Comparative study of the effects of two types of organic fertilizers (R1 and R4) and a chemical fertilizer (NPK) on some physico-chemical and biochemical parameters of lettuce (Latuca sativa L.)
Yao N’zué Benjamin1*, Kpata-Konan Nazo Edith1, Combo Agnan Marie-Michel1, Sossia Kossan Jules1, Tano Kablan2
1Department of Agroforestry, University Jean Lorougnon Guédé, Daloa, Côte d'Ivoire
2Department of Food Science and Technology, University Nangui Abrogoua, Abidjan 02, Côte d'Ivoire
*Corresponding Author: Yao N’zué Benjamin, University Jean Lorougnon Guede, Bp 150 Daloa, Côte D’ivoire
Received: 13 July 2020; Accepted: 27 July 2020; Published: 31 July 2020
Article Information
Citation: Yao N’zué Benjamin, Kpata-Konan Nazo Edith, Combo Agnan Marie-Michel, Sossia Kossan Jules, Tano Kablan. Comparative study of the effects of two types of organic fertilizers (R1 and R4) and a chemical fertilizer (NPK) on some physico-chemical and biochemical parameters of lettuce (Latuca sativa L.). Journal of Food Science and Nutrition Research 3 (2020): 181-194.
DOI: 10.26502/jfsnr.2642-11000048
View / Download Pdf Share at FacebookAbstract
The objective of this study was to evaluate the effect of two types of organic fertilizer (R1 and R4) and a commercial chemical fertilizer (NPK) on the nutritional composition of lettuce. For each type of organic fertilizer (R1 and R4), two concentrations were used (30% and 50%, v/v). The treatments [R1 30%, R1 50%, R4 30%, R4 50%, control and chemical (NPK)] were randomly assigned to units of a homogeneous experimental design. Three replicates were carried out per treatment. Twelve parameters were analysed: lipids, proteins, carbohydrates, total sugars, reducing sugars, fiber, moisture, ash, pH, vitamin C, polyphenols, flavonoids. Analyses show that the level of ash is high (1.01 ± 0.03%) in organic fertilizer R4 50% compared with chemical fertilizer (0.50 ± 0.02%) and the control (0.81 ± 0.08%). The results also showed a remarkable increase in antioxidant compounds (polyphenols and flavonoids), fiber and carbohydrates in lettuce fertilized with organic fertilizer R4 50% (polyphenols: 87.18 ± 0.74 mg/100 g, flavonoids: 1.44 ± 0.01 mg/100 g, fiber: 3.23 ± 0.14% and carbohydrates: 8.03 ± 1.64%) compared with the chemical fertilizer (polyphenols: 14.13 ± 0.33 mg/100 g, flavonoids: 0.82 ± 0.04 mg/100 g, fiber: 2.04 ± 0.10% and carbohydrates: 5.12 ± 0.05%) and the control (polyphenols: 55.80 ± 0.53 mg/100 g, flavonoids: 0.98 ± 0.01 mg/100 g, fiber: 2.39 ± 0.07% and carbohydrates: 5.27 ± 0.29%). The high amounts of phytochemicals, minerals and antioxidants recorded in this research give preference to the use of organic rather than inorganic fertilizers. In view of the results obtained, organic fertilizer R4 50% should be recommended to market gardeners.
Keywords
<p>Lettuce, Biological Fertilization, Chemical Fertilization, Physico-Chemical Parameters, Biochemical Parameters</p> <gdiv></gdiv>
Article Details
1. Introduction
The consumption of fruits and vegetables is considered by many authorities as a public health issue and is the subject of nutritional recommendations by the FAO and WHO [1]. Consumers are increasingly aware of the health benefits of fruit and vegetable consumption. Three arguments underpin the health benefits of fruits and vegetables. First, they are important contributors to nutrient intake [2]. Second, they have a protective effect against major chronic diseases such as cardiovascular, neurodegenerative and metabolic diseases and cancers [1, 3]. Finally, they have a low energy content, an aspect that is becoming crucial today with the rapid increase in overweight and obesity [4].
Moreover, according to United Nations projections, the world population of about 6.3 billion people today will reach nearly 8 billion in 2030 [5]. Agricultural production will therefore have to face a triple challenge, meeting the growing needs of the world population while preserving the nutritional quality of the food and at the same time preserving the environment and natural resources [6]. As agricultural land has reached its limit in many countries, this triple challenge can be met mainly through varietal improvement and associated cultivation techniques, including fertilization [6]. However, the intense and continuous use of chemical fertilizers creates plants that are vulnerable to insect attacks, as well as the appearance of weeds [7]. The use of chemicals such as pesticides and herbicides is used to protect plants. Possible risks are related in particular to the presence of pesticide residues in fruits and vegetables [1]. The latter is raising growing public concern, with the effect that regulatory measures are gradually being tightened to reduce their use [8]. In addition, nitrogen fertilization increases nitrate accumulation in vegetables such as lettuce [9, 10]. Also, inorganic fertilizers are relatively expensive and can potentially contaminate the environment [11]. One of the strategies for improving soil fertility, especially ferralitic soils that are low in nitrogen and phosphorus, is the use of organic fertilizers [12]. This practice contributes to increasing the stock of organic matter and increasing the cation exchange capacity and consequently the level of soil fertility [13]. It has also been proven that the application of organic fertilizers to market garden soils improves soil structure, increases soil water and nutrient retention capacity, stimulates microbial activity and increases crop yields [14, 15]. In addition, the results of [16] showed that the use of organic fertilizer as a fertilizer resulted in an increase in lettuce plant weight and height, total chlorophyll content, soluble protein content and soluble sugar content compared to the use of chemical fertilizer.
