Gelatine, present use and future applications: Decryption of a high-value multi-purpose by-product of the agro-food industry
Mouna Ambli, Barbara Deracinois, Rozenn Ravallec, Benoit Cudennec† and Christophe Flahaut†*
UMR Transfrontaliere BioEcoAgro N° 1158, Univ. Lille, Univ. Artois, INRAe, Univ. Liège, UPJV, YNCREA, Univ. Littoral Côte d'Opale, ICV-Institut Charles Viollette, F-59000 Lille and F-62300 Lens, France
†Contributed equally
*Corresponding Author: Dr. Christophe Flahaut, Université d’Artois, Faculté des Sciences Jean Perrin, Rue Jean Souvraz, 62300 Lens, France
Received: 12 September 2023; Accepted: 22 September 2023; Published: 08 December 2023
Article Information
Citation: Mouna Ambli, Barbara Deracinois, Rozenn Ravallec, Benoit Cudennec and Christophe Flahaut. Gelatine, present use and future applications: Decryption of a high-value multipurpose by-product of the agro-food industry. Journal of Food Science and Nutrition Research. 6 (2023): 179 - 207.
DOI: 10.26502/jfsnr.2642-110000144
View / Download Pdf Share at FacebookAbstract
According to a report from the United Nations on June 17th, 2019, the world population will increase by 2 billion people by 2100, resulting in a global population of nearly 11 billion people. How to achieve a sufficient and healthy diet while limiting the environmental impact of the food industry? Certain solutions emerge such as the valorisation of the circular economy and in particular the valorisation of collagen/gelatine-derived by-products. The novelty of this review lies in the integrative nature of the presentation of collagen and its derivatives such as gelatine and gelatine hydrolysates. It gathers critical insights into the subject that often do not appear in this genre of literature. This review therefore includes the historical context of its use, their physicochemical properties, the endogenous production of collagen. An entire chapter is dedicated to the different extraction and analysis methods currently available. The last chapter is devoted to the various scopes of application of bioactive peptides arising from collagen hydrolysis, varying from anti-oxidant, anti-hypertensive uses, as well as the numerous applications for the regulation of glucose metabolism.
Keywords
<p>Bioactive peptides, Collagen-derived peptides, DPP-IV inhibitory peptides, Food protein digestion, Gelatine hydrolysates, Glucose metabolism, Type 2 diabetes</p>
Article Details
Introduction
To meet the needs of the food and feed sectors, global demand for protein is expected to increase by approximately 40% by 2030, representing a 7% annual increase [1]. To meet this strong demand while limiting the economic and ecological impacts, alternatives such as the use of by-products from the vegetables, fishing, or meat industry would constitute significant protein resources that are presently still underexploited.
In the global functional food market and in light of societal developments and climatic change, the demand is mainly for so-called natural, non-ultra-processed products, with low environmental impacts and among these, collagen peptides have become a high priority must for many consumers. According to a new report from Grand View Research, Inc., the global collagen market is expected to reach 5.87 billion Euros by 2025, with a compound annual growth rate (CAGR) of 6.5% over the forecast period. The interest in collagen peptides generated from gelatine lies in their bioavailability and ease of digestion. They have many applications in health care, such as incorporation in designed formulas or as a powder that is to be diluted. Collagen peptides do not contain hormones, chemicals, or antibiotics. Their innocuousness is also one of the arguments for their consumption, whether by people who are ill or people concerned about their health. They are used by athletes for joint and tendon health during their daily workouts [2]. The increasing prevalence of several diseases such as obesity and diabetes [3], as well as a host of pathologies associated with metabolic syndrome, has led to a surge in demand for natural food sources containing such bioactive peptides as an early treatment option [4]. Bioactive peptides are used in a wide range of therapeutic areas, from alteration of the immune system to the gastrointestinal system to antihypertensive uses [5]. Indeed, the market for bioactive peptides, worth USD 48.65 billion in 2020, continues to grow and is expected to reach USD 95.71 billion by 2028, at a CAGR of 8.86% between 2021 and 2028 [6].
The novelty of this review lies in its propensity to discuss collagen under integrative aspects, which have often been eluded to date. First of all, via its historical use in ancient Egypt, Chinese traditional medicine, as well as in more contemporary times in Europe, to finally describe the different uses in our current societies. Then, the fundamental aspects of its structure and physicochemical properties, as well as the sources and methods of extraction, are addressed. Additional novelty is added regarding the methods of identifying peptides generated by the hydrolysis of food matrix to understand all the processes involved in the exploitation of such products, which are often hard to identify exhaustively in complex mixtures. Finally, a section on the bioactivities of collagen hydrolysates shows the versatility of their uses in today's and tomorrow's health, with the significant added value of these products, which is often overlooked in contemporary therapeutic arsenals, such as in glucose metabolism management for instance.
Collagen state of the art: History, structural, and physicochemical properties, biosynthesis, industrial processes, and different sources
Historical use of collagen: According to the literature, collagen and especially its hydrolysed form gelatine have been used by humans for thousands of years. In ancient Egypt, gelatine was extracted by boiling animal skins such as cowhides and bones [7]. This method produced glue that was then used for the confection of clothes and supplies [8], but it has also been found in coloured wall paintings dating back 4400 years [9]. In addition to this use, the consumption of gelatine extracted from bone broths was also a common practice in everyday life. The culinary use of gelatine quickly became commonplace in medieval Baghdadi culture, being incorporated into many preparations from the early 10th century [10]. In China, its use as an adhesive agent can be traced back to 1980-1450 BCE [11]. From a medicinal point of view, oriental and particularly traditional Chinese medicine describes the use of gelatine as far back as 771 BC [12]. The use of gelatine in the medical field was for a long time confined to China, Japan, and some East Asian countries.
