolerance to drought. Planta 232 (2010): 765-774.
- Levitt J. Responses of plants to environmental stresses. Volume II. Water, radiation, salt, and other stresses (1981): 607.
- Kumar V, Mahajan G, Chauhan BS. Rice weeds and their management. Rice Production Worldwide (2017): 361-392.
- Panda D, Mishra SS, Behera PK. Drought tolerance in rice: focus on recent mechanisms and approaches. Rice Sci 28 (2021): 119-132.
- Fukai S & Cooper M. Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crops Res 40 (1995): 67-86.
- Blum A, Shpiler L, Golan G, et al. Yield stability and canopy temperature of wheat genotypes under drought-stress. Field Crops Res 22 (1989): 289-296.
- Wang H, Inukai Y, Yamauchi A. Root development and nutrient uptake. Critical Rev Plant Sci 25 (2006): 279-301.
- Price AH, Cairns JE, Horton P, et al. Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J Exp Bot 53 (2002): 989-1004.
- Medrano H, Escalona JM, Bota J, et al. Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Ann Bot 89 (7): 895-905.
- Yeo ME, Cuartero J, Flowers TJ, et al. Gas exchange, water loss and biomass production in rice and wild Oryza species in well-watered and water-limiting growth conditions. Botanica Acta 110 (1997): 32-42.
- Zhou L, Ni E, Yang J, et al. Rice OsGL1-6 is involved in leaf cuticular wax accumulation and drought resistance. PloS one 8 (2013): e65139.
- Riederer M & Schreiber L. Protecting against water loss: analysis of the barrier properties of plant cuticles. J Exp Bot 52 (2001): 2023-2032.
- Henry A, Cal AJ, Batoto TC, et al. Root attributes affecting water uptake of rice (Oryza sativa) under drought. J Exp Bot 63 (2012): 4751-4763.
- Fleury D, Jefferies S, Kuchel H, et al. Genetic and genomic tools to improve drought tolerance in wheat. J Exp Bot 61 (2010): 3211-3222.
- Hasegawa PM, Jain M, Jenks MA. Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops. Springer Verlag (2007).
- Sullivan CY and Ross WM. Selecting for drought and heat resistance in grain sorghum (1979): 263-281.
- Messina CD, Sinclair TR, Hammer GL, et al. Limited-transpiration trait may increase maize drought tolerance in the US Corn Belt. Agronomy J 107 (2015): 1978-1986.
- Kozlowski TT. Responses of woody plants to flooding and salinity. Tree Physiol 17 (1997): 490-490.
- Allen MR & Ingram WJ. Constraints on future changes in climate and the hydrologic cycle. Nat 419 (2002): 224-232.
- Mitchell JFB, Wilson CA, Cunnington WM. On CO2 climate sensitivity and model dependence of results. Quarterly J Royal Meteorol Soci 113 (1987): 293-322.
- Voesenek LACJ, Sasidharan R, Weber A. Ethylene–and oxygen signalling–drive plant survival during flooding. Plant Biol 15 (2013): 426-435.
- Cao M, Linling Z, Junyi L, et al. Transcriptomic Profiling Suggests Candidate Molecular Responses to Waterlogging in Cassava. PLOS ONE 17 (2022): e0261086.
- Visser EJW, Voesenek LCJ, Vartapetian BB, et al. Flooding and plant growth: Preface. Ann Bot 91 (2003): 107–109
- Normile D. Reinventing rice to feed the world (2008): 330-333.
- Durack PJ, Wijffels SE, Matear RJ. Ocean salinities reveal strong global water cycle intensification during 1950 to 2000. Sci 336 (2012): 455-458.
- Vartapetian BB, Andreeva IN, Generozova IP, et al. Functional electron microscopy in studies of plant response and adaptation to anaerobic stress. Ann Bot 91 (2003): 155-172.
- Arbona V, Hossain Z, López-Climent MF, et al. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiologia Plantarum 132 (2008): 452-466.
- Perl-Treves R, Perl A. Oxidative stress: an introduction. In: Oxidative stress in plants. CRC Press (2001): 17-55.
- O'Hair SK. Tropical root and tuber crops. Horticultural Rev (1990): 157-196.
- Chandrasekara A & Kumar TJ. Roots and tuber crops as functional foods: a review on phytochemical constituents and their potential health benefits. Int J Food Sci (2016): 1-15
- Lin KHR, Tsou CC, Hwang SY, et al. Paclobutrazol pre-treatment enhanced flooding tolerance of sweet potato. J Plant Physiol 163 (2006): 750-760.