The overall objective of this study is to compare the effect of organic and chemical fertilizers on the nutritional composition of lettuce with a view to proposing the most suitable for the cultivation of this vegetable. More specifically, it first measures the effect of two types of organic fertilizers (R1 and R4) and one chemical fertilizer (NPK) on the evolution of a few physico-chemical, biochemical and antioxidant parameters of lettuce. And secondly to select the most suitable for the cultivation of this vegetable.
2. Material and Methods
2.1 Material
2.1.1 Vegetal material: The plant material used in this work is lettuce (Lactuca sativa L.) harvested from the experimental site of the Jean Lorougnon Guédé University, Daloa, Côte d'Ivoire (Figure 1).
2.1.2 Fertilizers used: Organic fertilizers are derived from the anaerobic digestion of the following substrates:
R1: human urine + cow's purse + cassava effluent.
R4: human urine + cassava effluent.
All these substrates were put into digesters in order to produce biogas. The discharges or digestates were used as fertilizers on which these studies were based. For each type of organic fertilizer, two weight concentrations were used (30% and 50%, v/v). Thus, the fertilizers R1 30%, R1 50%, R4 30% and R4 50% were obtained. In addition to these organic fertilizers, a commercial chemical fertilizer (NPK) was used as a fertilizer.
2.2 Methods
2.2.1 Experimental device and field experiments: On a homogeneous plot, a completely randomized experimental design was used (Figure 2). This design consisted of 18 experimental units. The assignment of treatments (R1 30%, R1 50%, R4 30%, R4 50%, control and chemical) to these units was randomized. In addition, 3 replicates were performed per treatment.

Figure 1: Mature lettuce.
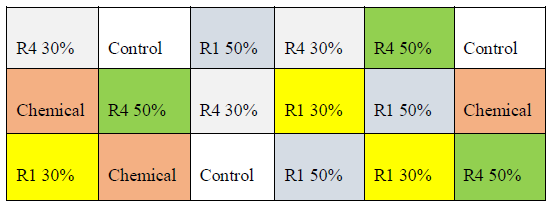
Figure 2: Experimental device.
2.2.2 Sampling: At maturity, eighteen (18) lettuce samples were taken randomly from the entire surface of the plot with three (3) samples per treatment. They were washed, wrapped in plastic film, stored in a cooler containing ice cubes and sent to the laboratory to measure the various physico-chemical, biochemical and antioxidant parameters of the lettuce.
2.2.3 Determination of Moisture and Dry Matter: The determination of moisture or dry matter was carried out by oven drying [17]. This method consists of evaporating the water contained in the raw material by drying in an oven at 105°C for 24 hours. Clean crucibles are dried in the oven and then cooled in a desiccator. The mass of the empty crucible is then measured. Five (5) g of lettuce contained in the crucible are then placed in an oven at 105 ± 2°C for 24 hours. The dried sample crucible assembly is cooled in the desiccator for 30 min. Then the mass of the crucible containing the dried sample of lettuce M2 is determined. The percentage of dry matter is calculated according to the following formula:
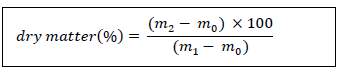
me: mass (g) of the sample
m1: mass (g) of the assembly (crucible + lettuce) before steaming
m2: mass (g) of the assembly (crucible + lettuce) after steaming.
2.2.4 Ashes: The ash content was determined by the AOAC method [17]. Mineral matter or ash is the residue obtained after the destruction of the organic substance by calcination. A silica crucible containing 5 g of lettuce is placed in a muffle furnace, previously heated at 550 ± 15°C for 12 hours. The resulting white ash is cooled with the crucible in a desiccator to room temperature and the crucible is weighed after cooling. The ash content is given by the following formula:
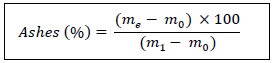
m0: mass (g) of the empty crucible
me: mass (g) of the sample
m1: mass (g) of the assembly (crucible + ashes) after incineration.
2.2.5 pH and titratable acidity: The pH of the sample was measured with a digital pH meter. Ten (10) g of lettuce were crushed in 50 mL of distilled water. The milled material is centrifuged at 3000 rpm for 30 min. The supernatant is collected in a jar and the pH is read on a digital display by dipping the pH meter electrode directly into the solution after calibrating the pH meter. The titratable acidity was determined according to the AOAC method [18]. This measurement is carried out by neutralisation of the total free acidity with sodium hydroxide solution (NaOH; 0.1 N). The neutralisation is monitored by means of a coloured indicator (phenolphthalein). The assay is stopped when the indicator turns pink/orange. Ten (10) g of lettuce are crushed in 50 mL of distilled water. The milled material is centrifuged at 3000 rpm for 30 min. The supernatant is collected in an Erlenmeyer flask, 3 drops of phenolphthalein are poured into the solution. The soda is added drop by drop until the solution turns pink/orange. The result is expressed by the following equation:
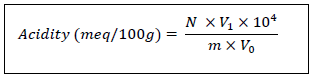
V1: Volume of NaOH paid at equivalence
V0: Volume of the test sample
m: Mass of fresh leaves
N: Normality of NaOH (0.1 N).
2.2.6 Total sugars and reducing sugars:
2.2.6.1 Total sugars: Total sugars were determined using the technique described by [19] using phenol and concentrated sulphuric acid. The ethanosoluble extract (100 µL) was collected and placed in a test tube. To this volume, 1 mL of phenol (5%, w/v) and 1 mL of concentrated sulphuric acid (97%) were added. The reaction medium was homogenized and allowed to cool for 5 min. The optical density reading was taken at 490 nm with the spectrophotometer against a control containing all products except the ethanosoluble extract. The optical density was converted to total sugars using the standard curve obtained from a glucose solution.
