Extension of Tamiflu Shelf-Life in Strategic Stockpile for Public Health
Keith DelMonte1, Chau To1, Kateryna Rashid1, Mike Sayers2, Michael Mendoza2, Fang Zhao1, Todd D Camenisch1*
1Department of Pharmaceutical Sciences, Wegmans School of Pharmacy, St. John Fisher College. Rochester, NY
2Monroe County Health Department. Rochester, NY, USA
*Corresponding Author: Todd D Camenisch, PhD, Pharmaceutical Sciences Department, Wegmans School of Pharmacy, St. John Fisher College. 3690 East Avenue, Rochester, NY, USA
Received: 30 September 2021; Accepted: 06 October 2021; Published: 14 December 2021
Article Information
Citation: Keith DelMonte, Chau To, Kateryna Rashid, Mike Sayers, Michael Mendoza, Fang Zhao, Todd D Camenisch. Extension of Tamiflu Shelf-Life in Strategic Stockpile for Public Health. Journal of Environmental Science and Public Health 5 (2021): 504-515.
View / Download Pdf Share at FacebookAbstract
Purpose: To determine whether stockpiles of expired Tamiflu in strategic national stockpiles are potent. Validation of drug stability and activity safeguards the public health to allow use of Tamiflu reserves during influenza outbreak.
Methods: USP method for oseltamivir phosphate was used to measure drug potency of stockpiled Tamiflu. Practical modifications to the dissolution method were used to evaluate the drug release./p>
Results: Chemical stability of three lots of expired Tamiflu indicated satisfactory drug potency meeting the USP acceptance criteria of the labeled claim. Dissolution testing showed that all Tamiflu lots passed the criteria of no less than 75% of labeled amount of drug dissolved. All samples analyzed had ~100% dissolution and chemical release attributes comparable to controls.
Conclusions: All expired lots of stockpiled Tamiflu tested were stable and potent based on USP guidelines. These stocks of Tamiflu could be authorized for use in response to influenza outbreak to protect the public health. This informs other local jurisdictions and health departments with an optimized protocol to validate additional stockpiled drug, and safeguard Tamiflu stocks by extending expiration dates as part of preparation strategies to protect the public health.
Keywords
<p>Tamiflu shelf-life; Stockpile; Public health; Oseltamivir phosphate</p>
Article Details
1. Introduction
The seasonal influenza extends from October to April each cycle. This seven month period is protracted during the emergence of novel influenza strains which often have two major peaks of activity coinciding with winter in the preceding year followed by a sequential upsurge in the fall. The 2020-2021 flu season was a historic low rate due to the universal precautions exercised for the COVID-19 pandemic [1]. The extreme minimal circulation of influenza virus has resulted in the low predictability of a seasonal strain and the subsequent development for a protective vaccine to the flu [2]. This could lead to severe influenza outbreaks within the next couple of years. Thus, the public health and availability of countermeasures is a major concern, and can be challenged due to shortages in medications such as oseltamivir phosphate, or Tamiflu.
The Food and Drug Administration publishes drugs including active pharmaceutical ingredients that are in shortage, or of critical concern on a weekly basis. Such shortages have been amplified by the COVID-19 pandemic with the extreme shortage of chloroquine and hydroxychloroquine stocks [3]. The H1N1 influenza pandemic of 2009 highlighted the need, as well as effectiveness, of Tamiflu. In 2018, there were shortages on the availability of Tamiflu in areas with high incidence of influenza (CDC, 2018). This underscores the importance to have this antiviral drug in stock to decrease severity and spread of outbreaks. In January 2014, in one week over 4,000 people died from the flu in part due to shortages of Tamiflu [4]. As recent as 2020, there was preventable death from flu complications in part from not receiving Tamiflu as prescribed [5]. These incidents highlight the necessity for stocks of the antiviral, Tamiflu, in sufficient quantities to safeguard against outbreaks of aggressive influenza.
