Urinary Biomonitoring for Detection of As and Cd Exposure in Adult Population - A Cross Sectional Study from Selected Area of West Bengal, India
AnupaYadav1&2, Aniruddha Mukhopadhayay2, Amit Chakrabarti1, AsimSaha1 and Pritha Bhattacharjee2*
1ICMR - Centre for Ageing and Mental Health (I-CAM),Division of Non-Communicable Diseases (NCD), Indian Council of Medical Research (ICMR);Block - DP-1, Sector V, Salt Lake Kolkata – 700091, India.
2Department of Environmental Science, University of Calcutta, Kolkata-700019, India.
*Corresponding Author: Pritha Bhattacharjee, Department of Environmental Science, Assistant Professor environmental Toxicogenomics, Molecular Biology and Human Genetics, University of Calcutta, Kolkata-700019, India.
Received: 11 March 2024; Accepted: 21 March 2024; Published: 16 May 2024;
Article Information
Citation: AnupaYadav, Aniruddha Mukhopadhayay, Amit Chakrabarti, AsimSaha and Pritha Bhattacharjee. Urinary Biomonitoring for Detection of As and Cd Exposure in Adult Population - A Cross Sectional Study from Selected Area of West Bengal, India. Journal of Environmental Science and Public Health. 8 (2024): 70-85.
View / Download Pdf Share at FacebookAbstract
Introduction: Monitoring of human exposure to environmental pollutants can be achieved by assessment of biological samples such as urine. Arsenic (As) and Cadmium (Cd) both metals are environmentally toxic in nature. Biomonitoring of these heavy metals in non-endemic areas of West Bengal is not well documented, at international level many human biomonitoring surveys have been well established.
Aim: To assess urinary arsenic (U-iAs) and urinary cadmium (U-Cd) among the adult population of West Bengal. And also endeavor to identify sociodemographic and lifestyle factors affecting exposure to environmental As and Cd.
Methods: This was a cross sectional study conducted in 258 adults. The data was collected through a structured questionnaire. U-iAs and U-Cd was analyzed by atomic absorption spectrometer (AAS).
Results: This study found predominantly low to moderate levels of U-iAs 4.55-40.95μg/L and U-Cd 0.01-1.7μg/L. About 5% individuals had U-iAs>27μg/L (reference value) and 7.4% individuals had U-Cd above human biomonitoring value (1μg/L). Urban residents had about 1.58-fold higher U-iAs than rural. Urban individuals, females involved in cooking and smoker traveler males had significantly (p<0.05 for each category) higher U-iAs and U-Cd than their counterparts.
Discussion: Cooking practice in females, smoking and travelling in males were reported as potential contributors to As and Cd exposure. Conclusion: This study provided data on U-iAs and U-Cd levels in study population that will help them to beware of toxic metals exposure and will be useful for policymakers in decision-making on interventions, enabling appropriate risk reduction strategies.
Keywords
<p>Urinary arsenic, Urinary cadmium, General population, Cooking, Smoking, Travelling, Rural, Urban, Environmental exposure</p>
Article Details
1. Introduction
Toxic metals such as As and Cd pose risk to human health due to their abundance in ambient environment and ubiquitous in biosphere. These metals have no physiological role in human and may results in intoxication at low level chronic exposure. The major sources of exposure to these heavy metals among general public is through dietary intake via food or water [1-5]. General public is usually exposed to inorganic As via drinking water and consuming contaminated food like rice and grain [6,7]. As per WHO, approximately 25% of the daily inorganic As (iAs) intake come from human diet and it was reported higher for men than women and children [8]. While exposure to organic arsenic mainly occurs from consumption of fish and sea food [9]. Inorganic form of As is more toxic, a non-threshold, and class 1 carcinogenic agent [10]. Several studies suggests strong correlation of non-carcinogenic cardiovascular and metabolic disease, developmental retardation, respiratory disease, hypertension, neurobehavioral function damage with As exposure [11-14].
Cd occurs naturally in earth crust, and further anthropogenic activities increased its level in the environment [15,16]. It is classified as category 1 human carcinogen by the international agency for research on cancer (IARC, 2012)[17]. Its' absorption in human from inhalation such as smoking was found higher (about 25%) than ingestion of contaminated food like rice, leafy vegetables, nuts and pulses (about 5-10%) [18,19]. Schwarz et al. (2014) reported average intake of Cd 1.46 μgkg-1 body weight week-1 and in high-end consumer it reached up to 2.35 μgkg-1 body weight week-1[20]. In view of public health, the Joint FAO/WHO Expert Committee on Food Additives (JECFA), had recommended provisional tolerable monthly intake (PTMI) of Cd is 25µg kg-1 body weight month-1 for human [21].
In 2005, Centre for Disease Control (CDC) defined human biomonitoring (HBM) as “the direct measurement of people's exposure to toxic substances by measuring the substances or their metabolites in human specimens”. HBM is mainly used to describe monitoring of chemicals’ or pollutants’ levels present in human fluids or tissues. This is a widely used tool to assess and evaluate exposure of environmental pollutants in general public belonging to any age group. Worldwide, many nations have successfully conducted studies on human exposure to environmental chemicals and established reference values for toxic metals ( As, Cd) to prevent future potential health risks associated with these metals. Perhaps, the oldest National Health and Nutrition Examination Survey (NHANES) in the United States was initiated in 1960s is still ongoing, followed by German Environmental Survey (GerES) in 1985 that was last conducted in 2014-2017, Belgian HBM Program since 2002, French National Human Biomonitoring Program (NHBP) started in 2007 last conducted in 2014-2016 and Korea National Survey for Environmental Pollutants in the Human Body (KorSEP) in 2005 [22].Other HBM programs from other countries such as Finnish HBM was initiated in 2012, Danish Human Biomonitoring Programme (DANBIOH) project launched in 2014 with collaboration with European countries, Canadian Health Measures Survey (CHMS) was launched in 2007 by Statistics Canada and latest one European Human Biomonitoring Initiative (HBM4EU) was initiated in 2017 involves 28 European Union countries. The major objective of all these studies were to identify the individuals with elevated levels of exposure to the given environmental pollutants and to identify potential health risks associated with these pollutants. In order to develop policies and interventions aimed at reducing exposure and improving public health.
At present many more countries from various part of the globe have started human biomonitoring studies, India fall short on this. Currently, India does not have any national HBM surveillance program. However, a few studies have been conducted to measure As in hair, nails, blood and urine among population of As endemic area in West Bengal, while rarely any study measured Cd in human urine in this region[23-26, 3].This background prompted us for the present study to obtain preliminary information on urinary levels of As and Cd among the residents of West Bengal, India included urban and rural areas.
2. Materials and Methods
2.1 Study population
This was a cross-sectional study, involved 258 healthy individuals (those did not diagnose with any medical illness included 129 males and 129 females).The random sampling was used for selection of study population. The study was conducted in urban (Kolkata city) and rural area (village Debipur, District purba Medinipur) of West Bengal. The urban area participants were selected from residential and commercial areas altogether. Urban area study sites were within 100meter of distance from traffic running road (traffic density was moderate). Rural study site was about 2km away from town or any traffic running road (i.e. traffic pollution could not be considered in that location). Sample and data collection was done during November 2021 and February 2022 to evaluate environmental exposure to As and Cd in adult individuals (22 to 80years; mean age 50years). Both working and non-working individuals were included in this study. The Inclusion criteria were, individual those did not have medical history of any chronic illness and did not have any direct occupational exposure to As and Cd. The exclusion criteria were, participants living in As and Cd endemic area, staying at current address less than one year and had any As or Cd toxicity symptoms.
2.2 Ethics
Prior to study, all participants were explained about purpose of work, confidentiality of their identity, unrestricted withdrawal; and only after that their participation consent was obtained. The study protocol was approved by Institutional Ethical Committee (approval number 15thIEC/CNCD/5.5 dated 15.07.2021; Ethical Committee of ICMR, Centre on Non-Communicable disease).
2.3 Data collection
During enrollment, participants were interviewed face-to-face at their doorstep using a structured questionnaire to gather information on their background, lifestyle, and health, including age, gender, monthly family income, education, employment, and lifestyle factors like smoking, cooking, and travel.