2.2.6.2 Reducing sugars: The reducing sugars was determined according to the method described by [20] using 3,5-dinitrosalicylic acid (DNS). A volume of 150 µl of ethanosoluble extract was taken and placed in a test tube. To this volume, 300 µL of DNS (3,5 dinitrosalicylic acid) was added. The mixture was brought to the boiling water bath for 5 minutes. Two milliliters of distilled water were added to the reaction medium after cooling for 5 min on the bench. The optical density reading was taken at 540 nm with the spectrophotometer against a control containing all the products except the ethanosoluble extract. The optical density was converted into the quantity of reducing sugars using a standard curve obtained from a glucose solution (1 g/L).
2.2.7 Fat: Lipids are extracted according to the method using SOXHLET [21]. The total lipids are extracted by hexane (organic solvent) from the lettuce grind. Ten (10) g of ground sample were introduced into a previously tarred and capped extraction cartridge and placed in the Soxhlet type extractor. Total lipid extraction was performed with 300 mL of hexane for 7 hours of boiling. Afterwards the hexane was evaporated using a rotary evaporator. Then the previously weighed extraction flask was dried in an oven at 100°C for 20 min and the whole (oil - flask) was weighed. The lipid content is determined by the following mathematical formula:
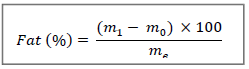
m0: mass (g) of the empty flask
me: mass (g) of the sample
m1: mass (g) of the assembly (flask + lipids) after evaporation.
2.2.8 Protein: The crude protein content is determined from the nitrogen content according to the Kjeldhal method [17]. It comprises a mineralization phase, followed by a distillation phase and a sulphuric acid titration phase in the presence of a colour indicator. First, a mass of 1 g of lettuce is weighed into a mineralization matra to which a pinch of the catalyst (selenium, copper sulphate (CuSO4) and potassium sulphate (K2SO4)) and 20 mL of concentrated sulphuric acid are added. Mineralization is carried out at 400°C for 2 hours in a digester. The resulting mineralization was cooled to room temperature and transferred to a 100 mL flask and made up with distilled water. After switching on the distiller, 10 mL of the mineralizate is withdrawn, to which 10 mL of NaOH (40%) is added. The mixture is placed in the tank of the distiller. The coolant extension is immersed in a beaker containing 20 mL boric acid with mixed indicator (methyl red + bromocresol green): the solution turns violet. Distillation is carried out for 10 minutes. At the end of the distillation, the solution turns green. The refrigerant extension is immersed in the beaker containing the distillate. The protein content is determined according to the following formula:
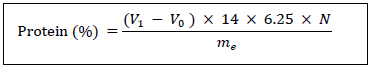
V0: volume (mL) of sulphuric acid solution poured for the blank test.
V1: volume (mL) of sulphuric acid solution poured for the test (sample).
N: normality of the sulphuric acid solution: 0,1.
me: mass (g) of the sample.
6.25: conversion factor of nitrogen to protein.
2.2.9 Fiber: The fiber content is determined according to the method of AOAC [17]. Two (2) grams of dried and crushed lettuce were homogenized in 50 mL of 0.25 sulphuric acid and boiled for 30 min and after 50 mL of 0.31N soda were added to the previous boiling mixture for another 30 min. The extract was then hot-filtered through a funnel with preweighed and residue-free filter paper. The residue was washed three times with hot distilled water and dried in an oven at 105°C for eight hours. The filter paper has been desiccated and weighed. It was calcined at 550°C for 3 hours in a muffle furnace. The ash was weighed. The fibre content is given by the following formula:
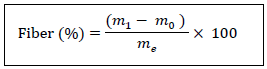
m1: mass (g) of the dried residue
m0: mass (g) of ash obtained
me: mass (g) of the sample.
2.2.10 Carbohydrates: Total carbohydrates as a percentage of fresh sample mass are obtained by the following relationship:
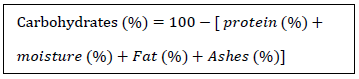
2.2.11 Vitamin C: The method used for the determination of vitamin C in our samples is that described by [22], which is based on the reduction of 2,6 DCPIP (2,6 dichlorophenol-indophenol) by this method. Ten (10) grams of samples are weighed and ground and then solubilized in 40 mL of metaphosphoric acid-acetic acid (2%; w/v). The resulting mixture is centrifuged at 3000 rpm for 20 min. The supernatant is introduced into a 50 mL volumetric flask and adjusted with boiled distilled water and cooled in the absence of air. A 10 mL test sample is placed in an Erlenmeyer flask and titrated with 2,6 DCPIP at 0.5 g/L to the persistent pink color. The 2.6 DCPIP solution is previously calibrated with a 0.5 g/L pure vitamin C solution. The vitamin C content of the sample is given in percent by the following expression:
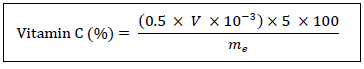
V: volume (mL) of 2,6 DCPIP poured at equivalency
me: mass (g) of the fresh leaf sample.
2.2.12 Extraction of phenolic compound: Phenolic compounds are extracted with methanol using the method of [23]. One (1) gram of ground sample is homogenized in 10 mL of methanol (70%; v/v). The resulting mixture is centrifuged at 1000 rpm for 10 min. The pellet is recovered in 10 mL of methanol (70%; v/v) and centrifuged again. The supernatants, collected in a 50 mL flask and adjusted with distilled water to the mark, constituted the total phenolic extract.