The Department of Defense, Center for Disease Control and Department of Health and Human Services have cooperative agreements to purchase and store potentially life-saving medications to be used in times of national emergency; the severity of which could exhaust local supplies at federal and state facilities [6]. Antibiotics, chemical antidotes, antitoxins, life-support medications, IV administration, airway maintenance supplies and medical/surgical items are stored under secure conditions in bunkers that are environmentally controlled [6]. This bulk of medications and medical supplies, collectively called ‘the strategic national stockpile (SNS)’, is a mechanism for the federal government to prepare for disasters that would require mass quantity of drugs to be dispensed to the population. In addition to Federal SNS, individual states are also authorized to store drugs and medical supplies in regional and county facilities, allowing for more efficient access in the time of crisis. Twenty five percent of the total purchase cost for local stockpiles is provided from the federal-national stockpile program to participating states to strategically store medical drugs and supplies in regional and county facilities [7]. The American Medical Association, FDA and pharmaceutical association reported on the risks and benefits of extending expiration dates for stockpiled medications [8].
It indicated that most drugs can retain potency beyond the initial recommended expiration by at least one year. A range of 12-60 months was identified across a spectrum of products. However, the actual stability in real-time of select drugs stored at local sites is not known and the study did not include Tamiflu.
Since oseltamivir phosphate (Tamiflu) effectively combats and prevents influenza virus, it is a component of strategic national and state stockpiles and a subject of this exploratory project. Tamiflu is a prescription medication approved by the FDA in 1999 to treat acute uncomplicated influenza A and B in patients who have experienced symptoms for no more than 48 hours [9].
It is also used as a prophylactic measure against influenza A and B virus in patients 1 year and older during flu season. Tamiflu is available in 30 mg, 45 mg and 75 mg strength capsules maintained at recommended storage at room termperature (20 – 25 °C)(8). In addition, an oral suspension (6 mg/ml) is available, but not a part of the current study. For the treatment of influenza in adults and adolescents, the recommended regimen is one 75 mg capsule twice daily for 5 days [9].
The chemical structure of oseltamivir phosphate is depicted in Figure 1. oseltamivir is an ester prodrug which converts readily after oral absorption to the active form oseltamivir carboxylate by esterases from the liver. This ester functional group may also undergo hydrolysis during storage depending on temperature, humidity, and integrity of the packaging. The USP Monograph of Tamiflu Phosphate (drug substance) lists two identified impurities, oseltamivir acid and oseltamivir phenol [10].
Oseltamivir acid is the protonated form of oseltamivir carboxylate as shown in Figure 2. oseltamivir phenol is an impurity likely derived from the synthetic process and not formed during storage. The USP Monograph of oseltamivir Phosphate Capsules (drug product) lists three identified impurities, A, B, and C [11].
Impurities A and B are the same as oseltamivir acid and oseltamivir phenol, respectively. Impurity C, as shown in Figure 2, is formed by N,N-acyl migration which can be accelerated to form if exposed to moisture. A forced degradation study performed by Junwal et al reported a total of 5 degradation products, including the USP Impurities A, B, and C as the major ones [12]. That study also confirmed that the in vitro degradation of oseltamivir to USP Impurities A and C is accelerated in acidic and basic conditions; however, these conditions should not occur under recommended storage parameters.
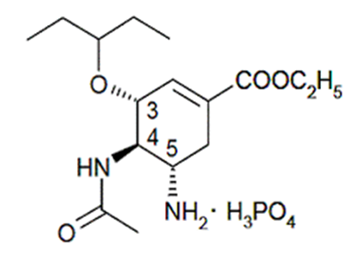
Figure 1: Chemical structure of oseltamivir phosphate.
Chemical structural formula of oseltamivir phosphate. The molecular weight is 312.4 for oseltamivir free base and 410.4 for oseltamivir phosphate salt. The strengths of all marketed products are expressed as the free base [2].
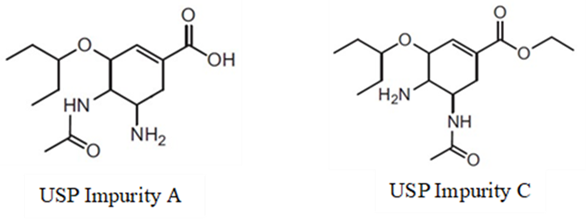
Figure 2: Chemical structures of USP Impurities A and C.
Impurity A is also the active form in vivo (from the ester hydrolysis of oseltamivir). It is also referred as oseltamivir carboxylate or oseltamivir acid.