It was not until the beginning of the 19th century that the extraction and use of gelatine were introduced in the Western world. First of all, in France, Denis Papin invented a more efficient method of extracting gelatine. Then, the English chemist Charles Hatchett carried out a further study on the use of acids on bones in 1806. From there, he developed a patent for the application, and it was used on a large scale from 1812 onward in Paris for food purposes. The methodology used at the time was divided into six stages. Two acid macerations (the first with hydrochloric acid) for 24 hours, then washing with cold water, followed by immersion in boiling water, a sodium carbonate solution, and finally washing with cold water until neutral and drying in an oven [13]. The first use of gelatine in the Western world for medical purposes was documented in France in the 19th century, with the use of gelatine as an excipient in the oral administration of bitter medicines such as syphilis treatments, pioneered by the pharmacist François Mothes, who filed a patent for the invention of the gelatine capsule in 1833 [14]. The dietary value of gelatine has been discussed at length by chemists, researchers, nutritionists, and others. It was used during the siege of Paris in 1870 by the Prussians when the resulting famine had to be contained. It was also prescribed in hospices to feed the most destitute and the sick in hospitals [13]. Although the nutritional value of gelatine has been debated for almost three centuries, at present, the recycling of various waste from the food industry [15-19] is leading to study of its physiological effects in greater depth, beyond the purely nutritional aspect.
Collagen and the gelatine derived from it are sources of very high added value that can be found in several fields of application. In medical areas, collagen films are used as a drug delivery system for infected corneal tissue [20,21], as well as in tissue engineering for wound healing [22] and cosmetology/dermatology [5,23]. Collagen solutions have significant advantages, notably their low production cost, and the large quantities indirectly produced by the food industry, as well as their bioavailability, biocompatibility, and lack of adverse effects [24].
Structural description: Collagens are water-soluble proteins present in connective tissues, skin, tendons, and bones. They are the most abundant fibrillar structural proteins, as they constitute up to 25% to 35% of the total proteins of the body [25]. The amino acid sequences of collagens and their structures are highly conserved in mammals. There are different types of collagen, and 29 are currently enumerated, of which the most abundant is type I collagen, which is the predominant form in the human body [26]. Up to now, the 29 types of collagens in vertebrates have been classified by their function and domain homology, as represented in table 1. They can be divided into fibrils, networks, beaded filaments, anchoring fibrils, and collagen associated with interrupted triple helix (FACIT) fibrils. Of these, the most common form is fibrillar collagen, which is present in most connective tissues [27].
Collagen conformation can be divided into four structures: the amino acid triplet Gly-Pro-Hyp, forming the primary structure; the secondary structure (the alpha-chain); the tertiary structure (triple helix conformation formed by two alpha-1 and one alpha-2 collagen chains); and the quaternary structure (fibrils) forming collagen fibres [28]; as illustrated in figure 1.
The conformation of collagen can be divided into four structures: the amino acid triplet Gly-Pro-Hyp, which forms the primary structure; the secondary structure (the alpha chain); the tertiary structure (triple helix conformation consisting of two alpha-1 and one alpha-2 collagen chains; and the quaternary structure (fibrils), which form the collagen fibres. GLY: glycine, PRO: proline and HYP: hydroxyproline. Illustration created with BioRender.com
The structure of the collagen alpha chain is determined by the amino acid sequence. Out of the 20 proteinogenic amino acids that can be found in collagen, glycine, proline, and hydroxyproline are the most frequent [29]. Indeed, the alpha chain consists of the repeating unit Gly-X-Y, where X is often proline (Pro) and Y is often hydroxyproline (Hyp). The content of proline and hydroxyproline is approximately 30% in mammalian gelatine, 22%–25% in warm water fish gelatine (Tilapia and Nile perch), and 17% in cold water fish gelatine (cod) [30,31]. Comparative studies on the rheological properties of terrestrial mammalian and fish gelatine concluded that the differences observed are due to the content of the specific imino acids, proline and hydroxyproline, which stabilise the conformation when gelatine forms a gel. Thus, a lower content of proline and hydroxyproline results in a less compact gel with a lower melting temperature [32-35]. Therefore, it is important to keep in mind the composition of the collagen molecule and also post-translational modifications (PTMs) such as hydroxylation of prolines, in order to make relevant and comprehensive identifications of the peptides generated following hydrolysis, using bioinformatic tool features accordingly.
|
Type |
Molecular form |
Tissue distribution |
Function |
|
I |
Fibrils |
Noncartilaginous connective tissues, e.g., skin, bone, tendon, dentin, ligaments, cornea |
Structural component |
|
II |
Fibrils |
Hyaline cartilage, vitreous body, nucleus pulposus |
Structural component |
|
III |
Fibrils |
Idem as type I |
Structural component |
|
IV |
Network |
Basement membranes |
Presynaptic organizer |
|
V |
Fibrils |
Idem as type I |
Structural component |
|
VI |
Beaded filaments |
Muscle, skin, cartilage, placenta, lungs, vessel wall, vessel |
Structural component |
|
VII |
Anchoring fibrils |
Skin, dermo–epidermal junction, oral mucosa, cervix |
Retaining dermal–epidermal adhesion |
|
VIII |
Network |
Descemet’s membrane |
Structural and signaling component |
|
IX |
FACIT |
Idem as type II |
Retaining tissue integrity |
|
X |
Network |
Hypertrophic cartilage, growth plate |
Regulating endochondral ossification |
|
XI |
Fibrils |
Idem as type II |
Structural component |
|
XII |
FACIT |
Tendon, skin, periodontal ligament |
Not clear |
|
XIII |
Transmembrane |
Epidermis, hair follicle, endomysium, intestine, chondrocyte, lung, liver, dermo–epidermal junction |
Regulating bone formation |
|
XIV |
FACIT |
Skin, tendon, vessel wall, placenta, lungs, liver |
Maintaining mechanical tissue |
|
XV |
Endostatins |
Fibroblast, smooth muscle cell, kidney, pancreas |
Not clear |
|
XVI |
FACIT |
Fibroblast, amnion, keratinocyte |
Protecting neurons against Ab toxicity |
|
XVII |
Transmembrane |
Dermo–epidermal junction |
Structural component |
|
XVIII |
Endostatins |
Lung, liver, vascular, epithelial basement membrane |
Associated with eye development and basement membrane integrity |
|
XIX |
FACIT |
Central neurons, human rhabdomyosarcoma |
Associated with hippocampal synapses |
|
XX |
FACIT |
Corneal epithelium, embryonic skin, sternum cartilage, tendon |
Not clear |
|
XXI |
FACIT |
Heart, placenta, stomach, jejunum, skeletal muscle, kidney,lung, pancreas, lymph node |
Not clear |
|
XXII |
FACIT |
Myotendinous junction, cartilage-synovial fluid, hair follicle–dermis |
Retaining tissue junctions |
|
XXIII |
Transmembrane |
Prostate |
Associated with prostate cancer |
|
XXIV |
Fibrils |
Cornea, bone |
Regulating osteoblast differentiation |
|
XXV |
Transmembrane |
Precursor protein for collagenous Alzheimer amyloid plaque component |
Associated with Alzheimer’s disease |
|
XXVI |
Beaded filaments |
Testis and ovary |
Associated with generation and modeling of tissues |
|
XXVII |
Fibrils |
Cartilage, skin, cartilage, cornea, retina, major arteries of the heart |
Cartilage calcification |
|
XXVIII |
Beaded filaments |
Peripheral nerves, skin calvaria, |
Not clear |
|
XXIX |
Beaded filaments |
Skin, lung, small intestine, colon |
Associated with atopic dermatitis |
Table 1: Types, forms, distribution, and functions of collagens [27].