2.2.13 Determination of Total Phenols: The method of [23], using Folin-ciocalteu, was used to determine total phenols. The reagent consists of a mixture of phosphotungstic acid (H3PW12O40) and phosphomolybdic acid (H3PMo12O40). It is reduced during the oxidation of the phenols to a mixture of blue oxides of tungsten and molybdenum. The coloration produced is proportional to the quantity of polyphenols present in the plant extracts. One (1) mL of methanolic extract is introduced into a test tube. To the contents of the tube, 1 mL of Folin-ciocalteu reagent is added. The tube is allowed to stand for 3 min and then 1 mL of 20% (w/v) sodium carbonate solution is added. The contents of the tube are made up to 10 mL with distilled water. The tube is placed in the dark for 30 min and the OD is read at 725 nm against a blank. A standard range established from a stock gallic acid solution (1 mg/mL) under the same conditions as the assay is used to determine the amount of phenols in the sample.
2.2.14 Determination of Flavonoids: The determination of flavonoids was carried out according to the method described by [24], which is based on the principle that flavonoids react with aluminium chloride in the presence of potassium acetate to give a yellow complex whose intensity is proportional to the amount of flavonoids present in the medium. A volume of 0.5 mL of methanolic extract is introduced into a test tube. To the contents of the tube are added successively 0.5 mL distilled water, 0.5 mL 10% aluminum chloride (w/v), 0.5 mL 1 M potassium acetate, and 2 mL distilled water. The tube is allowed to stand for 30 min in the dark and the optical density (OD) is read at 415 nm against a blank. A standard range established from a quercetin stock solution (0.1 mg/mL) under the same conditions as the assay is used to determine the amount of flavonoids in the sample.
2.2.15 Statistical Analysis: All experiments were performed in triplicate and the averages of the data were statistically analysed using the STATISCA 7.1 software. A one-factor analysis of variance (ANOVA) was performed to compare the means. Differences were considered significant for p ≤ 0.05 values. To separate the different samples, multiple comparison tests (Tukey HSD) were conducted.
3. Results and Discussion
3.1 Results
3.1.1 Effects of fertilizers on the physico-chemical parameters of lettuce: The different treatments significantly influenced (p ≤ 0.05) the water content of the lettuce. However, according to Tukey's HSD test, treatments with the organic fertilizers R1 30%; R1 50% and R4 30% with respective contents of 92.15 ± 0.54%; 91.98 ± 0.09% and 91.49 ± 0.37% are statistically identical. The lowest moisture content (90.55 ± 0.20%) is obtained in lettuce treated with the organic fertilizer R4 50%. Treatment with chemical fertilizer gives a moisture content of 93.26 ± 0.07%, which is the highest. This is statistically identical to the control (92.99 ± 0.27%) (Table 1). In addition, the ash contents obtained with the organic fertilizer treatments R1 30% (0.72 ± 0.02%) and R1 50% (0.74 ± 0.01%) are statistically identical. These contents are also identical to that of lettuce, which has not undergone any treatment (0.81 ± 0.08%). Treatment with chemical fertilizer had the lowest level (0.50 ± 0.02%). Treatment with organic fertilizers R4 30% and R4 50% resulted in ash contents of 0.99 ± 0.01% and 1.01 ± 0.03% respectively. These contents are also identical according to the Tukey HSD test at the 5% threshold. The latter treatments gave the highest ash content (Table 1). Concerning the pH (Table 1), the organic fertilizers R1 30%, R4 50% and chemical fertilizers gave a pH of 6.80 ± 0.00. These treatments recorded the highest value. On the other hand, R1 50% and R4 30% have a pH of 6.76 ± 0.00 which is statically identical to the pH of the control (6.77 ± 0.01).
3.1.2 Effects of fertilizers on the lettuce antioxidant compounds
Treatments with the organic fertilizers R1 30%, R1 50%, R4 30% and R4 50% gave the same vitamin C level (3.75 ± 0.00 mg/100 g). Compared with the treatment with chemical fertilizer (2.5 ± 0.00 mg/100 g), it is higher with a difference of 1.25 mg/100 g. Compared with the control (3.78 ± 0.06 mg/100 g), the treatment with organic fertilizers is identical to that with the control (3.78 ± 0.06 mg/100 g). The latter is superior to the treatment with chemical fertilizer according to the Tukey HSD test at the 5% threshold (Table 2). Statistical analysis showed a significant difference (p ≤ 0.05) in the polyphenol content for the different processed lettuce samples (Table 2). The treatment had a strong influence on the polyphenol content of each sample. Each sample is independent. Treatment with chemical fertilizer gives the lowest polyphenol content (14.13 ± 0.33 mg/100 g). In contrast, treatment with organic fertilizer R4 50% gives the highest content (87.18 ± 0.74 mg/100 g). Like polyphenols, the analysis of flavonoid content showed a significant difference (p ≤ 0.05). Treatments with the organic fertilizers R1 50% and R4 30% with a content of 1.31 ± 0.02 mg/100 g and 1.32 ± 0.01 mg/100 g respectively are statistically identical according to the Tukey HSD test. Treatment with the chemical fertilizer gives the lowest flavonoid content (0.82 ± 0.04 mg/100 g). The highest level was obtained with the organic fertilizer R4 50% (1.44 ± 0.01 mg/100 g). Treatment of the lettuce samples with the organic fertilizer R1 30% resulted in a flavonoid content of 1.17 ± 0.04 mg/100 g. The control recorded 0.98 ± 0.01 mg/100 g as the mean flavonoid level.