2.4 Description of variables
Individuals were grouped by gender first and then by age(<40years, 40-49years, 50-59years, 60-69years and >70years), cooking practice (NA or Yes), smoking habit (Yes or No), travelling activity (Yes or No) and study area (rural or urban). Here cooking practice means making food for their family twice daily. Travelling means to commute daily for their job and other purpose at least from 1to 3hours/day. The classification of individuals into low-income group (LIG) earning <20K/Month, middle-income group (MIG) earning up to 50K/Month, and high-income group (HIG) earning >50K/Month was based on their monthly family income. Women were again divided into two groups based on their 1) employment status: homemakers as well as working and only homemakers and 2) exposure to tobacco smoke: those were passively exposed(i.e. male member in their family were smoker and did smoke indoors) and those females were not exposed to any tobacco smoke. Men were also divided into two groups based on their smoking and travelling activity 1) smoker traveler and 2) non-smoker non-traveler. These classifications were done to better understand As and Cd exposure from ambient environment via cooking practice, tobacco smoke and traffic pollutants during daily commute.
2.5 Urine collection and analysis
Individuals (N=258) provided 50mL of the morning first (mid-stream) urine samples the day after their interview, collected using graduated and sterilized polypropylene urine containers (Tarson). After collection of urine samples, each sample was labeled with a code for the identification of participant and date of sample collection. The collected urine samples were brought to laboratory under refrigerated (4oC) condition. Urine samples were homogenized and then aliquoted for estimation of creatinine and heavy metals separately. Urine aliquot for estimation of heavy metals were acidified with concentrated HCl to pH 2 and stored at -20oC till further analysis, while creatinine was estimated on same day of urine collection.
To determine the U-iAs concentration, all collected urine samples (2mL) were digested with (6mL) ammonium peroxosulfate (2%, m/v) prepared in sodium hydroxide (3%, m/v) in microwave work station (Milestone, Ethos-D) using an optimized program as described by Sysalova et al.(2003)[27]. The final volume of digested urine sample was diluted to 10mL with deionized distilled water. The digested samples and working standards of As were prepared as per recommended analytical conditions and general information of Perkin Elmer metal analysis manual. Above prepared samples were ingested in FIAS-hydride generation atomic absorption spectrometer (Model: AAnalyst 800, Perkin Elmer, USA) and analysis was performed with the carrier (10% HCl) and reducing solution (0.2% NaBH4 in 0.05% NaOH). Atomization was done in a quartz cell at 900oC; absorbance was measured at 193.7nm (FIAS-Hydride: recommended analytical condition and general information, Perkin Elmer). A standard stock solution of arsenic(1000µg/L, traceable to SRM from NIST in HNO3) from Merck, Darmstadt, Germany was used. By diluting As stock solution calibration curve was prepared daily within range of U-iAs observed normally in general population [3,28,29]. Method detection limit was calculated by using standard deviation (10 replicates) of pooled urine sample (i.e. 3*SD).And 10% of urine samples were reanalyzed to verify accuracy. Quality control measure such percentage recovery study and intra/inter day precision measurement were also implemented. Recovery percentage was 80-110%. Intra-day coefficient of variation (CV%) was 1% for 1.0µg/L, 0.5% for 3 and 5µg/L, while inter-day CV% was 4% for 1.0µg/L, 5.1% for 3µg/L and 4.6% for 5µg/L. The limit of detection (LOD) and limit of quantification (LOQ) was 1.0µg/L and 3.8µg/L respectively. Results below LOD were replaced by value equal to half of the LOQ.
To determine the U-Cd, urine sample was diluted ten times with 5% (V/V) solution of ultrapure nitric acid (Suprapur, Merck-Germany) in ultrapure water. A mixed modifier containing 0.1% (W/V) Palladium (Merck, Germany) and 0.06% (W/V) Magnesium matrix modifier (Merck, Germany) was mixed with diluted urine for estimation of Cd. This solution was analyzed by Graphite Furnace-Atomic Absorption Spectrometer (Model: AAnalyst 800, Perkin Elmer, USA) as per the conditions described by Sauerhoff et al. (1996), absorbance was measured at 228.8nm [30]. A standard stock solution of Cd (1000µg/L, trace CERT® in nitric acid) from Merck, Darmstadt, Germany was used. Cd stock solution was diluted to prepare working standards, calibration curve was prepared daily within range of U-Cd observed normally in general population [28,29]. Quality control procedure was done in same way as for As estimation. Recovery percentage was 85-115%. Intra-day coefficient of variation (CV%) was1.8% for 0.3µg/L,1.5% for 1µg/L and 2.7% for 2µg/L, while inter-day CV% was5.2% for 0.3µg/L, 3.1% for 1µg/L and 4.1% for 2µg/L. LOD and LOQ was 0.2µg/L and 0.7µg/L respectively. Results below LOD were replaced by value equal to half of the LOQ.
The creatinine concentration in urine has been determined by Jaffe method based on photometric measurement of creatinine reaction with picric acid at 37oC [31]. The reagent kit for creatinine estimation was supplied by Arkray (ARKRAY, India). The photometric measurement was done with UV-visible spectrophotometer (Model: Lambda 45, Perkin Elmer, USA) at 520 nm.
2.6 Statistical analysis
We used Microsoft Excel for analysis of data and reported concentrations of U-iAs and U-Cd in micrograms per liter (µg/L) or micrograms per gram creatinine (µg/g creatinine). Kalmogrvo test was used to check data distribution of urine analysis results. We used descriptive statistic and two-tailed Mann-Whitney U-test (non-parametric test) to determine the significance of differences between two groups (in each category of variable) among study participants, SPSS software was used for this purpose.
3. Results
3.1 Characteristic of study individuals
The study initially involved a total of 383 individuals. However, 125 individuals excluded from the study due to refusal of responding the questionnaire, inadequate urine samples, damaged sample containers and urine creatinine falling below <0.3g/L or exceeding >3.0g/L as per the WHO(1999)[32]. Consequently, the final sample size of the study comprised of 258 individuals those were able to meet the criteria and participated in the present study.
Table1 presents socio-demographic characteristics of the study participants. The study included an equal number of male and female individuals with 129 (50%) individuals in each group. The majority of individuals belonged to the age group of 42-49years, 34.1% male and 32.6% female. Among the total individuals, the most prevalent educational qualification was high school, 26.7%. The majority of participants belonged to the lower-income group (LIG) included 62% male and 74.4% female in this category. Predominantly only females 79% were involved in cooking practice compared to males 6.2%. When it came to traveling, more males (61.2%) were frequent travelers than females(13.2%). In terms of smoking habit, 27.1% male individuals were smokers, while none of the female participant was smoker.
Table1: Demographic characteristics of study individuals (N=258)
|
Variables |
Male N (%) |
Female N (%) |
Total N (%) |
|
Total |
129 (50.0) |
129 (50.0) |
258 (100.0) |
|
Age Group (years) |
|||
|
<40 |
27 (20.9) |
27 (20.9) |
54 (20.9) |
|
40-49 |
42 (32.6) |
44 (34.1) |
86 (33.3) |
|
50-59 |
26 (20.2) |
27 (20.9) |
53 (20.5) |
|
60-69 |
22 (17.1) |
16 (12.4) |
38 (14.7) |
|
≥70 |
12 (9.3) |
15 (11.6) |
27 (10.5) |
|
Education |
|||
|
No Formal Education |
46 (35.7) |
9 (7) |
55 (21.3) |
|
Primary |
33 (25.6) |
29 (22.5) |
62 (24) |
|
High School |
32 (24.8) |
37 (28.7) |
69 (26.7) |
|
S Sec. School |
8 (6.2) |
19 (14.7) |
27 (10.5) |
|
College |
10 (7.8) |
35 (27.1) |
45 (17.4) |
|
Family Income |
|||
|
LIG |
96 (74.4) |
80 (62) |
176 (68.2) |
|
MIG |
29 (22.5) |
43 (33.3) |
72 (27.9) |
|
HIG |
4 (3.1) |
6 (4.7) |
10 (3.9) |
|
Cooking Practice |
|||
|
NA |
121 (93.8) |
27 (20.9) |
148 (57.4) |
|
Yes |
8 (6.2) |
102 (79.1) |
110 (42.6) |
|
Travelling |
|||
|
Yes |
79 (61.2) |
17 (13.2) |
96 (37.2) |
|
No |
50 (38.8) |
112 (86.8) |
162 (62.8) |
|
Smoking |
|||
|
Yes |
35 (27.1) |
0 (0) |
35 (13.6) |
|
No |
94 (72.9) |
129 (100) |
223 (86.4) |
Abbreviations: LIG, low income group; MIG, middle income group; HIG, high income group; NA, not applicable
3.2 Distribution of arsenic and cadmium in urine
Tables 2 and 3 present the results of U-iAs and U-Cd levels in µg/L and µg/g creatinine respectively. The U-iAs and U-Cd data was not normally distributed, while this data has a skewed significant distribution. The mean urinary levels of these metals among the study participants were found 14.18µg/L (95% CI: 12.96-15.96) for U-iAs and 0.52µg/L (95% CI: 0.48-0.56) for U-Cd. The mean urinary level was significantly higher in females than males for As (15.06µg/L; 95% CI: 14.10-16.03, p=0.001;Z-score=3.24), while the difference in urinary Cd levels between males and females was not statistically significant (0.48µg/L; 95% CI: 0.43-0.55). Moreover, residents of urban areas had significantly higher levels of U-iAs (15.80µg/L; 95% CI: 14.15-17.44, p=0.0001; Z-score=4.05) and U-Cd (0.52µg/L; 95% CI: 0.44-0.60, p=0.0001;Z-score=3.92) than the residents of rural area.