Physicochemical properties: Gelatine is a natural polymer similar to its collagen precursor. After collagen separation, gelatine can be extracted by either alkaline hydrolysis or acid hydrolysis. The latter determines the isoelectric point (pI) of the gelatine. With acid hydrolysis, gelatine is classified as type A, with an pI ≈ 5. Extraction with alkaline media yields type B gelatine, with an pI ≈ 9. Gelatine is classified as a physical gel [34]. That is to say, the interactions or bonds between the chains that make up the gel are physical in nature (van der Waals interactions and hydrogen bonding at E ≈ 2 kcal/mole). Some physical gels, such as alginate, are not thermoreversible. However, the binding energy of gelatine is relatively weak and can form thermoreversible gels. The most important physical properties of gelatine gels are strength and melting point. These are determined by molecular masses and complex interactions that depend on the amino acid composition and the ratio of α/β chains present in gelatine [36]. There is a strong correlation between gelatine gel strength and chain content. Indeed, differential rheological properties observed between mammalian and fish gelatines are mostly due to the content of the amino acids, proline and hydroxyproline, which are responsible for stabilisation of the gel network. Moreover, gelatines with a higher proportion of α-chains have higher gel strengths. On the other hand, a high percentage of peptides with molecular masses higher or lower than the α chain reduces the gel strength [37]. The gel strength of commercial gelatines is expressed in Bloom values, which is the weight in grams required by a specific plunger to push a standard temperature-controlled gel surface to a specific depth under standard conditions [38]. The gel strengths of commercial gelatines range from 100 to 300, but gelatines with Bloom values of 250 to 260 are the most desirable [39].
Fish gelatine typically has a Bloom value ranging from 0 to 270 (tested under standard Bloom test conditions), compared to the high Bloom values of bovine or porcine gelatine of 200-240. Certain gelatines from warm-water fish have been reported to have relatively high Bloom values, as has high-Bloom porcine gelatine [40]. Only gelatine from the skin of warm-water fish such as Tilapia [41-43] and grass carp [44] has a very high gel strength.
The wide range of Bloom values found in different gelatines is due to differences in the proline and hydroxyproline content of different types of collagens and is also related to the temperature of the animal's habitat. Badii and Howell showed that hydrophobic amino acids (Ala, Val, Leu, Ile, Pro, Phe, and Met) may also contribute to the high Bloom value of Tilapia fish gelatine [45].
Endogenous production: Collagen biosynthesis, from gene transcription in the nucleus to the aggregation of collagen heterotrimers into large fibrils, is a complex multistep process [46]. The endogenous production of collagen takes place in multiple successive stages, described hereafter and outlined schematically in figure 2, and is carried out within specialised cells, the fibroblasts.
From collagen genes in the nucleus (1), mRNA translation into ribosomal protein synthesis in the cytoplasm (2), post-translational modifications (PTMs) in the rough endoplasmic reticulum (3), vacuolar secretion (4), and maturation of tropocollagen into fibril formation, leading to collagen fibres in the extracellular matrix (5). Illustration created with BioRender.com
Nuclear level: At the nuclear level, the genes coding for type 1 collagen, COL1A1 and COL1A2, are transcribed into pre-mRNA and then capped at the 5'-end and polyadenylated at the 3'-end. The mRNAs are translated by ribosomes into collagen precursors, the α1 or α2 procollagens. They possess globular polypeptide domains at their ends, the N-terminal and C-terminal propeptides, 15 kDa on the N-terminal side and 30 kDa on the C-terminal side, respectively, which help maturation of the protein figure 2(1).
Endoplasmic reticulum and Golgi apparatus: These procollagens are introduced into the lumen of the rough endoplasmic reticulum during their synthesis by the presence of a signal peptide. After removal of the signal peptide by signal peptidase, several PTMs occur, such as the hydroxylation of proline and lysine by prolyl 3-hydroxylase, prolyl 4-hydroxylase, and lysyl hydroxylase, respectively. These three enzymes require cofactors to function: ferrous ions, 2-oxoglutarate, molecular oxygen, as well as ascorbic acid, the latter being of great importance in the synthesis of collagen by acting as an electron-donor that keeps iron in the ferrous state, thereby maintaining the full activity of collagen hydroxylases [47,48]. The 4-hydroxyprolines form hydrogen bonds between collagen polypeptides and thus play a key role in the thermal stability of the triple helix domain, but also that of the monomer and on a larger scale that of the collagen fibril [28]. Concomitantly the role of 3-hydroxyprolines still needs to be determined, but when prolyl 3-hydroxylase does not catalyse the addition of hydroxyl groups for various reasons (e.g., mutation, inhibition, etc.), their absence causes pathologies such as osteogenesis imperfecta [49]. In addition to their stabilising role, hydroxyprolines also provide attachment sites for carbohydrates. Through hydroxylysyl-galactosyltransferase and galactosylhydroxylysyl-glucosyltransferase enzymes, glucosyl- and galactosyl groups, respectively, can be transferred to the collagen molecules figure 2(3).