Pharmaceutical manufacturers establish the shelf life and expiration dates of the products as approved by FDA based on scientific evidence. Stockpiled medications are required to be replaced on a regular basis, accruing significant monetary value and waste. As a result, in 1986 the Federal Government established the shelf life extension program (SLEP) to permit usage of expired federal stockpile medications during times of national disasters such as bioterrorism, earthquakes, hurricanes and wildfires.
The U.S. Military has benefited greatly from SLEP in cost savings and extension of drug products in their stockpiles. The state stockpiles do not fall under federally mandated guidelines or does the SLEP program provide support to test and confirm extension of the shelf life of these vital reserves at the state level. The price for one package of Tamiflu containing 10 capsules of 75 mg, which is usual course of treatment, ranges from $100-200.
It is apparent that it would be exorbitant to replace Tamiflu in stockpiles once the drug is nearing its expiration date or has expired especially if concurrent with another pandemic. Lyon et al previously reported findings on the stability profile of drug products such as antibiotics and potassium iodide tablets beyond their labeled expiration dates; however, Tamiflu was not part of that comprehensive study [8].
Several lots of Tamiflu are currently stored in the secure facility at Monroe County Department of Public Health facility with expiration dates ranging from 2011 to 2020. Lot U4033-01 expired on 10/31/2011; lot 531741 expired on 11/30/2017; and lot 589654U1 expired on 09/30/2020. The most recent update from the Department of Health and Human Services does not include the above mentioned lots of Tamiflu in the list of lot numbers that have scientifically supportable expiration date extensions [13].
The New York State stockpiles of Tamiflu does not fall under federally mandated stockpile guidelines, nor does SLEP system provide support and guidance on shelf life extension for the currently stored medications under county and state jurisdiction.
The aim of this project was to evaluate the quality of several Tamiflu stocks stored at the Monroe County Department of Public Health in New York in order to establish the chemical stability and activity for data to be used decisions related to public health response to influenza.
2. Materials and Methods
2.1 Materials
|
Material |
Source |
|
Tamiflu 75 mg capsules |
Genentech, NDC 0004-0800-85 Lot# U4033, EXP: 10/2011 (packaged in bottles, 10 capsules each) Lot# 531741, EXP: 11/2017 (packaged in blister packs, 10 capsules each) Lot# 589654U1, EXP: 09/2020 (packaged in blister packs, 10 capsules each) Lot# 589654U1, EXP: 09/2020 Lot# 531741, EXP: 11/2017 Lot# U4033, EXP: 10/2011 |
|
Tamiflu phosphate, 98% |
Acros Organics, Cat. No. 461170050 Lot# A0400559 (purity 99.5% based on Certificate of Analysis) |
|
Potassium phosphate monobasic, HPLC grade |
Fisher Scientific, CAS 7779-77-0, Cat. No. P286-1 Lot# 064592 |
|
85% (w/w) phosphoric acid, HPLC grade |
Fisher Chemical, CAS 7664-38-2, Cat. No. A260-500 Lot# 184413 |
|
10.0 N hydrochloric acid |
Ricca Chemical Company, Cat. No. 3770-32 Lot# 2812503 |
|
Methanol, HPLC grade |
Fisher Chemical, CAS 67-56-1, Cat. No. A454-4 Lot# 161218 |
|
Acetonitrile, HPLC grade |
Fisher Chemical, CAS 75-05-8, Cat. No. A996SK-4 Lot# 158908 |
|
Type I (ultrapure) water |
Prepared in-house using a Milli-Q Direct 8 system from Millipore Sigma |
|
Millex-HN syringe filter, 0.45 µm Nylon membrane |
Millipore Sigma, Cat. No. SLHNX13NL |
2.2 Assay (Potency) method
2.2.1 High performance liquid chromatography (HPLC) analysis: The HPLC method from the compendial USP monograph for oseltamivir phosphate capsules was used to measure drug potency in the Tamiflu capsules and potential degradation products [6]. Practical modifications were used for optimal analysis. An LC-2010A system from Shimadzu Scientific Instruments (Marlborough, Massachusetts) was equipped with a C8, 5 µm, 100 Å, 250 x 4.6 mm column from Phenomenex (Torrance, California) as the stationary phase. The mobile phase consisted of Solution A:methanol:acetonitrile (62:24.5:13.5). Solution A was prepared by dissolving 6.8 g potassium phosphate monobasic in approximately 980 mL water; the pH of the solution was adjusted to 6.0 using 1N KOH; the solution was brought to a total of 1000 mL with water followed by filtration through a 0.22 µm membrane. Additional HPLC parameters are described in the table below.