Table 2: Urinary (µg/L) arsenic and cadmium without creatinine correction in study individuals by gender and study area wise
|
N(%) |
Mean ± SD |
Range |
Median |
CI 95% (Lower & Upper Limits) |
Percentile |
|||
|
25th |
75th |
95th |
||||||
|
Arsenic (As) |
||||||||
|
Total |
258 (100%) |
14.18±6.75 |
4.55-40.95 |
12.7 |
12.96-15.39 |
8.95 |
17.4 |
27.3 |
|
Male |
129(50%) |
13.29 ±5.95 |
4.80-38.30 |
12.7 |
12.26-14.32 |
8.8 |
16.9 |
24.62 |
|
Female |
129(50%) |
15.06±6.76 |
4.55-40.95 |
12.7 |
14.10-16.03 |
9.1 |
18.65 |
30.57 |
|
Urban |
186(72%) |
15.80±6.75 |
4.80-40.95 |
13.75 |
14.15-17.44 |
10.5 |
19.25 |
28.97 |
|
Rural |
72(28%) |
9.99±4.78 |
4.55-30.55 |
8.68 |
9.31-10.68 |
6.45 |
11.4 |
19.15 |
|
Cadmium (Cd) |
||||||||
|
Total |
258 (100%) |
0.52±0.35 |
0.01-1.7 |
0.41 |
0.48-0.56 |
0.17 |
0.62 |
1.14 |
|
Male |
129(50%) |
0.45±0.30 |
0.01-1.52 |
0.38 |
0.42-0.52 |
0.16 |
0.62 |
1.14 |
|
Female |
129(50%) |
0.48±0.34 |
0.01-1.7 |
0.42 |
0.43-0.55 |
0.18 |
0.6 |
1.11 |
|
Urban |
186(72%) |
0.52±0.35 |
0.01-1.7 |
0.43 |
0.44-0.60 |
0.17 |
0.54 |
0.88 |
|
Rural |
72(28%) |
0.38±0.23 |
0.01-0.99 |
0.31 |
0.35-0.41 |
0.16 |
0.61 |
1.14 |
Table 3: Urinary (µg/g) arsenic and cadmium after creatinine correction in study individuals by gender and study area wise
|
N (%) |
Mean ± SD |
Range |
Median |
CI 95% |
Percentile |
||||
|
( Lower & Upper Limits) |
25th |
75th |
95th |
||||||
|
Arsenic (As) |
|||||||||
|
Total |
258 (100%) |
9.73±4.92 |
2.17-36.32 |
8.96 |
9.13-10.34 |
5.99 |
12.44 |
17.77 |
|
|
Male |
129(50%) |
8.46±4.64 |
2.17-36.32 |
9 |
7.66-9.26 |
5.16 |
10.34 |
16.59 |
|
|
Female |
129(50%) |
11.01±4.88 |
3.64-34.24 |
8.92 |
10.16-11.85 |
6.8 |
13.94 |
19.11 |
|
|
Urban |
186(72%) |
10.17±5.22 |
2.17-36.32 |
9.29 |
8.97-11.38 |
6.07 |
13.55 |
18.05 |
|
|
Rural |
72(28%) |
8.61±3.86 |
2.82-21.12 |
7.76 |
8.05-9.16 |
1.26 |
1.39 |
1.42 |
|
|
Cadmium (Cd) |
|||||||||
|
Total |
258(100) |
0.37±0.28 |
0.04-2.37 |
0.28 |
0.34-0.41 |
0.25 |
0.45 |
0.75 |
|
|
Male |
129(50%) |
0.35±0.24 |
0.01-1.19 |
0.28 |
0.30-0.39 |
0.25 |
0.5 |
0.78 |
|
|
Female |
129(50%) |
0.33±0.26 |
0.01-2.37 |
0.28 |
0.29-0.38 |
0.25 |
0.43 |
0.7 |
|
|
Urban |
186(72%) |
0.37±0.28 |
0.04-2.37 |
0.27 |
0.31-0.44 |
0.23 |
0.45 |
0.77 |
|
|
Rural |
72(28%) |
0.33±0.20 |
0.01-.86 |
0.28 |
0.30-0.35 |
0.25 |
0.45 |
0.69 |
|
Table 4: Comparison of urinary arsenic and cadmium levels with other countries
|
Country |
U-As 95th percentile |
U-Cd 95th percentile |
Study Population |
Study Period |
References |
|
India |
27.3µg/L |
1.14µg/L |
Population (age 22-87yrs) |
2021-2022 |
This study |
|
South Korea |
119.7µg/L |
3.11µg/L |
Adult (age ≥20yrs) |
2008 |
Lee et al. 2012 |
|
Belgium |
48.8µg/L |
- |
Adult (age ≥18yrs) |
2010-2011 |
Hoet et al. 2013 |
|
Flanders |
90.0µg/L |
- |
Adult (age 20-40yrs) |
2007-2011 |
Schoeters et al. 2012 |
|
USA |
52.4µg/L |
0.87µg/L |
Male and Female (age ≥6yrs) |
2011-2012 |
CDC 2009 |
|
Canada |
76.0µg/L |
1.80µg/L |
Population (age 3-79yrs) |
2009-2011 |
CHMS 2013 |
|
Malaysia |
260.0µg/L |
1.50µg/L |
Adult (age 18-88yrs) |
2017-2018 |
Zurahamin et al. 2021 |
|
Canada |
27µg/L |
- |
Population (age 3-79yrs) |
2007-2013 |
Saravanabhavan, et al. 2017 |
|
Flanders |
- |
0.44µg/L |
Non-Smoking Adult (age 18-69yrs) |
1997-1999 |
Schoeters et al. (2012) |
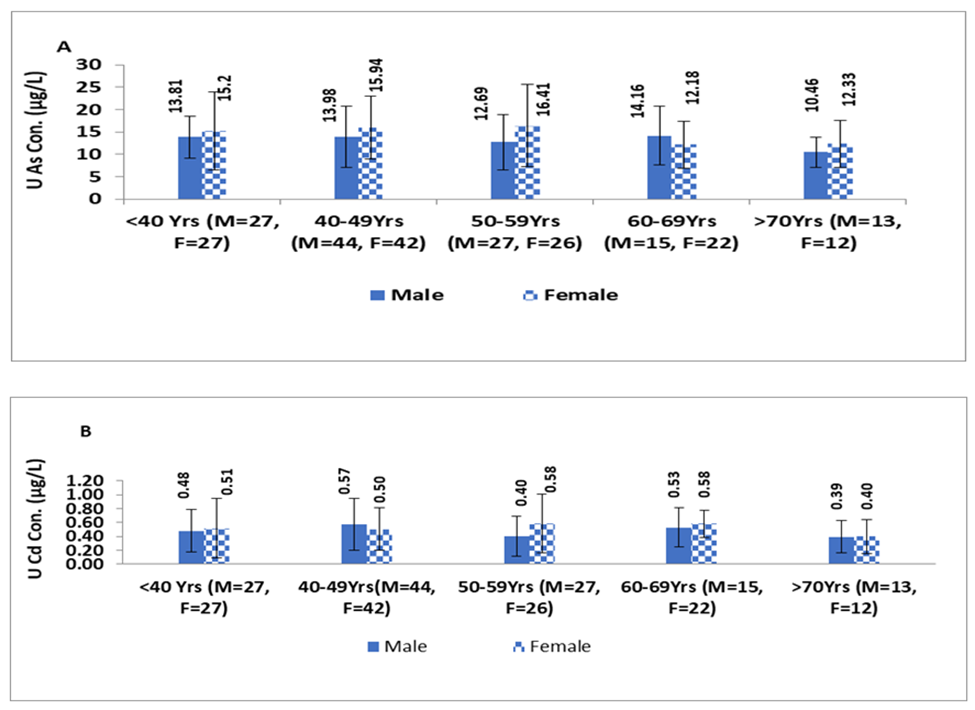
Figure 1, illustrates that females tend to exhibit higher levels of U-iAs and U-Cd than males across all age groups. This difference was statistically significant between males and females only in the age groups of <40 years and 50-59years for U-iAs levels (p=0.003;Z-score=2.85 and p=0.05; Z-score=1.94, respectively). However, the difference in U-Cd levels between males and females was not significant in any of the age groups.