The presence of disulphide bridges also plays a structure-stabilizing role, on the N-terminal propeptide side, the bridges are intracatenal, whereas, on the C-terminal propeptide side, they are formed between the three alpha polypeptide chains. The C-terminal propeptide plays a crucial role in the assembly of the three alpha chains into the trimeric collagen monomers. Indeed, the formation of disulphide bonds between each C-terminal propeptide provides the alignment and initiation of the formation of the triple helix in the direction of the N-terminal extremity figure 2(3). This step relies on the activity of peptidyl-prolyl cis-trans-isomerase and collagen-specific chaperones (HSP47) [50,51]. Once procollagen is formed in the Golgi apparatus, procollagen molecules are secreted in the extracellular environment via secretory vacuoles figure 2(4).
Extracellular matrix: Two specific peptidases cleave the N-terminal and C-terminal propeptides, namely procollagen N-proteinase and procollagen C-proteinase, respectively, allowing the formation of mature tropocollagen [52]. After cleavage of the propeptides, tropocollagen bundles aggregate into fibrils near the cell surface. The formation of aldehydes at the level of collagen telopeptides by lysyl oxidase allows the formation of covalent cross-links via the substitution of a carbonyl group for the amine group of a lysine residue, which results in spontaneous bridging into several tropocollagen bundles. Minerals, such as hydroxyapatite crystals, are present between the fibrils. The monomers are 300 nm long and there are 40 nm gaps between consecutive monomers, which results in the characteristic striated appearance of type I collagen fibrils by electron microscopy. Finally, collagen fibrils aggregate into collagen fibres. Altogether, a collagen fibre’s molecular mass is 300,000 Da, the length is 280 nm, and the width is 1.4 nm [53].
Collagen hydrolysate obtention processes: The process of obtaining low-molecular-size collagen hydrolysates requires several treatments, as described below. Raw materials such as bones, cartilage, skin and tendons, and scales from different by-product sources (cattle, poultry, and aquaculture industries) are first pre-treated after grinding to remove fat and minerals. The pre-treatment process ensures removal of non-collagenous materials and enrichment of collagen as gelatine). However, as a result of the presence of cross-linked collagen in connective tissue, the collagen dissolves very slowly, including in boiling water. Furthermore, the triple helix conformation makes collagens very resistant to most proteases [29]. Thus, gentle pre-treatment is often required to disrupt the triple helix structure to extract the collagen (or gelatine).
Unlike fish-derived collagen, mammalian collagen generally requires a higher pH to remove non-collagenous proteins [54]. Lipids are also partially eliminated during this process [55]. Nevertheless, to achieve maximal lipid removal, organic solvents, such as chloroform-methanol [56] and hexane-extraction [57], are usually required. The main issue with the use of organic solvents is the potential for adverse effects on public health, the environment, and general safety [58].
Acid pre-treatment for demineralisation followed by alkaline neutralisation can provide a neutral extraction medium, resulting in a high yield of gelatine that has desirable gelling properties [59]. An example in the literature has demonstrated the benefits of using phosphoric acid instead of acetic acid (AcOH) to disrupt skin tissues from unicorn leatherjackets (Aluterus monoceros) [60].
Once the pre-treatment stage has been completed, the process continues with extraction of the collagen. There are several different methods, as depicted in figure 3, and the most common ones are those based on the solubilisation of collagen in acidic solutions, with or without the help of enzymes such as pepsin, and alkali solutions [61]. There are also options to optimise this extraction, such as the application of heat treatment or high-pressure [58].
Collagen extraction methods are varied and are tailored to meet specific demands. These methods are used on many sources of bovine, porcine, and marine origin. Among these extraction methods are those using conventional methods via acid extraction and enzyme-aided acid extraction and also non-typical methods such as fermentation, deep eutectic solvent (DES), ultrasound, electrodialysis, and supercritical fluid (SFE) extraction. Illustration created with BioRender.com
Acid extraction methods: Acid treatment is used to extract type 1 collagen from porcine tissues and fish skins. The different concentrations of acid tend to result in thorough extraction and change of the physicochemical parameters of the matrices by decreasing their final pH, modifying the electrostatic interactions and thus affecting their structures. This also has an impact on the solubility of the final product [26].
The literature describes several protocols for the extraction of marine and fish collagen/gelatine, which generally involves use of acid solutions. Indeed, the H+ ions liberated by the acids allow water molecules to be attracted to the collagen fibres, which are thus trapped between the charged polar groups by hydrogen bonds or by the uncharged polar groups and the negatively charged atoms by electrostatic forces. The collagen fibre thereby becomes saturated with water and swells, ultimately causing it to dissolve [62].
The method using AcOH is regularly used. Indeed, literature data describe the extraction of collagen from several aquatic species, both marine and freshwater: grass carp (Ctenopharyngodon idella) skin [26], Alaska pollock skin [59]; swim bladders of yellowfin tuna (Thunnus albacares) [63], catla (Catla catla) and rohu (Labeo rohita) skins [64]; small-spotted catshark skin [65]; Tilapia (Oreochromis niloticus) skin and scale [66]; scales of five species from Vietnam and Japan lizard fish (Saurida spp.) horse mackerel (Trachurus japonicus), grey mullet (Mugil cephalis), flying fish (Cypselurus melanurus), and yellow black seabream (Dentex tumifrons) [67]; three Vietnamese freshwater fish skins [68]; elasmobranch by-products [69]; and jellyfish (Acromitus hardenbergi) [70].
Most of these methods use concentrations of AcOH ranging from 0.1 to 1 M. This method is not the only one used since other solutions are also described, such as the use of hydrochloric, citric, and lactic acid [71].