- Hwang SY, Lin HW, Chern RH, et al. Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regulation 27 (1999): 167-172.
- Lin KHR, Weng CC, Lo HF, et al. Study of the root antioxidative system of tomatoes and eggplants under waterlogged conditions. Plant Sci 167 (2004): 355-365.
- Asada K. Ascorbate peroxidase–a hydrogen peroxide-scavenging enzyme in plants. Physiologia plantarum 85 (1992): 235-241.
- Kauss H, Jeblick W, Ziegler J, et al. Pretreatment of parsley (Petroselinum crispum L.) suspension cultures with methyl jasmonate enhances elicitation of activated oxygen species. Plant Physiol 105 (1994): 89-94.
- Effa Effa BW. Effets de la symbiose endomycorhizienne sur la tolérance au stress hydrique chez le riz. Université de Montpellier: thèse de doctorat, Ecophysiologie et adaptation des plantes (2020): 238.
- Anand K, Pandey GK, Kaur T, et al. Arbuscular mycorrhizal fungi as a potential biofertilizers for agricultural sustainability. J Applied Biol Biotechnol 10 (2022): 90-107.
- Frac M, Hannula SE, Belka M, et al. Fungal biodiversity and their role in soil health. Front Microbiol 9 (2018): 707.
- Mahmud K, Missaoui A, Lee K, et al. Rhizosphere microbiome manipulation for sustainable crop production. Curr plant Biol 27 (2021): 100210.
- Finlay RD. Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J Exp Bot 59 (2008): 1115-1126.
- Rambelli A. The rhizosphere of mycorrhizae. Ectomycorrhizae: Their Ecol Physiol (1973): 299-343.
- Linderman RG. Mycorrhizal interactions in the rhizosphere. In: The Rhizosphere and Plant Growth: Papers presented at a Symposium held May 8–11, 1989, at the Beltsville Agricultural Research Center (BARC), Beltsville, Maryland. Springer Netherlands (1991): 343-348.
- Bonfante P & Genre A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat commun 1 (2010): 48.
- Jakobsen I & Rosendahl L. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytologist 115 (1990): 77-83.
- Smith SE, and Read DJ. Mycorrhizal Symbiosis. 3. ed., Repr. Amsterdam: Elsevier/Acad. Press (2009)
- Nakagawa T & Imaizumi-Anraku H. Rice arbuscular mycorrhiza as a tool to study the molecular mechanisms of fungal symbiosis and a potential target to increase productivity. Rice 8 (2015): 1-9.
- Fortin JA, Plenchette C, Piché Y. Les mycorhizes : la nouvelle révolution verte (2011) :1-21.
- Manjunath A, Hue NV, Habte M. Response of Leucaena leucocephala to vesicular-arbuscular mycorrhizal colonization and rock phosphate fertilization in an Oxisol. Plant and soil 114(1989): 127-133.
- Tawaraya K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Science and Plant Nutrition 49 (2003): 655-668.
- Gavériaux JP. Les glomeromycota. Bull Soc Mycol Nord Fr 92 (2012): 1-17.
- Plassard C, Robin A, Le Cadre E, et al. Améliorer la biodisponibilité du phosphore : comment valoriser les compétences des plantes et les mécanismes biologiques du sol. Innovations Agronomiques 43 (2015): 115-138.
- Toju H, Peay KG, Yamamichi M, et al. Core microbiomes for sustainable agroecosystems. Nat Plant 4 (2018): 247-257.
- Bever JD. Preferential allocation, physio-evolutionary feedbacks, and the stability and environmental patterns of mutualism between plants and their root symbionts. New Phytologist 205 (2015): 1503-1514.
- Wang W, Shi J, Xie Q, et al. Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Mol Plant 10 (2017): 1147-1158.
- Khan AG. Occurrence and importance of mycorrhizae in aquatic trees of New South Wales, Australia. Mycorrhiza 3 (1993): 31-38.
- Miller SP, Sharitz RR. Manipulation of flooding and arbuscular mycorrhiza formation influences growth and nutrition of two semiaquatic grass species. Functional Ecol 14 (2000): 738-748.
- Harley JL. The significance of mycorrhiza. Mycol Res 92 (1989): 129-139.
- Søndergaard M & Laegaard S. Vesicular–arbuscular mycorrhiza in some aquatic vascular plants. Nat 268 (1977): 232-233.
- Keeley JE. Endomycorrhizae influence growth of blackgum seedlings in flooded soils. Am J Bot 67 (1980): 6-9.
- Sengupta A, Chaudhuri S. Arbuscular mycorrhizal relations of mangrove plant community at the Ganges river estuary in India. Mycorrhiza 12 (2002): 169-174.