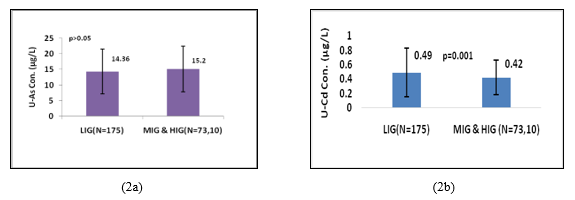
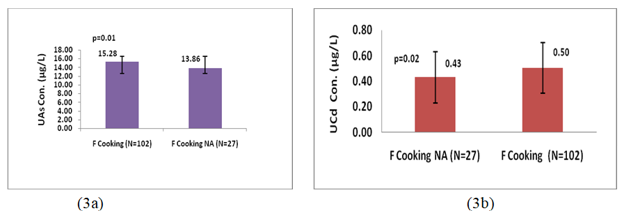
Figure 2a, shows that there was no statistically significant difference in U-iAs levels between LIG group (14.36µg/L) and merged MIG and HIG group (15.25µg/L). On the other hand Figure2b, shows that U-Cd levels in LIG individuals (0.49µg/L) were significantly (p=0.001; Z-score=3.03) higher than those in merged MIG and HIG group (0.42µg/L). Female individuals who were involved in cooking practice had significantly higher levels of U-iAs (p=0.01; Z-score=2.64) and U-Cd (p=0.02;Z-score=1.98) than those who were not involved in cooking practice (Figure 3a and 3b).
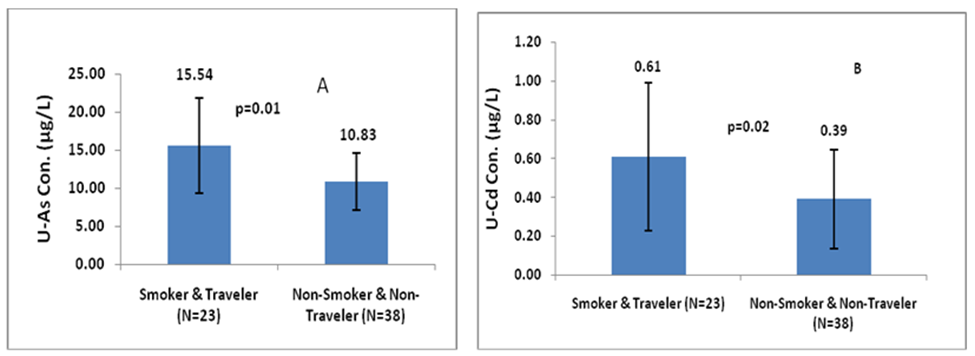
Figure 4a and 4b shown that male individuals who were smokers and involved in daily travelling had higher levels of U-iAs and U-Cd (15.54µg/L and 0.61µg/L, respectively) compared to those who did not smoke and did not travel daily (10.83µg/L and 0.39µg/L, respectively). The difference in U-iAs and U-Cd levels between these two groups were statistically significant p=0.002 (Z-score=-2.99) and 0.02 (Z-score=-2.20), respectively.
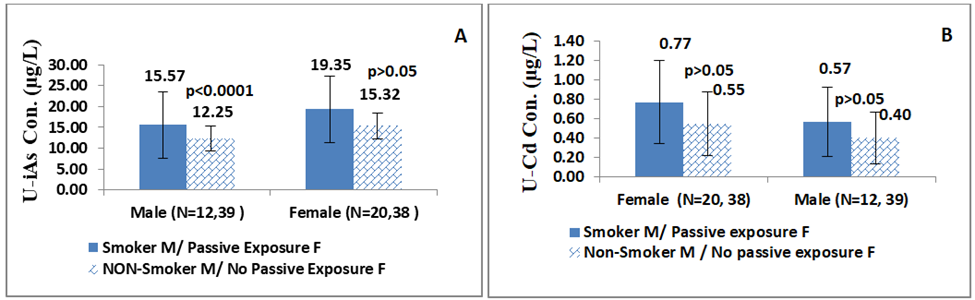
Smokers male had significantly higher levels of U-iAs (15.57µg/L) than non-smokers (12.25µg/L) with p< 0.0001 (Z-score=4.05), FigureS1a. On the other hand, females who had passive exposure to tobacco smoke had higher U-iAs levels (19.35µg/L) than those who did not have passive exposure (15.32µg/L), but the difference was not statistically significant. U-Cd levels were higher in smoker males (0.57µg/L) compared to non-smoker males(0.40µg/L). Similarly, females who were exposed to passive tobacco smoke had higher U-Cd levels (0.77µg/L) than those who didn’t have passive exposure (0.55µg/L), FigureS1b. However, there was no significant difference in U-Cd levels for tobacco smoke exposure between the two groups either in males or females.
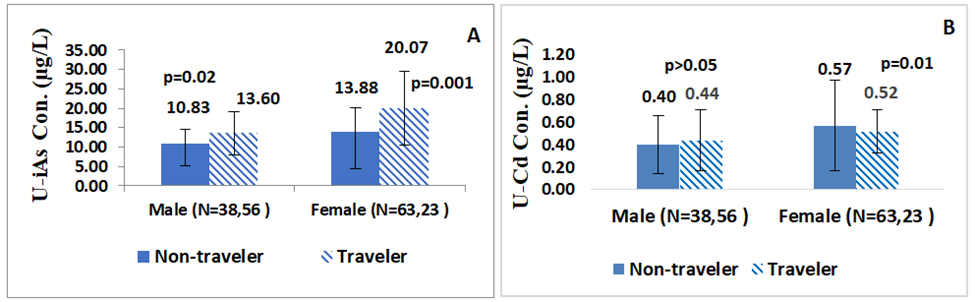
The results showed that the mean levels of U-iAs were higher in traveler males (13.60µg/L) and females (20.07µg/L) than non-travelers (males: 10.83µg/L; females: 13.88µg/L), FigureS2a. This difference in U-iAs concentration was statistically significant between traveler and non-traveler group for both males(p=0.02;Z-score=2.27)and females(p=0.01; Z-score=-3.10). Similarly, the mean levels of U-Cd were higher in traveler males (0.44µg/L) and females (0.52µg/L) than in non-travelers (males: 0.40µg/L; females: 0.57µg/L), FigureS2b. Non-traveler females had a significantly higher concentration of U-Cd (p=0.01; Z-score=1.96) than traveler females, but this difference was not significant in the case of males.
4. Discussion
This study aimed to estimate U-iAs and U-Cd levels in the residents of West Bengal, India. It was well reported that As contamination possess serious health issue in West Bengal population and most of the studies were conducted among the population of As affected areas to monitor As exposure (via drinking water mainly followed by rice and vegetables).While in the present work, we primarily quantified As and Cd levels in the urine of West Bengal residents from non-endemic areas including both rural and urban sites. Urban dwellers were exposed to toxic metals from traffic and industries, while rural residents serve as a control group to compare levels of As and Cd. This baseline data will contribute in identifying the individuals with high exposure between 22-80years of age.