Acid and enzyme-aided acidic collagen extraction: As an example, the extraction of collagen from the swim bladder of Atlantic cod (Gadus morhua) has been carried out using two different methods. The first method is with AcOH, and the second involves combining AcOH and pepsin. The authors demonstrated that the combined approach increased the final yield from 5.72% to 11.14% [72]. Other studies of the combined use have demonstrated their effectiveness in the extraction of collagen from Tilapia skins and gills [73] and from Chilean mussel byssus [74].
Various extraction methods: Unusual processes such as fermentation for collagen extraction have been described for Nile Tilapia skins [75], or by electrodialysis for Pufferfish (Takifugu flavidus) skins [76]. The latter appears to be rather promising as it allows this extraction to be carried out efficiently and cost-effectively, notably by reducing the amount of water waste by 95% while still maintaining a high extraction yield.
Deep eutectic solvent (DES) extraction is a method involving a two-compound tandem: a hydrogen bond receptor and a hydrogen bond donor. This approach favours the use of non-toxic, abundant, and biodegradable green solvents such as choline chloride, oxalic acid, urea, and ethylene glycol, among others, allowing extraction of high-value-added products from numerous by-products from plants, mammals, and aquatic sources. A study on the extraction of collagen from cod skins using a mixture of oxalic acid and choline chloride resulted in a yield of almost 90% [77], thus demonstrating the benefits of this extraction technique versus conventional ones.
Supercritical fluid extraction (SFE) is based on the use of fluids at pressures and temperatures beyond the critical point, allowing the capacities of a solvent to be modified through physical change. CO2 is most often used as it is inexpensive, stable, and has high availability, as well as rather simple conditions of use. Studies from the same author provided evidence of the unambiguously better performance of SFE versus acid and pepsin-aided AcOH extraction methods on cod skins [72].
Among the various unconventional methods, several studies have investigated the use of ultrasound to improve collagen extractability. Ultrasound induces cavitation in the liquid solvent, creating micro bubbles that in turn cause tissue damage, thereby increasing the tissue/solvent contact area. The higher the frequency, the smaller the bubbles and the more effective they are. This method is suitable not only for marine sources but also for plant and animal sources [78], although the harsh conditions of ultrasound could affect the physicochemical and molecular characteristics of the extracted collagen.
Extrusion-assisted extraction (EAE) increases the yield of collagen extracted from Tilapia scales by two- to three-fold compared with a simple acid extraction, making this method relevant in the context of tissue pre-treatment [79].
After centrifugation, the contaminating particles and the remaining fat are eliminated [80], and hydrolysis can be performed, whether enzymatic, physical, or chemical. The batches are decolourised and deodorised with activated carbon and then filtered. The salts are removed from the hydrolysates via ion-exchange columns, which are then concentrated by vacuum evaporation [58]. The hydrolysates are sterilised by heat treatment before pulverisation and then packaged [81], as depicted in figure 4.
The first step consists of washing the tissues, and the second step is a pre-treatment to remove non-collagenous proteins and fat as well as minerals. The collagens can then be extracted by several processes according to the manufacturer such as heat, acid, or enzyme-aided acidic methods, or the less conventional methods discussed above. The extracted gelatine is recovered after centrifugation and filtration, as well as decolourisation and deodorisation. Batches are deionized and sterilized to avoid contaminations before being dried and pulverized.
All of these extraction methods are carried out with the aim of reducing or even eliminating the release of solvents that are harmful to the environment and, via the "One Health" principle, preserving human and animal health at the same time. These innovative methods also reduce the energy costs associated with extraction by reducing the heating time of the matrices and the addition of costly enzymes and by implementing methods such as sonication, which provide a more efficient way of obtaining high-quality peptides of interest at a lower energy cost. These aspects are all the more important as the current challenges linked to the energy crisis do not allow these issues to be ignored at any scale of society.
Sources of collagen and gelatin: Collagen can be retrieved from skin, bones, and tendons of several sources, such as porcine, bovine, avian, and marine species. Many applications have been described in the literature due to intrinsic features such as biocompatibility and degradability [82]. Bovine collagen is mostly used for extra-oral wounds [83], and marine collagen such as Tilapia skin is used as a xenograft after burn injuries [84]. With its low immunogenicity and reduced chance of rejection, it is a material of choice for several fields of application such as surgery and cosmetology, drug delivery, and food [85,86].
Due to the variability of collagen sources, the versatility of use is equally broad, like no other available protein. Nevertheless, the use of animal collagen has been severely compromised over the past several decades due to the emergence of severe diseases. Sanitary crises, such as the emergence of bovine spongiform encephalopathy (BSE) in 1985, forced the market to be more cautious in its use [87]. The use of mammalian collagen also increases the risk of immune reaction in 3% of the population, as well as the transmission of zoonoses such as foot and mouth disease and transmissible spongiform encephalopathy [46]. Additionally, in several cultures and religious backgrounds, such as Jews, Muslims, and Hindus, porcine and/or bovine collagen use is prohibited or subjected to special religious requirements [88]. Thus, for the past three decades, marine collagens (in blue) as alternatives have emerged as a solution of choice as the number of scientific researches had increased over time (figure 5).
Articles indexed in PubMed (https://pubmed.ncbi.nlm.nih.gov) with the keywords “porcine collagen”, “bovine collagen”, and “marine collagen” published between 1926 and 2021 (last accessed on August 2022) and the time when the BSE outbreak occurred, inducing an increase of research interest for marine collagen in the late 1990s.
As the interest in marine collagen sources increased in the past 30 years, two categories emerged: vertebrate and invertebrate marine animals. The latter category is the most widely exploited, such as molluscs comprising cuttlefish and mussels, sponges, echinoderms comprising sea urchins, starfish, jellyfish, and crustaceans such as prawns [89-94]. Regarding vertebrate animals, the added value of such sources lies in the possibility to use by-products derived from industrial processes [95,96] such as scales and skin.
Analytic aspects and identification methods for peptides derived from agro-food
Background
Food scientists have faced new challenges in the past several decades. To this end, they rely on methods developed in medical, pharmacological, and/or biotechnological fields. Thus, advanced analytical methodologies, omics approaches, and bioinformatics, often combined with in vitro, in vivo, and/or clinical trials, are applied to investigate topics in food science and nutrition.