3.1.3 Effects of fertilizers on the biochemical parameters of lettuce: Statistical analysis showed a significant difference (p ≤ 0.05) in the lipid content of the samples analysed. Apart from the sample treated with the chemical fertilizer with a value of 0.09 ± 0.01%, which is statistically identical to the control (0.1 ± 0.01%), the other samples are independent. The results are for R1 30% (0.11 ± 0.02%), R1 50% (0.13 ± 0.01%), and R4 30% (0.14 ± 0.01%). The lipid content is higher in the sample treated with the organic fertilizer R4 50% (0.16 ± 0.01%) (Table 3). Like lipids, the analysis of protein content showed a significant difference (p ≤ 0.05). The lowest protein content was recorded in the samples treated with chemical fertilizer (1.02 ± 0.01%), statistically identical to the samples treated with organic fertilizer R1 30% (1.03 ± 0.02%). The control gave a significantly lower protein content of 0.82 ± 0.06% than the others. The highest protein content was obtained in the samples treated with the organic fertilizer R4 50% (1.25 ± 0.05%). The protein contents obtained with the treatments with the organic fertilizers R1 50% and R4 30% are 1.11 ± 0.01% and 1.15 ± 0.06% respectively (Table 3). Carbohydrate analysis revealed a significant difference at the 5% cut-off in Tukey's HSD test. Of the six (6) treatments, four (4) were statistically identical at the 5% Tukey HSD threshold. These were control treatments (5.27 ± 0.29%); chemical fertilizer (5.12 ± 0.05%); R1 30% (5.70 ± 0.24%); and R1 50% (6.04 ± 0.11%) (Table 3). Only treatments with the organic fertilizer R4 30% (6.23 ± 0.43%) and R4 50% (8.03 ± 1.64%) differ at the 5% threshold according to the Tukey HSD test. In addition, statistical analysis shows that total sugar levels differ significantly (p ≤ 0.05) from one treatment to another (Table 3). Treatment with chemical fertilizer gives a total sugar level of 1.18 ± 0.02%, which represents the minimum content among the samples analyzed. It is statistically identical to the control (1.22 ± 0.03%). Treatments with the organic fertilizers R1 30%, R1 50% and R4 30% give total sugar contents of 1.46 ± 0.06%, 1.56 ± 0.04% and 1.62 ± 0.04% respectively, which are intermediate contents. The highest total sugar content (1.84 ± 0.04%) comes from treatment with the organic fertilizer R4 50%.
The reducing sugar content of lettuce is statistically identical regardless of the type of treatment (Table 3). The results are: control (0.14 ± 0.01%), R1 30% (0.13 ± 0.03%), R4 50% (0.13 ± 0.01%), R4 30% (0.12 ± 0.03%), R1 50% (0.15 ± 0.01%) and chemical fertilizer treatment (0.21 ± 0.16). Treatment therefore had no significant effect on the reducing sugar content of lettuce (P ≥ 0.05). Analysis of fiber content revealed a significant difference (p ≤ 0.05) (Table 3). The treatment therefore had a strong influence on the fiber content of each sample. The treatment with the organic fertilizer R1 30% was the lowest (1.97 ± 0.00%). When the treatment is carried out with the organic fertilizers R1 50% and R4 30%, the fiber contents are 2.63 ± 0.26% and 2.69 ± 0.19%, respectively, statistically identical to each other and to the control (2.39 ± 0.07%). Treatment with organic fertilizer R4 50% gives the highest fiber content (3.23 ± 0.14%). The chemical fertilizer gives a fiber content of 2.04 ± 0.10%.
|
Treatments |
Physico-chemical parameters |
||
|
Moisture (%) |
Ashes (%) |
pH |
|
|
Control |
92.99 ± 0.27b |
0.81 ± 0.08a |
6.77 ± 0.01b |
|
Chemical (NPK) |
93.26 ± 0.07b |
0.50 ± 0.02c |
6.80 ± 0.00a |
|
R1 30% |
92.15 ± 0.54a |
0.72 ± 0.02a |
6.80 ± 0.00a |
|
R1 50% |
91.98 ± 0.09a |
0.74 ± 0.01a |
6.75 ± 0.01b |
|
R4 30% |
91.49 ± 0.37a |
0.99 ± 0.01b |
6.76 ± 0.00b |
|
R4 50% |
90.55 ± 0.20c |
1.01 ± 0.03b |
6.80 ± 0.00a |
For each mean, the values with the same letters in the same column are statistically identical to the 5% threshold according to Tukey's HSD test.
Table 1: Effect of treatments on the physico-chemical parameters of lettuce.
|
Treatments |
Antioxidant compound (mg/100 g) |
||
|
Vitamin C |
Polyphenols |
Flavonoids |
|
|
Control |
3.78 ± 0.06a |
55.80 ± 0.53b |
0.98 ± 0.01c |
|
Chemical (NPK) |
2.5 ± 0.00b |
14.13 ± 0.33a |
0.82 ± 0.04b |
|
R1 30% |
3.75 ± 0.00a |
77.00 ± 1.10d |
1.17 ± 0.04d |
|
R1 50% |
3.75 ± 0.00a |
83.06 ± 0.61e |
1.31 ± 0.02a |
|
R4 30% |
3.75 ± 0.00a |
74.79 ± 0.77c |
1.32 ± 0.01a |
|
R4 50% |
3.75 ± 0.00a |
87.18 ± 0.74f |
1.44 ± 0.01e |
For each mean, the values with the same letters in the same column are statistically identical to the 5% threshold according to Tukey's HSD test.