The results found that the 95th percentile value of urinary arsenic in total study individuals, females, and urban individuals was higher than the RV95value (27µg/L) [3,24,33]. The 95th percentile value of U-iAs was 27.3µg/L for total study individuals, 24.62µg/L for males, 30.57µg/L for females, 28.97µg/L for urban and 19.15µg/L for rural individuals. On the other hand, the 95th percentile value of U-Cd was 1.14µg/L for total study individuals, 1.14µg/L for males, 1.11µg/L for females, 0.88µg/L for urban individuals and 1.14µg/L for rural individuals. The 95th percentile value of U-Cd for all study participants was higher than the human biomonitoring (HBM) value of 1µg/L [33], except for urban individuals. However, in urban individuals the 95th percentile value of U-Cd was still higher than the RV95 (0.8µg/L) [33].The results of 95th percentile values of U-iAs and U-Cd from present study were compared with international research work such as Germany, South Korea, Belgium, Flanders, USA, Canada and Malaysia, as presented in Table4. Present work found that the 95th percentile value of urinary arsenic was similar to the observation made in the general Canadian public (27µg/L, 95th percentile value) [34]. However, it was higher than the value found in German population (15µg/L) [35]. Additionally, the value of the 95th percentile found in the present study population was 9.6, 2.8, 4.4, 2, 3 and 1.8 times less than the 95th percentile value found in the population of Malaysia, Canada, South Korea, USA, Flanders and Belgium respectively[36-41].U-Cd 95th percentile value in the Indian population was about 2.5 and 1.3 times higher than those in Flanders and USA [41,37]. On the other hand, in the Indian population, U-Cd 95th percentile value was 2.7, 1.6 and 1.3 times lower than South Korea, Canada and Malaysia populations [38, 40, 42]. The discrepancies in values reported from international work might be explained by differences in environmental exposures as well as the unique diet of each country individuals. This was apparent in several works conducted globally, as the values in the present study were similar to those reported in other nations, but might also be higher or lower depending on the study site. These results suggested that the present study population had higher levels of As and Cd exposure compared to populations in some other countries, but lower levels compared to others. It is important to continue monitor and minimiz exposure to environmental sources of As and Cd to reduce the associated health risk.
Biswas et al. (2012, 2019) reported that urban residents of West Bengal(Kolkata City), India consuming arsenic contaminated (0.024-0.324mg/Kg) foodstuffs, that exceed the recommended limit of As in rice (0.2mg/Kg) by the European Union and Ministry of Health, although it was less than threshold limit (0.5mg As/Kg) of fresh matter for leafy vegetables by the Ministry of Health, Czech Republic Commission Regulation (EC) 2015/1006 [43, 3, 44].These levels of As ingested by urban residents of WB via foodstuffs were consistent with the levels found in food commodities (0.007-0.373mg/Kg) grown in arsenic-endemic areas of WB [45]. This suggests that contaminated food may be transported to urban centres, such as Kolkata city. Consequently, urban residents (28.97µg/L)had a relatively high 95th percentile value of U-iAs compared to rural residents (19.15µg/L). It is important to note that chronic exposure to low levels of As can also have adverse health effects. Studies had shown a dose-dependent risk of prostate cancer in populations consuming water containing As ranging from 0.33 to 16.23µg/L[46, 47]. Suppressed cognitive function was observed due to As exposure (mean 6.42µg/L) and endocrine dysfunction was found due to intake of water containing As content ≤1.7 to 15.5µg/L [48, 49]. The quantity and type of food consumed by individuals such as rice, vegetables, fish and seafood were directly related to the excretion of urinary As concentration [50, 51]. Lee et al. (2012) found significantly higher levels of urinary As among those individuals who consumed fish three days before providing the urine sample than those who did not consume fish [40].
In this study, U-iAs and U-Cd levels were higher in females than males, this difference was significant (p=0.001)only for U-iAs without creatinine correction. Similar results were reported for gender differences in other studies [52, 53]. Same patterns of occurrence were reported for urinary As in Korea [22], for U-Cd in Spain, Canada, Germany and Northern California [54-57]. The results might be influenced by various factors such as creatinine levels higher in males than in females[58]. Other factors like reproductive hormones and methylation of ingested As might also influence the levels of U-iAs in males and females [59]. However, we did not analyze the potential impact of gender-based dietary differences on U-iAs and U-Cd levels. It has been reported that women have higher Cd absorption rates due to depletion in iron levels during menstruation, that might resulted in higher U-Cd in women [54, 60, 61].
It was found that levels of both As and Cd were higher in females compared to males across all age groups, except for the age group of 60-69years for As and 40-49years for Cd. The difference in U-iAs levels between males and females was statistically significant for individuals <40years and those aged 50-59years (p=0.003 and p=0.05, respectively); Although the concentration variation trend was non-linear for both metals in males and females across all age groups. The highest levels of U-iAs were found in the age group of 50-59years for females and 60-69years for males, while the levels of U-Cd had the highest value in the age group of 60-69years for both males and females. In females, childbearing age influenced the arsenic excretion in urine [62]. Our findings also suggested that maternal age was positively associated with urinary As concentration, these results were consistent with previous research from Los Angeles and Canada [63, 64]. The influence of gender and age on urinary arsenic levels might provide valuable insight into differences in arsenic methylation efficiency between males and females, particularly during childbearing age. Additionally, these results suggested that maternal age and sex hormones might play an important role in arsenic methylation and could help to explain why females exhibit greater arsenic methylation efficiency than males. Cd has a long biological half-life(30years) in the renal system, which means it took longer time to eliminate from the body, leading to its accumulation over time, particularly in the kidney, liver, and bones [65-67]. Studies also reported high U-Cd in the urine of elder individuals than the younger ones [52,68].
Based on family income no significant difference was observed in levels of U-iAs among study individuals, although individuals of MIG and HIG had higher levels of U-iAs than those in LIG. These differences might be attributed to the variation in dietary habits and lifestyle behaviour. Stojsavljevic et al. (2019) studied difference in dietary intake and found elevated blood arsenic levels among individuals who consumed fish more than twice a week compared to those who consumed fish less than twice a week[69]. Gamble et al. (2005) and Steinmaus et al. (2005) reported that improper nutrition had a significant adverse impact on arsenic metabolism, while present study results could not align with their findings[70, 71]. Other factors, such as individual differences in one-carbon metabolism and the activity of arsenic methyltransferases, might be important for environmental arsenic exposure [72, 73]. Sometimes, inhibition in the activity of arsenic methyltransferases could also be responsible for a longer retention time of As in the body [74]. Therefore, economic status might play a role in the urinary excretion of arsenic, but other factors were also crucial in determining the level of exposure to environmental As. U-Cd levels were significantly (p=0.001) higher in LIG individuals than in merged MIG/HIG groups’ individuals. It seems individuals who belong to LIG might be at a higher risk of exposure to environmental Cd. These findings highlighted the importance of addressing socioeconomic inequalities. Studies reported that dietary intake of micronutrients such as zinc and iron plays an important role in Cd absorption among individuals exposed to environmental Cd [75, 76]. Bioaccumulation of Cd increase in the human body with a nutrition-deprived state and it was a potential cause of heavy metal toxicity prevalence in the under nutrition condition [77].
The study results found levels of urinary As and Cd were significantly (p=0.01; p=0.02, respectively) higher in females who were involved in cooking practice than those who were not involved in cooking practice. These findings suggested that cooking practice might be an important route of inhalation exposure to toxic air pollutants such as As and Cd in female participants. Among the female participants who were engaged in cooking practice, 64% were using clean fuel (LPG), while 36% were using solid biomass fuel (wood/dry leaf, kerosene and coal). These observations indicated that the burning of solid biomass fuel for cooking purposes had emitted As and Cd, which cause more exposure among female individuals who were using biomass as cooking fuel and resulted in elevated urinary As and Cd in them. Burning of arsenic-containing wood in open spaces or homes resulted in an increased concentration of As in the ambient air [78]. Jia et al. (2012) monitored burning of crop residue containing arsenic, release gaseous compounds of arsenic into the atmosphere[79]. Studies found that the absorption rate of Cd through the inhalation route was higher (25%) than through ingestion (5-10%) [18,19]. Furthermore, U-Cd(median 0.31-1.6µg/g creatinine) had been associated with negative effects on bone mineral density [80, 81].Gallagher et al. (2010) found positive association between breast cancer in female and concentration of U-Cd 0.37µg/g creatinine in them [82]. Likewise, urinary arsenic 3.4μg/L in females was associated with delivering a low birth weight baby [83].Therefore, present work observations recommended that measures should be taken to reduce exposure to As and Cd during cooking by use of ventilation systems or cooking in well-ventilated areas to minimize inhalation exposure mainly in females.