- Wang Y, Huang Y, Qiu Q, et al. Flooding greatly affects the diversity of arbuscular mycorrhizal fungi communities in the roots of wetland plants. PloS One 6` (2011): e24512.
- Nielsen KB, Kjøller R, Olsson PA, et al. Colonisation and molecular diversity of arbuscular mycorrhizal fungi in the aquatic plants Littorella uniflora and Lobelia dortmanna in southern Sweden. Mycol Res 108 (2004): 616-625.
- Sudová R, Rydlová J, Ctvrtlíková M, et al. The incidence of arbuscular mycorrhiza in two submerged Isoëtes species. Aquatic Bot 94 (2011): 183-187.
- Sah S, Reed S, Jayachandran K, et al. The effect of repeated short-term flooding on mycorrhizal survival in snap bean roots. Hort Sci 41 (2006): 598-602.
- Yang H, Koide RT, Zhang Q. Short-term waterlogging increases arbuscular mycorrhizal fungal species richness and shifts community composition. Plant and Soil 404 (2016): 373-384.
- Zheng FL, Liang SM, Chu XN, et al. Mycorrhizal fungi enhance flooding tolerance of peach through inducing proline accumulation and improving root architecture. Plant, Soil Environ 66 (2020): 624–631.
- Wirsel SG. Homogenous stands of a wetland grass harbour diverse consortia of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 48 (2004): 129-138.
- Lekberg YLVA, Koide RT, Rohr JR, et al. Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J Ecol 95 (2007): 95-105.
- Helgason T, Fitter AH. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J Exp Bot 60 (2009): 2465-2480.
- Dumbrell AJ, Nelson M, Helgason T, et al. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J 4 (2010): 337-345.
- Tan SL & Ambak K. A lysimeter study on the effect of watertable on cassava grown on peat. MARDI Res J 17 (1989): 37-142.
- Gupta MM, Chourasiya D, Sharma MP. Diversity of arbuscular mycorrhizal fungi in relation to sustainable plant production systems. Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications. Soil & Agroecosystems 2 (2019): 167-186.
- Noort MW, Renzetti S, Linderhof V, et al. Towards sustainable shifts to healthy diets and food security in sub-Saharan Africa with climate-resilient crops in bread-type products: A food system analysis. Foods 11 (2022): 135.
- Thanni B, Merckx R, De Bauw P, et al. Spatial variability and environmental drivers of cassava—arbuscular mycorrhiza fungi (AMF) associations across Southern Nigeria. Mycorrhiza (2022): 1-13.
- Szabados L & Savouré A. Proline: a multifunctional amino acid. Trends plant Sci 15 (2010): 89-97.
- Kemble AR & Macpherson HT. Liberation of amino acids in perennial rye grass during wilting. Biochem J 58 (1954): 46.
- Daniell H, Wurdack KJ, Kanagaraj A, et al. The complete nucleotide sequence of the cassava (Manihot esculenta) chloroplast genome and the evolution of atpF in Malpighiales: RNA editing and multiple losses of a group II intron. Theoretical and Applied Genetics 116 (2008): 723-737.
- Alves-Pereira A, Zucchi MI, Clement CR, et al. Selective signatures and high genome-wide diversity in traditional Brazilian manioc (Manihot esculenta Crantz) varieties. Sci Rep 12 (2022): 1268.
- Yamauchi T, Shimamura S, Nakazono M, et al. Aerenchyma formation in crop species: a review. Field Crops Res 152 (2013): 8-16.
- Takahashi H, Yamauchi T, Colmer TD, et al. Aerenchyma formation in plants. Low-oxygen stress in plants: Oxygen sensing and adaptive responses to hypoxia (2014): 247-265.
- Evans DE. Aerenchyma formation. New Phytologist 161 (2004): 35-49.
- Tavares EQ, De Souza AP, Romim GH, et al. The control of endopolygalacturonase expression by the sugarcane RAV transcription factor during aerenchyma formation. J Exp Bot 70 (2019): 497-506.
- Pruitt KD, Brown GR, Hiatt SM, et al. RefSeq: an update on mammalian reference sequences. Nucleic Acid Res 42 (2014): D756-D763.
- Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34 (2018): 3094-3100.
- Gmakouba T, Koussao S, Traore ER, et al. Analyse de la diversité agromorphologique d’une collection de manioc (Manihot esculenta Crantz) du Burkina Faso. Int J Biol Chemical Sci 12 (2018): 402-421.
- Smith SE & Smith FA. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 104 (2012): 1-13.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 