Table 2: Effect of treatments on the antioxidant compounds of lettuce.
|
Treatments |
Biochemical parameters (%) |
|||||
|
Fat |
Protein |
Carbohydrates |
Total sugars |
Reducing sugars |
Fibers |
|
|
Control |
0.10 ± 0.01a |
0.82 ± 0.06d |
5.27 ± 0.29a |
1.22 ± 0.03a |
0.14 ± 0.01a |
2.39 ± 0.07a |
|
Chemical (NPK) |
0.09 ± 0.01a |
1.02 ± 0.01a |
5.12 ± 0.05a |
1.18 ± 0.02a |
0.21 ± 0.16a |
2.04 ± 0.10bc |
|
R1 30% |
0.11 ± 0.02ab |
1.03 ± 0.02a |
5.70 ± 0.24a |
1.46 ± 0.06b |
0.13 ± 0.03a |
1.97 ± 0.00b |
|
R1 50% |
0.13 ± 0.01bc |
1.11 ± 0.01ab |
6.04 ± 0.11a |
1.56 ± 0.04bc |
0.15 ± 0.01a |
2.63 ± 0.26a |
|
R4 30% |
0.14 ± 0.01cd |
1.15 ± 0.06bc |
6.23 ± 0.43ab |
1.62 ± 0.04c |
0.12 ± 0.03a |
2.69 ± 0.19a |
|
R4 50% |
0.16 ± 0.01d |
1.25 ± 0.05c |
8.03 ± 1.64b |
1.84 ± 0.04d |
0.13 ± 0.01a |
3.23 ± 0.14d |
For each mean, the values with the same letters in the same column are statistically identical to the 5% threshold according to Tukey's HSD test.
Table 3: Effect of treatments on biochemical parameters of lettuce.
4. Discussion
The treatment of lettuce with organic fertilizers significantly influenced its moisture content. However, the values obtained with these treatments are lower than those recorded with the chemical fertilizer and the control. Regardless of the treatment considered, the results are lower than those of [25] who, after removal of the outer leaves of a fresh iceberg lettuce, found 95.6 g water/100 g lettuce for the composition of the remaining edible part (83% by weight). This difference is probably due to the part of the plant used. Indeed, in our work the whole plant was used in contrast to [25] who only used the interior of the lettuce, which was fresher. In addition, bottom ash is the residue of inorganic compounds remaining after incineration of a sample containing organic substances of animal, vegetable or synthetic origin. These represent about 1 to 5% of the mass of a food on a wet basis. The ash results recorded in this study (0.5-1.01%), are lower than those of [26] who found an ash content of 2.82% when estimating heavy metal levels in green leafy vegetables. This could either be due to the method of cultivation or it could be plant-dependent, since the ash content they refer to here is generalized to leafy vegetables. Similarly in Bangladesh, [27] were able to achieve an ash content of 11.26 ± 0.3% in lettuce. In this case, it could be envisaged that this difference may be due to climate, cultural conditions and highly regionally dependent. Leafy vegetables contain different types of minerals that are very important for health [27]. The ash, therefore gives an overall perception of these minerals.With respect to pH (Table 1), the values are similar to those of [28] who, working on the absorption of copper and zinc in lettuce planted in lignite-based soils and in lignite bottom ash mixtures, found a pH of between 6.64 ± 0.78 and 6.89 ± 0.98. The results of this study showed that organically fertilized lettuce generally had higher antioxidant levels than lettuce under mineral fertilization and the control (unfertilized) for all samples. Treatments with the organic fertilizers R1 30%, R1 50%, R4 30% and R4 50% all gave the same vitamin C level (3.75 ± 0.00 mg/100 g). When comparing the vitamin C content obtained with organic fertilizers with the vitamin C content obtained with treatment with chemical fertilizer (2.5 ± 0.00 mg/100 g), it is higher with a difference of 1.25 mg/100 g. Compared with the control (3.78 ± 0.06 mg/100 g), the treatments with organic fertilizers are identical to the control. The latter is superior to the treatment with chemical fertilizer. The results of our work are contrary to the results obtained by [16]. These authors showed that the use of organic fertilizer increased the vitamin C content of lettuce by more than 12%. Moreover, the treatments had a strong influence on the polyphenol content of each sample. Treatment with chemical fertilizer gives the lowest polyphenol content (14.13 ± 0.33 mg/100 g). In contrast, treatment with organic fertilizer R4 50% gives the highest content (87.18 ± 0.74 mg/100 g). Like the polyphenols, the treatments significantly influenced (p ≤ 0.05) the flavonoid content of all samples (p ≤ 0.05). Treatments with the organic fertilizers R1 50% and R4 30%, with a content of 1.31 ± 0.02 mg/100 g and 1.32 ± 0.01 mg/100 g respectively, are statistically identical in the Tukey HSD test. Treatment with the chemical fertilizer gives the lowest flavonoid content (0.82 ± 0.04 mg/100 g). The highest level of flavonoids was obtained with the organic fertilizer R4 50% (1.44 ± 0.01 mg/100 g). Treatment of the lettuce samples with the organic fertilizer R1 30% gave a flavonoid content of 1.17 ± 0.04 mg/100 g. The control recorded 0.98 ± 0.01 mg/100 g as the mean flavonoid level. These results are consistent with the findings of [29], who found that tomatoes treated with organic fertilizers had higher antioxidant content than those treated with mineral fertilizers and controls. Similarly, [30] found in their work a higher antioxidant activity of 5.5 mmol TE/100 g of fresh jujubes under organic fertilization, while this was only 4 mmol TE/100 g of fresh jujubes under mineral fertilization. The increase in secondary metabolites and antioxidant potential may be caused by the presence of major and minor elements in organic fertilizers whereas mineral fertilizer contains only three basic mineral elements (nitrogen, potassium and phosphorus) [31]. With respect to the effect of fertilizers on the biochemical parameters of lettuce, statistical analysis showed a significant difference (p ≤ 0.05) in the lipid contents of the samples analysed. Apart from the sample treated with the chemical fertilizer with a value of 0.09 ± 