Study results have shown that smokers had higher levels of U-iAs and U-Cd than non-smokers. However, the difference was statistically significant only for U-iAs (p=0.0001), not for U-Cd. Passive exposure to tobacco smoke among females also caused elevated levels of U-iAs and U-Cd, but the difference was not significant. Other researchers also found non-significant differences in urinary arsenic levels between smokers and non-smokers [52, 84].It was important to note that other factors such as age and diet might also impact the results and the lack of significance did not necessarily rule out an association between smoking and levels of urinary As and Cd. Jarup et al. (2009) found that non-smoker and unexposed population had U-Cd higher than 0.5µg/g creatinine [85]. According to the Agency for Toxic Substances and Disease Registry (ATSDR,1993) smoking one cigarette might resulted in the ingestion of about 0.25µg of As [86]. Additionally, tobacco smoke might have a negative influence on arsenic methylation efficiency, especially in bidi smokers, where tar and nicotine were found in higher quantities compared to cigarette smoke [87]. Several studies had reported higher levels of urinary As and Cd in smokers than non-smokers [52, 88, 89].
Our study found that both male and female travelers had significantly (p=0.01) higher levels of U-iAs than their non-travelling counterparts, indicating that daily commuting in urban areas was a significant source of traffic-related pollution exposure. While traveler males and females also had higher levels of U-Cd, the difference was not significant. Interestingly, among females, those who were non-travelers had significantly higher levels of U-Cd compared to travelers (p=0.01). However, such results might be due to some other underlying reason such as age of non-traveler females. Importantly, there is currently limited literature on the association between U-iAs, U-Cd and traffic-related exposure making this study a valuable addition to public health research. Moreover, previous studies had reported the presence of considerable As in particulate matter (29 to 39µg/g; 2 to 29µg/g) from Kolkata city in West Bengal, reflecting the presence of As in the urban environment due to high traffic transport and industrial activities [90, 91]. Therefore, in addition to dietary intake of As and Cd from food, individuals who were commuting regularly for work or other purposes have a higher exposure to environmental As and Cd than those who stay at home or not travelled on a routine basis.
The study results clearly revealed that smokers and traveler individuals were at a significantly higher risk of exposure to As and Cd (p= 0.01 and 0.02, respectively). These findings suggested that both tobacco smoke and traffic pollution were potential sources of environmental exposure to As and Cd via inhalation route of exposure. Consequently, these individuals were at a greater risk of developing health issues due to low level chronic As and Cd exposure compared to non-smokers and non-traveler individuals. These results emphasized the importance of reducing exposure to environmental pollutants to mitigate the risk of adverse health outcomes associated with As and Cd exposure.
According to the Human Biological Monitoring Commission of the German Federal Environment Agency reference value 95 (RV95) represent the upper margin of background exposure in the general population to a given environmental toxin at a given time. Reference value was only used to establish the guideline value for the general population with increased body burden to environmental toxins and could not be used to evaluate the health-related criteria [92]. The German HBM Commission also defined human biomonitoring values (HBM I and HBM II) based on toxicology and epidemiology work, which could be useful for establishing health-related biological exposure limits. HBM I represents the concentration of environmental toxins in biological samples that do not pose any risk of adverse health effects for individuals in the general population [92]. On the other hand, HBM II represents the concentration of environmental toxins in biological samples that could pose an increased risk of adverse health effects in susceptible individuals within the general population [92]. These values provide a framework for assessing the potential health risks associated with exposure to environmental toxins. As per HBM Commission of the German Federal Environment Agency for U-Cd, the established HBM-I and RV95 were 1µg/L and 0.8µg/L respectively. Among present study individuals the estimated exposure was approximately 52% of the HBM-I and 65% of the RV95 [33]. However, based on gender and study sites the high-end exposed individuals (95th percentile) exceeded both the HBM and RV95 values for U-Cd. In the present study, 7.4% of the individuals had U-Cd levels above the recommended value (1µg/L) for human biomonitoring, while 13.5% had U-Cd levels above the reference value (RV95, 0.8µg/L) [33]. These prevalence percentages were similar (7.7%) to those reported by Munoz et al. (2022), while higher (4.9%) than those reported by Lopez-Herranz et al. (2016)[57,88].Gallagher et al. (2010) diagnosed breast cancer in females, who had U-Cd levels ≥0.37µg/g creatinine [82]. In this study, the mean level of U-Cd was 0.37µg/g for total individuals and urban individuals, which was consistent with the findings of Gallagher et al. (2010)[82]. These findings of the study highlighted that elevated levels of U-Cd in general populations could be a cause of concern.
The German HBM Commission has established a reference value (RV95) 27µg/L for U-iAs, the estimated exposure among the individuals in this study was approximately 53% of the RV95 [33]. However, no HBM value was established for As because it was a non-threshold carcinogen toxin. In the present study, 5% of the individuals had U-iAs levels above RV95. Literature work revealed that at lower levels U-iAs had been found positively associated with various health aspects. For instance, Wu et al. (2013) reported that U-iAs < 15.40µg/g creatinine was associated with an increased risk of urothelial carcinoma in males[93]. Powers et al. (2018)found a positive association between U-As (1.33µg/g creatinine) and restrictive lung function [94]. Profili et al. (2018) found a positive association between U-As 1.8-15.5µg/L and circulatory disease [95]. Wang et al. (2016) observed a link between low-level environmental arsenic exposure and unexplained male infertility (urinary arsenic levels 41.37µg/g creatinine in the case group and 1.79µg/g creatinine in the control group) [96].Gilbert-Diamond et al. (2016) observed that U-As 3.4µg/L was associated with delivering a low birth weight child [83]. The information from the literature survey on urinary arsenic was associated with health risks and hence the results of the present study (i.e. mean U-iAs 14.18µg/L; 9.73µg/g creatinine) emphasizes the importance of U-iAs monitoring and implementation of strategic efforts to minimize environmental emission of As in order to reduce the health risks among the West Bengal residents.
The present study indicated the presence of low-to-moderate environmental exposure to As and Cd among the residents of West Bengal, primarily high exposure was found in urban areas individuals and females. In European and other Asian countries HBM data had seen a reduction in levels of environmental toxins since they had implemented reduction measures for air pollutants after conducting the first HMB study. Such as USEPA had reduced W-As levels from 50μg/L to 10μg/L in January 2006. Therefore, the present study findings provided useful information about U-iAs and U-Cd for the residents of West Bengal that could help them to be aware of toxic metals’ body burden, this data could also be used for the new researchers in the field of health risk assessment studies and also would be helpful for the policymakers, so that appropriate exposure and risk reduction strategies could be executed.
Limitations
Our study had some limitations such as i) small sample size, ii) only total As was measured in urine instead of As speciation. Thus the interpretation of urinary As may be less accurate, iii) details for food consumption were not collected for depth information on dietary intake of As and Cd, iv) estimation of As and Cd was done by AAS instead of using a sophisticated and sensitive instrument such as inductively coupled plasma mass spectrometer (ICP-MS), v) As and Cd measured in urine only; which reflected recent exposure rather than hair and nails which could reflect chronic exposure vi) water samples were not taken for estimation of As and Cd.
5. Conclusions
In conclusion, results of the urinary arsenic and cadmium obtained from the present study provided information on the body burden of As and Cd in the study population and also about group of individuals who were at risk of high exposure of these toxic metals. Gender, age, family monthly income, cooking, travelling and smoking habits influenced urinary levels of As and Cd. Cooking, travelling and smoking were potential factors that had affected the levels of As and Cd in urine among the study individuals. Here, we did not conduct As speciation analysis hence further work should include analysis of organic and inorganic arsenic species. In addition to that, future studies should include dietary questionnaires to collect detailed information on food consumption. Individuals younger than 20years of age (children and adolescents) should be included in the study for more comprehensive information.
Statements and Declarations
Funding: The authors affirm that this manuscript was prepared solely with institutional funding and no additional external grants, funds or support were received in the course of its preparation.
Conflict of Interest: The Authors declare no conflict of interest.
Acknowledgement
The authors express their gratitude to the director in charge and officer in charge of the Indian Council of Medical Research-Centre for Ageing and Mental Health in Kolkata, India, for providing the necessary support to conduct this research. Additionally, the support and assistance extended by the department of environmental science at the University of Calcutta, West Bengal, India, in facilitating the execution of the study is deeply appreciated. The authors also wish to acknowledge their colleagues, fellow scholars and office staff for their valuable contributions.
References
- ATSDR Toxicological Profile for Cadmium. Atlanta, GA (2012).