Within this framework, several research projects are directed toward the study of peptides, which are significant and multifaceted components of the human diet. They can be present naturally in food, but they can also be formed from protein precursors during physiological processes, for example, gastrointestinal digestion (GID) or food processing such as fermentation or enzymatic hydrolysis [97]. Chemical hydrolysis is more commonly used in the industry nowadays, but biological processes using enzymatic hydrolysis are more promising when products with high nutritional value and improved functionality are required. In addition, enzymatic hydrolysis generates less waste and is less costly and time-consuming [61].
Peptidomics applied to food is hence emerging as an important area of food science. These technologies include the use of peptide separation techniques coupled with mass spectrometry and bioinformatics for sequencing, identification, and quantification of peptides as well as the PTMs present on them [99]. The term "Omics" derives from the word "ome" from the Greek terminology -ωμα ("-oma"), which denotes a totality. As a result, when the totality of objects in a given biological system (such as genes, proteins, peptides, or metabolites) is combined with the suffix "ome", the result is genome, transcriptome, proteome, peptidome, and metabolome, respectively [99]. In 1996, Marc Wilkins first defined the proteome as the set of proteins encoded by a genome at a given time and in a given environment [100]. The addition of the suffix "omics" (genomics, transcriptomics, proteomics, peptidomics, metabolomics, etc.) then defines the analytical technologies that make it possible to explore the totality of these different biological molecules [101]. From then on, the combination of these approaches constitutes a multi-omics approach [102], also called integrative omics or pan-omics, which allows integration of data from various omics platforms to highlight the interrelationships of the biomolecules involved and their functions [103].
In response to advances in omics technologies, research in the field of food science has developed the concept of "foodomics" approaches to traceability, authenticity [104], well-being, health, and food safety, which are synonymous with consumer confidence in the agro-food sector [105]. Foodomics is defined as a discipline that studies the fields of food and nutrition through the application of omics technologies to, for example, characterize and demonstrate the beneficial effects of innovative food products on human health [106,107]. One of the main challenges is, therefore, to improve our currently limited understanding of the interaction of food components with genes, lipids, proteins, sugars, metabolites, etc. and the consequences of these interactions. This knowledge will undoubtedly in the future allow a rational design of strategies to achieve a beneficial impact on, for example, human health [108], but also to ensure food safety by determining/measuring harmful components or organisms that may be present in food at very low concentrations and much more.
Food peptidomics
The term peptidomics was introduced at the beginning of this century to represent the various peptide analysis technologies for the complete identification of endogenous peptides in a biological sample [109] and was subsequently rapidly applied in the clinical field and food industry. Clinical peptidomics thus relates to the analysis of peptides from a cell, organ, or organism, whereas food peptidomics relates to the analysis of the entire pool of peptides present in food items or generated during processing, storage, or GID of foods [110].
Thus, different workflows have proven useful to help identify, characterise, and quantify peptides in a particular approach. Among these, peptidomics has demonstrated that allergenicity due to the presence of certain food protein allergens (e.g., wheat gluten, β-lactoglobulin, milk casein, collagen [111] can be greatly reduced after protein cleavage into peptides. Liang and colleagues showed by in vitro ELISA that the immunoreactivity due to cow milk allergenicity could be significantly reduced by a two-hour enzymatic incubation [112]. These findings have many applications, such as food formulations containing hydrolysed proteins for feeding infants and children with food allergies or intolerances. In the same context, peptidomics has also been applied to monitor the extent of proteolysis to ensure that allergenic epitopes are destroyed [112].
Furthermore, the quality, safety, and authenticity of foods can also be studied using peptidomics [113]. As the sequence of proteins in original and altered products differs to a certain extent, the disparities are, therefore, reflected through peptidomics tools to identify peptides that can serve as biomarkers [114]. For example, most commercial gelatines are manufactured from porcine or bovine skin and/or bones. However, in some cases, such as outbreaks of bovine spongiform encephalopathy, it may be necessary to differentiate between bovine and porcine gelatine for safety considerations. Another aspect is that some religions ban the consumption or use of pork derivatives. Therefore, it is necessary to develop biomarker-based analytical methods to trace the species origin of gelatine [115,116].
Peptidomics is also an asset for studying the sensory aspects of hydrolysates. Indeed, work on predicting the bitterness of milk hydrolysates using peptidomics made it possible to identify the peptides responsible for bitterness, combined with a targeted bioinformatics approach and consumer sensory tests [117]. However, sensory study combined to peptidomics dedicated to collagen peptide study are not numerous [118]. On the other hand, studies have highlighted that such peptides from food-derived proteins may, beyond their nutritional role, confer additional health benefits [119,120].
In this respect, food peptidomics has identified bioactive peptides found in different types of foods such as milk [121], soy [122], and egg white [123]. They can also be derived from agro-food by-products, which refer to the parts discarded after processing for direct consumption, such as fish by-products [124] containing collagen for instance in tilapia and halibut skin gelatines [125], Atlantic salmon skin collagen [126] and boarfish [127], meat by-products [128], and plant by-products (pulp, kernels, skins, etc.) [129]. Once peptide bioactivity has been identified, the potential activities in the organism, can be assessed after GID. However, many studies take the reverse approach, which is all the more interesting, as the whole hydrolysate is tested. Indeed, a protein matrix digested with in vitro simulated GID is tested for various bioactivities (e.g., inhibition of DPP-IV, ACE, anti-microbial activity, etc.) and then the potential bioactive peptides are identified by the use of methods that allow their identification, such as peptidomics. Thus, bioactive peptide sequences have been identified from bovine haemoglobin after GID [130] or from Tilapia by-product [131], halibut [125], Alaska pollock [132], and even Blue whiting [133,134].
Understanding and identification of food peptide components of the peptidome have become crucial for the study of food allergenicity, biomarker discovery, combating fraud, identification of bioactive peptides, study of the digestome, as well as valorisation of agro-food by-products [110].