|
HPLC System |
Shimadzu LC-2010A HT |
|
Column |
Phenomenex Luna 5 µm C8(2) 100Å, 250 x 4.6 mm |
|
Column Temp. |
50°C |
|
Mobile Phase |
Methnaol, acetonitrile and Solution A (245: 135: 620) |
|
Flow Rate |
1.2 mL/min |
|
UV Detection |
207 nm |
|
Inject Vol. |
15 μL |
|
Run Time |
30 min |
Table 1: Parameters of HPLC.
The standard solution of oseltamivir phosphate, 1 mg/mL based on free base, was prepared by dissolving 66.0 mg oseltamivir phosphate powder in mobile phase to a total volume of 50 mL. The amount of oseltamivir phosphate powder was calculated based the percent free base (76.12%) and the lot purity (99.5%) of the oseltamivir phosphate powder material. Seven replicates of HPLC testing of the standard solution were performed to verify the suitability of the system.
To prepare the sample solutions, three bottles/blister packs were selected randomly from each lot of expired Tamifluâ. Five capsules were retrieved from each bottle or blister pack, and the fill contents were pooled, weighed, and triturated to a fine powder. Calculation was performed to determine the amount of capsule powder which contained a theoretical amount of 50 mg oseltamivir free base. This amount of the capsule powder was weighed and transferred into a 50-mL volumetric flask.
About 30 mL mobile phase was added to the flask followed by 10 minutes of sonication. Additional mobile phase was added to bring the final volume to 50 mL. After thorough mixing, 1.0 mL aliquot was withdrawn and filtered using a 0.45 μm Nylon syringe filter. The filtrate was analyzed by the HPLC method. Each lot of expired Tamiflu was prepared in triplicate for analyses.
2.3 Dissolution method
The dissolution method from the USP Monograph of Oseltamivir Phosphate Capsules was used with practical modifications to evaluate the drug release performance from the Tamiflu capsules [11]. The method parameters are listed in the table below. The dissolution media was prepared by mixing 200 mL 10 N hydrochloric acid with 19.8 L purified water in a 20-L carboy. Because the placebo excipients were not available for this study, the analysis of the dissolution samples was changed from direct UV measurement at 240 nm to the HPLC method used for the potency assay (with an increased injection volume of 50 µL). The placebo excipients were previously observed to interfere with the drug absorption at 240 nm wavelength.
The standard solution was prepared by dissolving 99.0 mg oseltamivir phosphate powder (equivalent to 75.0 mg free base) in 900 mL dissolution media at 37 °C. This amount of oseltamivir phosphate powder was determined based the percent free base (76.12 %) and the lot purity (99.5 %) of the oseltamivir phosphate powder material.
For sample analysis, six capsules from each lot of Tamifluâ were placed into metal sinkers and dropped into vessels individually. After 20 minutes, an aliquot of ~ 3 mL was taken from each vessel, and passed through a 0.45 µm nylon syringe filter, and analyzed by HPLC (75 µL injection volume).
|
Dissolution System |
Varian VK 7000 with 7 vessels |
|
Apparatus |
Apparatus 2 (paddle), 50 rpm |
|
Media |
0.1 N hydrochloric acid, 900 mL, 37°C |
|
Time |
20 min |
|
Analysis |
HPLC method as described in Assay Method with an increased injection volume of 75 µL |
Table 2: Parameters of dissolution analysis.
3. Results
This experimental study evaluated the chemical stability and drug release properties of three different Tamiflu lots in the Monroe County Department of Public Health stockpile. The chemical stability relates to the potency of the drug and was analyzed by high performance liquid chromatography (HPLC) according to the USP. The HPLC also measures potential degradation products in the capsules which may vary from select lots and storage conditions. The dissolution method measures the amount of drug released from the capsules in a liquid medium simulating the stomach pH and temperature.