- Biswas A, Deb D, Ghose A, et al. Dietary arsenic consumption and urine arsenic in an endemic population: response to improvement of drinking water quality in a 2-year consecutive study. Environ Sci Pollut Res 21 (2014): 609-619.
- Biswas A, Swain S, Chowdhury NR, et al. Arsenic contamination in Kolkata metropolitan city: perspective of transportation of agricultural products from arsenic-endemic areas. Environ Sci Pollut Res 26 (2012}: 22929-22944.
- Clemens S, Aarts MG, Thomine S, et al. Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18 (2013): 92-99.
- Cadmium dietary exposure in the European population. Euro Food Safety Authority 10 (2012): 2551-2588.
- Argos M, Kalra T, Rathouz PJ, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376 (2010): 252-258.
- Liao CM, Lin TL, Hsieh NH, et al. Assessing the arsenic-contaminated rice (Oryza sativa) associated children skin lesions. J Hazard Mater 176 (2010): 239-251.
- Kapp RW. Arsenic Toxicology (2018).
- Francesconi KA, Kuehnelt D. Determination of arsenic species: a critical review of methods and applications, 2000-2003. Analyst 129 (2004): 373-395.
- IARC Monographs on the Identification of Carcinogenic Hazards to Humans 184 (2004): 84-86.
- Das D, Bindhani B, Mukherjee B, et al. Chronic lowlevel arsenic exposure reduces lung function in male population without skin lesions. Int J Public Health 59 (2014): 425-574.
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, et al. Rejoinder: Arsenic exposure and prevalence of type 2 diabetes: updated findings from the National Health Nutrition and Examination Survey, 2003-2006. Epidemiol 10 (2009): 816-820.
- Wasserman GA, Liu X, Parvez F, et al. Arsenic and manganese exposure and children's intellectual function. Neurotoxicol 32 (2011): 450-457.
- Zhang C, Mao G, He S, et al. Relationship between long term exposure to low level arsenic in drinking water and the prevalence of abnormal blood pressure. J Hazard Mater 262 (2010): 1154-1158.
- Faroon O, Ashizawa A, Wright S, et al. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. Toxicological Profile for Cadmium. Agency for Toxic Substances and Disease Registry (US), Atlanta (2012).
- Xu Y, Morel FMM, Sigel A, et al. Cadmium: from toxicity to essentiality (2013).
- International Agency for Research on Cancer (IARC). Cadmium and Cadmium Compounds. IARC (2012).
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Cadmium. Update. Atlanta (1999).
- Reeves PG, Chaney RL. Bioavailability as an issue in risk assessment and management of food cadmium: A review. Sci Total Environ 398 (2010):13-19.
- Schwarz MA, Lindtner O, Blume K, et al. Cadmium exposure from food: the German LExUKon project. Food Additives & Contaminants: Part A 31 (2014): 1038-1051.
- FAO, World Health Organization, & WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Seventy-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization (2016).
- Park C, Hwang M, Kim H, et al. Early snapshot on exposure to environmental chemicals among Korean adults—results of the first Korean National Environmental Health Survey (2009-2011). Int J Hygiene Environ Health 219 (2016): 398-404.
- Banerjee S, Dhar S, Sudarshan M, et al. Investigating the synergistic role of heavy metals in Arsenic-induced skin lesions in West Bengal, India. J Trace Element Med Biol 75 (2023): 127103.
- Joardar M, Das A, Mridha D, et al. Evaluation of acute and chronic arsenic exposure on school children from exposed and apparently control areas of West Bengal, India. Exposure and Health 13 (2020): 33-50.
- Kumar A, Ali M, Kumar R, et al. High arsenic concentration in blood samples of people of village GyaspurMahaji, Patna, Bihar drinking arsenic-contaminated water. Exposure and Health 12 (2020): 131-140.
- Pandey PK, Yadav S, Pandey M. Human arsenic poisoning issues in central-east Indian locations: biomarkers and biochemical monitoring. Int J Environ Res Public Health 4 (2007): 15-22.
- Sysalova J, Spevackova V. A study of sample mineralization methods for arsenic analysis of blood and urine by hydride generation and graphite furnace atomic absorption spectrometry. Central Euro J Chemist 1 (2003): 108-120.
- Aprea MC, Apostoli P, Bettinelli M, et al. Urinary levels of metal elements in the non-smoking general population in Italy: SIVR study 2012-2015. Toxicol Lett 298 (2018): 177-185.
- Pino A, Amato A, Alimonti A, et al. Human biomonitoring for metals in Italian urban adolescents: data from Latium Region. Int J Hygiene Environ Health 215 (2012): 185-190.
- Sauerhoff ST, Grosser ZA, Carnrick GR. The determination of chromium and cadmium in urine by graphite furnace atomic absorption. Atomic Spectroscopy-Norwalk Connecticut 17 (1996): 225-228.
- Peake M, Whiting M. Measurement of serum creatinine-current status and future goals. Clin Biochemist Rev 27 (2006): 173-184.
- World Health Organization (WHO) Biological Monitoring of Chemical Exposure in the Workplace 1 (1999).
- Schulz C, Wilhelm M, Heudorf U, et al. Human biomonitoring commission of the German federal environment agency. Update of the reference and HBM values derived by the German human biomonitoring commission. Int J Hygiene Environ Health 215 (2011): 26-35.
- Saravanabhavan G, Werry K, Walker M, et al. Human biomonitoring reference values for metals and trace elements in blood and urine derived from the Canadian Health Measures Survey 2007-2013. Int J Hygiene Environ Health 220 (2017): 189-200.
- Wilhelm M, Ewers U, Schulz C. Revised and new reference values for some trace elements in blood and urine for human biomonitoring in environmental medicine. Int J Hygiene Environ Health 207 (2004): 69-73.
- Anual ZF, Sham MN, Ambak R, et al. Urinary concentrations of metals and metalloids in Malaysian adults. Exposure and Health 13 (2021): 391-401.
- Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention, Atlanta, GA (2009).
- Second Report on Human Biomonitoring of Environmental Chemicals in Canada 2009-2011; CDC (2009) Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention, Atlanta, GA (2013).
- Hoet P, Jacquerye C, Deumer G, et al. Reference values and upper reference limits for 26 trace elements in the urine of adults living in Belgium. Clin Chemist Lab Med 51 (2013): 839-849.
- Lee JW, Lee CK, Moon CS, et al. Korea National Survey for Environmental Pollutants in the Human Body 2008: heavy metals in the blood or urine of the Korean population. Int J Hygiene Environ Health 215 (2012): 449-457.
- Schoeters G, Colles A, Den Hon E, et al. The Flemish Environment and Health Study (FLEHS) Second Survey (2007-2011): Establishing Reference Values for Biomarkers of Exposure in The Flemish Population. Issues Toxicol 9 (2012): 135-165.
- Zurahamin FA, Sham MN, Ambak R, et al. Urinary concentrations of metals and metalloids in Malaysian adults. Exposure and Health 13 (2021): 391-401.
- Biswas A, Biswas S, Santra SC. Risk from winter vegetables and pulses produced in arsenic endemic areas of Nadia District: field study comparison with market basket survey. Bullet Environ Contamin Toxicol 88 (2021): 909-914.
- Commission Regulation (EC) 2015/1006 as regards maximum levels of inorganic arsenic in food stuffs. European Commission, Brussels (2015).
- Roychowdhury T, Uchino T, Tokunaga H, et al. Survey of arsenic in food composites from an arsenic-affected area of West Bengal, India. Food Chem Toxicol 40 (2013): 1611-1621.
- Bulka CM, Jones RM, Turyk ME, et al. Arsenic in drinking water and prostate cancer in Illinois counties: An ecologic study. Environ Res 148 (2016): 450-456.
- Roh T, Lynch CF, Weyer P, et al. Low-level arsenic exposure from drinking water is associated with prostate cancer in Iowa. Environ Res 159 (2017): 338-343.
- Edwards M, Hall J, Gong G, et al. Arsenic exposure, AS3MT polymorphism, and neuropsychological functioning among rural dwelling adults and elders: a cross-sectional study. Environ Health 13 (2014): 15.
- Pan WC, Seow WJ, Kile ML, et al. Association of low to moderate levels of arsenic exposure with risk of type 2 diabetes in Bangladesh. Am J Epidemiol 178 (2012): 1563-1570.