Current strategies for collagen-derived peptide characterisation
From an analytical point of view, after the cleavage of the proteins, the samples are usually centrifuged to remove insoluble material. The peptide-enriched mixture is then subjected to separation and/or fractionation processes for characterisation. Note that the prerequisites for gas chromatography (GC) separation of molecules are volatility of the analytes and their thermal stability. As peptides are not naturally volatile enough and, therefore, require chemical derivatisation to vaporise, they are not suitable for GC separation [135,136]. For this separation purpose, liquid chromatography (LC) has been widely used. However, capillary electrophoresis (CE) and non-capillary electrophoresis (peptide isoelectric focusing) have also been applied, albeit to a lesser extent due to the accumulation of peptides on the walls of the CE capillary as well as due to the potential precipitation of analytes for non-capillary techniques, which leads to a deterioration in analytical performance (loss of efficiency and non-repeatability) [137].
Depending on their physicochemical properties, a distinction is made among the liquid chromatography methods between (i) steric (or size)-exclusion chromatography (SEC); (ii) ion-exchange chromatography (IEX), and (iii) reverse-phase partition chromatography (RP). In this context, separation can be carried out at low pressure (< 6 bar) with constant flow and applied mainly for preparative fractionation techniques or at high pressure (HPLC) ranging from 100 to 300 bar used particularly for analytical purposes in order to obtain better separation efficiency and resolution.
One-dimensional chromatographic separation methods
Steric-exclusion chromatography (SEC) allows separation of peptides according to their hydrodynamic volume, which takes into account their shape and size. Although the molecules migrate at the same speed, peptides with a low hydrodynamic radius have access to a larger part of the total pore volume of the stationary phase than solutes with a high hydrodynamic radius, which will, therefore, be eluted first. The choice of the column is crucial, as the porosity and volume of the particles determine the separation capacity of this technique using an isocratic elution mode. Dextran gel (Sephadex®), polyacrylamide gel (Bio-Gel®), or polystyrene gel (Styragel®) resins can be used. The mobile phase must be capable of solubilising the sample. It should also be noted that the sample should not be too concentrated, as the gel matrix pores may become saturated [138]. Therefore SEC is currently used to characterise the peptide molecular mass distribution of collagen hydrolysates [139,140] but also the lot-to-lot reproducibility of collagen hydrolysates [141] as well as the purification of collagen peptides according to their average steric hindrance [142,143].
Ion-exchange chromatography (IEX) separates peptides according to their overall charge. The separation is based on a reversible interaction between the charged peptides and a resin of opposite charge. Elution of the molecules is then carried out using a pH or salt concentration gradient (NaCl or KCl). The choice of the type, composition, and pH of the mobile phase, which is usually a buffer, is important, as it affects the elution of the peptides and the shape of the peaks. The use of cationic or anionic columns is described in the literature [144,145]. The choice of strategy depends on the stability of the peptides at a given pH [146]. In overall, IEX is currently used to purify collagen peptides from various origins and test different bioactivities [147-150].
Reverse-phase (RP) chromatography is based on the use of an apolar stationary phase that interacts with hydrophobic analytes through a polar mobile phase generally composed of water/acetonitrile or water/methanol. Thus, the elution of peptides is based on their hydrophobicity. The most commonly used RP columns include silicas grafted with linear chains of 8 or 18 carbon atoms (C8 and C18). RP-chromatography is used to purify one or several peptide fractions or just one peptide [151-153]. However, the greatest advantage of this method is its compatibility with the electrospray ionisation (ESI) source in mass spectrometry (MS) for collagen peptide identification [154-156]. To achieve increased chromatographic resolution and mass detection sensitivity, nanoLC separations have been reported [157,158]. The reduced internal diameter of the column (< 100 μm) as well as the reduced mobile phase flow rate (a few nL.min-1) lead to increased chromatographic resolution and analyte concentration, which improve detection and mass fragmentation [159-161].
Multidimensional chromatographic separation methods: To improve the analysis of complex food peptidomes, most studies perform separations by orthogonal or multidimensional chromatographic systems. However, these separations present many challenges such as the need for automated or semi-automated systems, the need for specific interfaces, and the compatibility of different mobile phases from one technique to another [162]. SEC and IEX are often applied as a first step for fractionation prior to subsequent peptide characterisation by RP-HPLC since they can be directly combined with MS. Studies adopting this strategy have successfully identified functional peptides produced from different sources; for example, from loach fish protein hydrolysate [163] or carp muscle hydrolysate. Sample prefractionation using two-dimensional offline LC (IEX followed by SEC) was used to test the bioactivities of the fractions. Subsequently, high-resolution RP-HPLC-MS/MS combined with bioinformatics tools identified the peptide sequence Pro-Ser-Tyr-Val in loach protein hydrolysate and Pro-Ser-Lys-Tyr-Glu-Pro-Phe-Val in carp muscle hydrolysate as antioxidant sequences [164].
Multi-hydrolysis strategy: In complex matrices of proteins, such as the milk micellar caseins, the identification of particular proteins and their PTMs requires successive and complementary identification methods. Indeed, by combining a four-phase approach, combining a partial-, double-enzymatic dephosphorylation and endoGluC hydrolysis, the authors were able to increase the number of identified peptides to 90% but also increase the identification of PTMs (especially phosphorylation), making this protocol a rapid and reliable method [165].
Analytical techniques for peptide identification
Tandem mass spectrometry: After chromatographic fractionation, the detection of peptides depends on their physicochemical properties. Among these, their ultraviolet (UV) absorbance capacity is mainly used through UV-visible-Diode Array Detectors (DAD). However, this mode of detection does not allow the identification of peptides. On the other hand, MS/MS is the ideal detector for the identification of molecules, including peptides (figure 6).
The peptides are first positively ionised in the source and then a precursor ion beam is selected in the first MS1 analyser. This ion beam is then activated and fragmented in the CID collision cell in order to be analysed in the MS2 mass analyser to acquire the MS/MS spectrum. The peptide sequence is finally mainly reconstructed from the series of b- and y-fragment ions obtained.