0.01%, which is statistically identical to the control (0.1 ± 0.01%), the other samples are independent. The results are for R1 30% (0.11 ± 0.02%), R1 50% (0.13 ± 0.01%), and R4 30% (0.14 ± 0.01%). The lipid content is higher in the sample treated with the organic fertilizer R4 50% (0.16 ± 0.01%). These results are lower than those of [32] who found a fat content of 0.48 ± 0.2 g/100 g in leaf samples of Amaranthus spinosus L. The results of this study are in agreement with those of [33] who found a level of 0.14 g per 100 g of lettuce, as lettuce is a very low-fat vegetable. Protein content is low in our samples compared to that obtained by [32] in leaves of Amaranthus spinosus L. (7.90 ± 0.01%). Indeed, the lowest protein level is recorded in the samples treated with chemical fertilizer (1.02 ± 0.01%), statistically identical to the samples treated with organic fertilizer R1 30% (1.03 ± 0.02%). The control gave a protein content of 0.82 ± 0.06%, which was significantly lower than the others. The highest content was obtained in the samples treated with organic fertilizer R4 50% (1.25 ± 0.05%). The intermediate contents are obtained with the organic fertilizers R1 50% and R4 30% 1.11 ± 0.01% and 1.15 ± 0.06% respectively. The results of the present study are in agreement with those of the [5], which in one of its reports found a value of 1.1 g of protein per 100 g of lettuce. Similarly, these values are close to those of [33], which mentions 0.9 g of protein per 100 g of lettuce as the standard value. For carbohydrates, the analysis revealed a significant difference. Of the six (6) treatments, four (4) were statistically identical (5.27 ± 0.29%; 5.12 ± 0.05%; 5.70 ± 0.24%; 6.04 ± 0.11%). Only the treatments with the organic fertilizer R4 30% (6.23 ± 0.43%) and R4 50% (8.03 ± 1.64%) differ. These results are higher than those of [33], which have a value of 2.96 g/100 g of lettuce. Similarly, [25] in their work on lettuce found a carbohydrate level of 1.9 g/100 g when they removed the outer part. This difference could be due to plant variety, amount of sunlight and water supply. Indeed, carbohydrates are formed naturally during photosynthesis. In addition, statistical analysis shows that total sugar levels differ significantly (p ≤ 0.05) from one treatment to another. Treatment with chemical fertilizer gives a total sugar content of 1.18 ± 0.02%, which represents the minimum content among the samples analyzed. It is statistically identical to the control (1.22 ± 0.03%). Treatments with the organic fertilizers R1 30%, R1 50% and R4 30% give total sugar contents of 1.46 ± 0.06%, 1.56 ± 0.04% and 1.62 ± 0.04% respectively, which are intermediate contents. The highest total sugar content (1.84 ± 0.04%) comes from treatment with the organic fertilizer R4 50%. The use of organic fertilizer improves the total sugar content of lettuce by 33% when comparing the R4 50% treatment (1.84 ± 0.04%) to the control treatment (1.22 ± 0.03%). This is consistent with the results of [34] who found a 21% improvement. On the other hand, analysis of fiber content revealed a significant difference (p ≤ 0.05). The treatment therefore had a strong influence on the fiber content of each sample. The treatment with organic fertilizer R4 50% gives the highest fiber content (3.23 ± 0.14%). The result obtained with the organic fertilizer R4 50% is identical to that of [32] who obtained a fiber content (3.20 ± 0.2%) in the leaves of Amaranthus spinosus L.
5. Conclusion
The objective of this work was to select, if possible, the type of fertilizer that gives the best results in terms of nutritional qualities for the production of lettuce, based on relevant parameters. Three (3) nutritional parameters were measured or calculated. These are biochemical parameters (lipids, proteins, carbohydrates, total sugars, reducing sugars and fibre), physico-chemical parameters (moisture, ash, pH and acidity) and antioxidant compounds (Vitamin C, polyphenols and flavonoids). The analyses carried out have indicated that not all the parameters defined have had the same levels of discrimination and either cannot be used for selection or may lead to selection. For example, acidity, pH and non-discriminating reducing sugars cannot be used to make an input selection. The control soil indicates that there is no need for chemical or organic fertilizers. For organic fertilizers, their use will be an alternative to that of chemical fertilizers. Apart from these three (3), organic fertilizer R4 at 50% is the best. These results conclusively suggest that lettuce is a good source of water, minerals, and vitamin C. The high amounts of phytochemicals, minerals and antioxidants recorded in this research give preference to the use of organic rather than inorganic fertilizers. Therefore, the use of organic fertilizers in lettuce cultivation should be encouraged for better nutritional quality. Organic fertilizer R4 50% should therefore be offered to market gardeners.
References
- Marie-Jo A, Caillavet F, Causse M, et al. Fruits and vegetables in the diet. Stakes and determinants of consumption. Collective scientific expertise, summary of the report, INRA (France) (2007): 80.
- Tinjaka MAM. Effect of electron beam irradiation on quality and shelf life of Tommy Atkins mango (Mangifera indica L.) and blueberry (Vaccinium corymbosum L.). Thesis Submitted to the Office of Graduate Studies of Texas A&M University (2005): 307.
- Djioua T. Improving the preservation of 4th range mangoes by applying heat treatments and using storage in a modified atmosphere. Doctoral thesis University of Avignon and the Pays de Vaucluse (2010): 169.
- Grimplet J. Functional genomics and quality markers in apricot. Doctoral thesis, National Polytechnic Institute of Toulouse (2004): 253.