- Aguilera I, Daponte A, Gil F, et al. Biomonitoring of urinary metals in a population living in the vicinity of industrial sources: a comparison with the general population of Andalusia, Spain. Sci Total Environ 407 (2008): 669-678.
- Becker K, Schulz C, Kaus S, et al. German Environmental Survey 1998 (GerES III): environmental pollutants in urine of the German population. Int J Hygiene Environ Health 206 (2003): 15-24.
- Chaumont A, Voisin C, Deumer G, et al. Associations of urinary cadmium with age and urinary proteins: further evidence of physiological variations unrelated to metal accumulation and toxicity. Environmental Health Perspect 121 (2013): 1047-1053.
- Lindberg AL, Ekstrom EC, Nermell B, et al. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ Res 106 (2018): 110-120.
- Gunier RB, Horn-Ross PL, Canchola AJ, et al. Determinants and within-person variability of urinary cadmium concentrations among women in northern California. Environ Health Perspect 121 (2013): 643-649.
- Haines DA, Saravanabhavan G, Werry K, et al. An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007-2019. Int J Hygiene Environ Health 220 (2017): 13e28.
- Schwarz, Markus A, Oliver Lindtner, et al. "Cadmium exposure from food: the German LExUKon project." Food Additives & Contaminants 6 (2014): 1038-1051.
- Munoz JB, Lope V, de Larrea-Baz NF, et al. Levels and determinants of urinary cadmium in general population in Spain: Metal-MCC-Spain study. Environ Res 210 (2022): 112959.
- Cocker J, Mason HJ, Warren ND, et al. Creatinine adjustment of biological monitoring results. Occup Med 61 (2011): 349-353.
- Lee BK, Kim YH. Sex-specific profiles of blood metal levels associated with metal-iron interactions. Saf Health Work 5 (2014): 113-117.
- Julin B, Vahter M, Amzal B, et al. Relation between dietary cadmium intake and biomarkers of cadmium exposure in premenopausal women accounting for body iron stores. Environ Health 10 (2011): 105.
- Vacchi-Suzzi C, Eriksen KT, Levine K, et al. Dietary intake estimates and urinary cadmium levels in Danish postmenopausal women. PLoS One 10 (2015): e0138784.
- Lindberg AL, Kumar R, Goessler W, et al. Metabolism of low dose inorganic arsenic in a Central European population—influence of gender and genetic polymorphism. Environ Health Perspect 115 (2007): 1081-1086.
- Ettinger AS, Arbuckle TE, Fisher M, et al. Arsenic levels among pregnant women and newborns in Canada: Results from the Maternal-Infant Research on Environmental Chemicals (MIREC) cohort. Environ Res 153 (2017): 8-16
- Farzan SF, Howe CG, Chavez TA, et al. Demographic predictors of urinary arsenic in a low-income predominantly Hispanic pregnancy cohort in Los Angeles. J Expo Sci Environ Epidemiol 31 (2021): 94-107.
- Cadmium in food - Scientific opinion of the Panel on Contaminants in the Food Chain. EFSA J 980 (2009): 1-139.
- Nordberg GF, Fowler BA, Nordberg M, et al. Handbook on the Toxicology of Metals. 3rd ed. Burlington, MA: Academic Press. Elsevier (2007).
- Health risks of heavy metals from long-range transboundary air pollution. Copenhagen (2007).
- Sun H, Wang D, Zhou Z, et al. Association of cadmium in urine and blood with age in a general population with low environmental exposure. Chemosphere 156 (2012): 392-397.
- Stojsavljevic A, Borkovic-Mitic S, Vujotic L, et al. The human biomonitoring study in Serbia: background levels for arsenic, cadmium, lead, thorium and uranium in the whole blood of adult Serbian population. Ecotoxicol Environ Safety 169 (2019): 402-409.
- Gamble MV, Liu X, Ahsan H, et al. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect 113 (2005): 1683-1688.
- Steinmaus C, Carrigan K, Kalman D, et al. Dietary intake and arsenic methylation in a US population. Environ Health Perspect 113 (2005): 1153-1159.
- Brosnan J, Jacobs R, Stead L, et al. Methylation demand: a key determinant of homocysteine metabolism. Acta biochimica polonica 51 (2004): 405-413.
- Ueland PM, Holm PI, Hustad S. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med 43 (2005): 1069-1075.
- Vahter M.. Variation in human metabolism of arsenic. In: Chappell, W.R., et al. (Eds.), Arsenic Exposure and Health Effects. Elsevier (1999): 267-279.
- Kim K, Melough MM, Vance TM, et al. The relationship between zinc intake and cadmium burden is influenced by smoking status. Food Chem Toxicol 125 (2019): 210-216.
- Kippler M, Goessler W, Nermell B, et al. Factors influencing intestinal cadmium uptake in pregnant Bangladeshi women-a prospective cohort study. Environ Res 109 (2009): 914-921.
- Das A, Das A. Heavy metals in common food items in Kolkata, India. Euro-Mediterranean J Environ Integrat 3 (2018): 1-9.
- Vishwakarma YK, Tiwari S, Mohan D, et al. A review on health impacts, monitoring and mitigation strategies of arsenic compounds present in air. Cleaner Engineering Technol 3 (2021): 100115.
- Jia Y, Huang H, Sun G.-X, et al. Pathways and relative contributions to arsenic volatilization from rice plants and paddy soil. Environ Sci Technol 46 (2012): 8090-8096.
- Akesson A, Bjellerup P, Lundh T, et al. Cadmium-induced effects on bone in a population-based study of women. Environ Health Perspect 114 (2006): 830-834.
- Schutte R, Nawrot TS, Richart T, et al. Bone resorption and environmental exposure to cadmium in women: a population study. Environ Health Per 116 (2008): 777-783.
- Gallagher CM, Chen JJ, Kovach JS. Environmental cadmium and breast cancer risk. Aging (Albany NY) 2 (2010): 804-814.
- Gilbert-Diamond D, Emond JA, Baker ER, et al. Relation between in utero arsenic exposure and birth outcomes in a cohort of mothers and their newborns from New Hampshire. Environ Health Per 124 (2016): 1299-1307.
- Ikeda M, Moriguchi J, Ezaki T, et al. Smoking-induced increase in urinary cadmium levels among Japanese women. Int Arch Occup Environ Health 78 (2005): 533-540.
- Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238 (2009): 201-208.
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic. Atlanta: Agency for Toxic Substances and Disease Registry (1993): 57-73.
- Khan MM, Aklimunnessa K, Kabir MA, et al.Tobacco consumption and its association with illicit drug use among men in Bangladesh. Addiction 101 (2006): 1178-1186.
- Lopez-Herranz A, Cutanda F, Esteban M, et al. Cadmium levels in a representative sample of the Spanish adult population: the BIOAMBIENT.ES project. J Exposure Sci Environ Epidemiol 26 (2016): 471-480.
- Pan Y, Ding C, Zhang A, et al. Distribution of manganese, cobalt and molybdenum in blood and urine among general population in 8 provinces of China. Chinese J Prev Med 48 (2014): 784-790.
- Kar S, Nath B, Samal AC, et al. Arsenic in urban particulates-A case study in Kolkata metropolis. Curr Sci 90 (2006): 158-160.
- Samanta G, Chattopadhyay G, Mandal BK, et al. Air pollution in Calcutta during winter-A three-year study. Curr Sci (1998): 123-138.
- Ewers U, Krause C, Schulz C, et al. Reference values and human biological monitoring values for environmental toxins: Report on the work and recommendations of the Commission on Human Biological Monitoring of the German Federal Environmental Agency. Int Arch Occup Environ Health 72 (1999): 255-260.
- Wu CC, Chen MC, Huang YK, et al. Environmental tobacco smoke and arsenic methylation capacity are associated with urothelial carcinoma. J Formosan Med Assoc 112 (2018): 554-560.
- Powers M, Sanchez TR, Grau-Perez M, et al. Low to moderate arsenic exposure and respiratory health in American Indian Communities. Ann Am Thoracic Soci 15 (2018): S128-S129.
- Profili F, Nuvolone D, Barbone F, et al. Health effects among a cohort exposed to low-level arsenic in a geothermal area of Tuscany, Italy. Int Arch Occup Environ Health 91 (2018): 971-979.
- Wang X, Zhang J, Xu W, et al. Low level environmental arsenic exposure correlates with unexplained male infertility risk. Sci Total Environ 571 (2016): 307-313.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 