The HPLC results, summarized in table 3, indicated satisfactory drug potency with all lots meeting the USP acceptance criteria of 90.0% - 110.0% of the labeled claim. Two unknown peaks, at relative retention times of 0.53 and 0.58, were detected in negligible quantity (< 0.2% total HPLC peak area). These unknown peaks were also present in the standard solution at similar levels. Specifically, lot U4033 had 100.1%, lot 531741 had 99.6% and lot 589654U1 had 99.5% detected pharmaceutical ingredient of the labeled claim.
|
Lot Number |
Expiration Date |
% Label Claim (n = 3) |
|
U4033 |
Oct 2011 |
101.3% ± 1.4% |
|
531741 |
Nov 2017 |
100.6% ± 0.9% |
|
589654U1 |
Sep 2020 |
100.9% ± 0.9% |
Table 3: Chemical stability.
|
Lot Number |
Eexpiration Date |
% Released (n = 6) |
|
U4033 |
Oct 2011 |
100.1% ± 2.0% |
|
531741 |
Nov 2017 |
99.6% ± 0.9% |
|
589654U1 |
Sep 2020 |
99.5% ± 4.3% |
Table 4: Dec 2020 Drug release properties.
The results from dissolution testing are summarized in table 4. All three Tamiflu lots met the passing criteria of no less than 75% of labeled amount of drug dissolved after 20 minutes.
Thus, all Tamiflu samples analyzed had approximately 100% dissolution and chemical release attributes compared to unexpired controls. The three lots of stockpiled Tamiflu demonstrated chemical stability beyond the expiration dates with potency detected to the level of the new Tamiflu phosphate control.
4. Discussion
This study reveals that expired reserves of Tamiflu may retain potency and could be used to protect public health in response to influenza outbreak. The novelty of this study is that it presents a practical approach to test expired drug products with modified compendia methods. The detection of the chemical stability and potency had three main practical modifications implemented in this study. First, for the preparation of the “Standard solution”: a high purity grade of oseltamivir phosphate drug powder was used in place of USP oseltamivir ohosphate reference standard. This was mainly due to the excessive cost of USP RS ($1600/g), whose main consumers are for-profit companies in the pharmaceutical industry. In contrast, the source used for this study costs $26/g, which is reasonable for government and non-profit organizations. It is important to obtain the purity data from the certificate of analysis for each lot and adjust the quantity calculation based on purity. For example, the purity was 99.5% for the lot of control oseltamivir phosphate used in this study, and the quantity used to prepare the “Standard solution” was adjusted by dividing the theoretical amount by 0.995. Second, for the preparation of the “Sample solution”, the “Mobile phase” was used instead of the “Diluent”. For HPLC analysis in general, it is a well-established best practice to use the mobile phase as the sample diluent whenever possible to obtain optimal chromatography. In this study, it was confirmed that when the mobile phase was used as the sample diluent, the retention time fluctuation was minimized (data not shown). In addition, oseltamivir phosphate is an ester prodrug, which is prone to hydrolysis at low and high pH environments.
The “Diluent” had a measured pH of about 2.8, which could potentially accelerate the hydrolysis degradation. In contrast, the “Mobile Phase” has a measured pH of 6.8 and it was used in these experimental studies. Finally, three independent sample replicates were prepared and analyzed to calculate the mean and relative standard deviations for each lot tested.
The analysis of dissolution had two modifications. The first is the same preparation modifications for the sample solution as noted above. The second was that the “Excipient solution” was not prepared, because the placebo mixture was not commercially available. This appears to be a common problem when USP tests are performed on drug products with proprietary formulations, including both brand and generic products. Even though the excipient names are listed in the product labeling, the exact technical grades and quantities are not disclosed. As such, it is nearly impossible for an independent testing laboratory to prepare the appropriate “excipient solution” without obtaining the placebo mixture directly from the manufacturer of the drug product. To overcome this issue, the detection method of the dissolution test was modified in this study. Instead of direct UV detection, the dissolution samples were analyzed by the same HPLC method used for the assay of potency. Since the HPLC method separates the drug from the excipients prior to the in-line UV detection, there was no need for the “Excipient solution”. The HPLC sample injection volume was increased from 15 µL to 75 µL to account for the lower drug concentration in the dissolution samples.