- Food balance sheets. Manuel (2003): 96.
- Latiri K. Fertilization: Fertilizers and agricultural production. Workshop on the management of potassium fertilization, research findings and perspectives, Tunis December 10 (2002): 9.
- Lavinia W, Harrie O. The effluent: fertilizer par excellence. A study on the results and uses of the effluent. Hivos People Unlimited (2014): 52.
- Chayma O. Effects of UV-C radiation on the response of romaine lettuce Lactuca sativa var Claudius to biotic and abiotic constraints. Co-supervised doctoral thesis: University of Tunis el Manar, Faculty of Sciences of Tunisia Department of Biological Sciences, University of Avignon and the Pays de Vaucluse, Agrosciences Center of Avignon (2014): 178.
- Van Der Boon J, Pieters JH, Slangen JHG, et al. The effect of nitrogen fertilization on nitrate accumulation and yield of some field vegetables. In Eds.: Lambers H, Neeteson JJ, Stulen I. Fundamental, Ecological and Agricultural Aspects of Nitrogen Metabolism in Higher Plants, Martinius Nijhoff, Boston, États-Unis (1986): 489-492.
- Vermeulen J, Delvaux J, Vlassak K. Effet de la fumure azotée et des conditions de croissance sur la teneur en nitrate des légumes à feuilles. Revue Agriculture 40 (1987): 879-893.
- Masarirambi MT, Mandisodza FC, Mashingaidze AB, et al. Influence of plant population and seed tuber size on growth and yield components of potato (Solanum tuberosum). International Journal of Agriculture and Biology 14 (2012): 545-549.
- Koné B, Oikeh S, Diatta S, et al. Response of interspecifics and sativa upland rice to Mali phosphate rock and soluble phosphate fertilizer. Journal archives of Agronomy and Soil Science 57 (2010): 421-434.
- Weber J, Karczewska A, Drozd J, et al. Agricultural and ecological aspects of sandy soil as affected by the application of municipal solid waste composts. Soil Biology and Biochemistry 39 (2007): 1294-1302.
- Kowaljow E, Mazzarino MJ. Soil restauration in semarid Patagonia: chemical and biological response to different compost quality. Soil Biology and Biochemistry 39 (2007): 1580-1588.
- Amadji GL, Saïdou A, Chitou L. Recycling of residues in compost to improve coastal sandy soil properties and cabbage shoot yield in Bénin. International Journal of Biological and Chemestry Sciences 3 (2009): 192-202.
- Zhang E, Lin L, Liu J, et al. The effects of organic fertilizer and inorganic fertilizer on yield and quality of lettuce. Advances in Engineering Research 129 (2017): 907-910.
- Official methods of analysis. 15th Edition, Association of Official Analytical Chemists, Washington, DC, USA (1990): 200-210.
- Official methods of Analysis of AOAC International, 17th Edn. AOAC International Washington, D C (2000): 2200.
- Dubois M, Gilles KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Division of biochemistry 28 (1956): 350-356.
- Bernfeld P. Amylase β and α (Assay method). In Eds.: Colowick SP, Kaplan N. Methods in enzymology I. Academic, New York (1955): 149-154.
- Collection of French standards. Quality control of dairy products. Afnor, Paris-la-Defense (1986): 1030.
- Pongracz G, Weiser H, Matzinger D. Tocopherols- Antioxydant. Fat Science Technology 97 (1971): 90-104.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidant substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymological 299 (1999): 152-178.
- Meda A, Lamien CE, Romito M, et al. Determination of total phenolic, flavonoid and proline in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry 91 (2005): 571-577.
- Holland B, Unwin JD, Buss DH. Vegetables, herbs and spices: Fifth supplement to Mc Cance and Widdowson’s. The Composition of Foods, London (1991): 146.
- Prisacaru AE, Apostol LC, Ropciuc S. Estimation of heavy metal levels in green leafy vegetables purchased from suceava. Journal of Faculty of Food Engineering 16 (2017): 234-238.
- Kamal K, Tanvir M, Md AR. Chemical Composition of Some Leafy Vegetables of Bangladesh. Dhaka University Journal Sciences 61 (2013): 199-201.
- Suthep S, Prat I, Pisit V, et al. Uptake of Copper and Zinc in Lettuce (Lactuca sativa L.) Planted in Sida Soil and Lignite Bottom Ash Mixtures. Naresuan University Journal: Science and Technology 3 (2016): 31-42.
- Abdoulaye S, Christophe D, Moumouni K, et al. Influence of organic and mineral fertilizers on the antioxidants and total phenolic compounds level in tomato (solanum lycopersicum) var. mongal f1. Journal of Experimental Biology and Agricultural Sciences 4 (2016): 414-420.
- Wu CS, Gao QH, Kjelgren RK, et al. Yields, Phenolic Profiles and Antioxidant Activities of Ziziphus jujube Mill. In Response to Different Fertilization Treatments. Molecules 18 (2013): 12029-12040.
- Mohd IH, Hawa JZ, Ehsan K, et al. Impact of Organic and Inorganic Fertilizers Application on the Phytochemical and Antioxidant Activity of Kacip Fatimah (Labisia pumila Benth). Molecules 18 (2013): 10973-10988.
- Mofunanya AAJ, Ebigwai JK, Bello OS, et al. Comparative Study of the Effects of Organic and Inorganic Fertilizer on Nutritional Composition of Amaranthus spinosus L. Asian Journal of Plant Sciences 14 (2015): 34-39.
- Food composition databases (2015).
- Chen JH. The combined use of chemical and organic fertilizers and /or biofertilizer for crop growth and soil fertility. National Chung Hsing University, Taiwan (2006): 11.


 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 