Similar to most oral tablet or capsule products, there are two important quality tests under the Performance Tests category of the USP monograph for oseltamivir phosphate capsules: Dissolution and Uniformity of Dosage Units. Uniformity of dosage units was not analyzed in this study, because it is usually performed only once after production to confirm that each unit (capsule in this case) contains the labeled amount of drug. Once established, uniformity of unit dosage products is not expected to change over storage. In most stability testing programs, this test is only performed at time zero and not at other time points. All lots of Tamiflu in this study were released by the manufacturer and were presumed to have passed the uniformity test.
Based on the data from the HPLC and dissolution experiments in this study, the three Tamiflu lots tested from the Monroe County Department of Public Health stockpile appear to meet the USP quality standards. Importantly, FDA guidance released in June 2020 states that stocks of 75 mg Tamiflu lots can be used for 10 years after expiration with a maximum of 15 years extension for emergency response use [14]. The confidence in extension of these antiviral drugs is more confident if scientific evidence is provided to support the stability of the compound particularly if it is to be deployed for use in an emergency response. The current SARS-CoV2 pandemic and strain on the SNS as reported in the New York Times [15], places greater emphasis for preparedness to influenza outbreaks with existing stockpiled medications if real-time data can support use beyond expiration. Collectively, the data from this study suggests that in a case of influenza epidemic in the region, these stockpiled lots could be considered for use based on data supporting stability from this ‘shelf-life’ study. The three lots tested in the current study have expirations of 2011, 2017 and 2020 which are within the fifteen year extension period supported by the FDA for federally-regulated SNS. It is recommended that other state and county departments of public health with stockpiled Tamiflu stocks be analyzed for chemical stability and potency to support official declaration for extension of the expiration dates and appropriate relabeling for the local stockpile. Annual testing should be performed to monitor each lot for effective confidence in these Tamiflu reserves. The current study provides critical readiness data on Tamiflu reserves that have retained the potency and meets the criteria for emergency use in case of an influenza outbreak in the region, and serves as a model for other non-federal jurisdictions to follow in order to protect the public health during the next influenza pandemic.
Author Contribution(s)
TDC, conceptualization and design, execution of study. Writing of manuscript.
KD, conceptualization and acquisition of materials and supplies; editing manuscript.
FZ, supervision and execution of analytical testing, interpretation of results and data.
MS and MM, provided materials and interpretation of results.
KR and CT, equally contributed to performing experiments, collecting data.
Conflicts of Intrest
Authors have no disclosures of conflicts of interests.
References
- World Health Organization. Influenza Update-388 (2021).
- Hilton J. The pandemic dramatically reduced flu cases. That could backfire. Politico (2021).
- Choo EK, Rajkumar SV. Medication Shortages during the COVID-19 Crisis: What We Must Do. Commentary, Mayo Clinic Proc 95 (2020): 1112-1115.
- https://www.dailymail.co.uk/news/article-5379261/Shortage-Tamiflu-flu-kills-4-000-Americans-week.html)(CDC statistic, Feb. 2014)
- https://www.insider.com/child-dies-flu-anti-vaxxers-advice-facebook-2020-2
- Strategic National Stockpile. phe.gov. U.S. Department of Health and Human Services (2018).
- Courtney B, et al. Maximizing State and Local Medical Countermeasure Stockpile Investments through the Shelf Life Extension Program. Biosecurity and Bioterrorism 7 (2009): 1.
- Lyon RC, Taylor JS, et al. Stability profiles of drug products extended beyond labeled expiration dates. J Pharm Sci 95 (2006): 1549-1560.
- Tamiflu (Tamiflu phosphate) capsules, package insert. Genentech, Inc., South San Francisco, CA.
- Tamiflu Phosphate monograph. In: USP42-NF37. United States Pharmacopeia (2019).
- Tamiflu Phosphate Capsules monograph. In: USP42-NF37. United States Pharmacopeia (2019).
- Junwal M, Sahu A, Handa T, et al. ICH guidance in practice: Degradation behaviour of Tamiflu phosphate under stress conditions. J of Pharm and Biomedical Analysis 62 (2012): 48-60.
- Commissioner of the. EUA Archive [Internet]. U.S. Food and Drug Administration. FDA (2010).
- FDA Notice on Expiration Dating Extension. Available at: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/expiration-dating-extension.
- Hamby C, Stolberg SG. U.S. Stockpiled anthrax vaccine but neglected needs for a virus. Front page, New York Times (2020).


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 