Molecules are ionised in the mass spectrometer ionisation source and then a beam of precursor ions is selected in a first mass analyser (MS1) and focused in the mass spectrometer collision region where it is then fragmented by high or low energy input, which can be done in many ways, such as collision-induced dissociation (CID). The kinetic energy is then transformed in part into vibrational energy which leads to the fragmentation of the peptides. The fragments produced are then sorted by a second analyser (MS2) in order to acquire an MS/MS mass spectrum of the fragment ions (the abscissa is a mass/charge ratio scale (m/z) and the ordinate the relative intensity of the ions). The sequencing of the peptides is finally determined on the basis of their fragmentation profiles mainly through the series of b- and y- ions generated. These b- and y- ions correspond to the location of the positive charge with respect to the N-terminus and C-terminus of the peptide, respectively. Finally, the difference in mass between consecutive ions of the same series allows determination of the identity and the sequence of amino acids.
Among the various ionisation methods, the most widely used are: matrix-assisted laser desorption ionisation (MALDI) and ESI, which are soft ionisation techniques that generate little or no fragmentation in the source. Subsequently, high-resolution (HR) mass analysers such as time-of-flight (TOF), quadrupole-TOF (Q-TOF), orbitrap, and Fourier transform-ion cyclotron resonance (FT-ICR) are the analysers of choice. The high resolution provides greater measurement accuracy, good mass measurement accuracy, and unambiguous determination of peptide charge states.
MALDI involves the ionisation of analyte molecules assisted by a chemical matrix that can be activated by laser energy absorption [167]. The activated molecules of the matrix (derivatives of cinnamic or benzoic acid) and the analyte are vaporised, ionised, and released into the vacuum of the source. It is often combined with a TOF analyser, which measures the mass of intact peptides.
MALDI-TOF instruments equipped with an ion selector are able to provide fragment ions produced from precursor ions that spontaneously decay in flight by post-source decay (PSD) [168]. The analysis of these so-called metastable ions leads to the determination of the peptide sequence. However, the long spectrum acquisition time and the low resolution of the ion selectors represent major drawbacks of PSD. This is problematic when analysing complex mixtures such as peptidomes of food matrices. For these reasons, the deployment of MALDI-TOF-TOF techniques improves this PSD process by ion fragmentation activation [168]. Fragment ions of given peptides are thus generated by high-energy CID in a cell collision before they enter the reflectron. These ions, therefore, penetrate the reflectron substantially and allow complete fragment ion spectra to be acquired in a single acquisition at a fixed reflectron voltage [169]. For example, peptidome characterisation applying this technique was done on collagen peptides [170,171] and a Tilapia by-product [172]. For this latter, the results revealed the identification of a total of 799 peptides. Due to the complexity of the sample, a prefractionation method by RP-HPLC or SEC is required [173]. As the samples have to be crystallised in the matrix, the chromatographic system cannot be coupled directly to the mass spectrometer. The fractions are, therefore, collected and co-crystallized with the matrix to the MALDI target. The MALDI-TOF-TOF-MS analysis is then performed offline.
ESI-MS/MS is a more common source-ionisation-mass analyser combination for peptide identification in MS/MS. It consists of generating multi-charged ions by applying a high voltage at the end of the capillary to produce an aerosol from a conductive analyte solution and generate molecular ions in the sample for MS and MS/MS analysis [174]. Its direct association with RP-HPLC to analyse complex samples offers advantages in terms of time, selectivity, and high sensitivity and makes this technique the most commonly used compared to MALDI-TOF-MS/MS, especially for the collagen peptides which are composed of redundant amino acid sequences [152,175].
Coupled with HR analysers such as Q-TOF or FT-MS (LTQ-Orbitrap and FT-ICR), RP-HPLC-ESI-MS/MS provides a technique capable of screening almost the entire peptide pool. Its high sensitivity and ability to detect even trace amounts of peptides in complex matrices provide the most promising approach to analysing the food peptidome [149,176,177]. For example, a study characterising the peptidome of sheep milk led to the identification of 257 peptides through RP-HPLC-ESI-Q-TOF [178], and another study on donkey milk led to the identification of 1330 peptides by nano HPLC-ESI-LTQ-Orbitrap [179], where nano HPLC corresponds to the miniaturisation of the HPLC method to increase the resolving power of the chromatographic device. For these different types of instruments, the low-energy CID activation method was applied to obtain typical MS/MS mass spectra from each selected peptide ion.
After the investigations, the results are provided reliably, however, there is an urgent need to develop appropriate search databases and algorithms to exhaustively identify the food peptidome since each peptidomics approach is complementary to another [180] because of the complexity of agro-food peptide mixtures [181,182].
Bioinformatics data analysis: The analytical data is further processed and exploited through bioinformatics software owing to a large data set of up to thousands of ion spectra produced from a single sample. These software packages use mathematical algorithms and statistical tools to archive, retrieve, and analyse HPLC-MS/MS data [183]. Routine software for peptide identification includes Mascot, Sequest, Progenesis QI for proteomics, PEAKS® Studio, FlexAnalysis, X!Tandem, OMSSA, and MaxQuant [184]. Peptide identification can be performed according to two principles: (i) database searching, which compares the m/z ratio list from experimentally acquired MS/MS spectra with theoretical lists of predicted peptide fragments for each peptide contained in the database, the best known of which are UniProtKB and PepBank; and (ii) de novo sequencing, which presents itself as an alternative, given that peptide sequences may be absent from the databases and due to certain limitations of peptidomics. This allows determination of the peptide sequence directly from the m/z ratio of the fragmentation spectra. For this purpose, tools can be used to perform sequencing for each MS/MS spectrum such as PEAKS® Studio, PepNovo, MS-Blast, and MassHunter [118,185-187].
Advantages and challenges of peptidomics approaches
Peptidomics has revealed its advantages in the food industry, which produces both food items and by-products, both of which are rich in peptide precursor proteins. On the one hand, in the context of direct feeding of products, allergenicity is reduced and the improvement of functional properties such as solubility, improved digestibility, or intestinal absorption can contribute to solving certain problems such as poor absorption of proteins in the body [188]. On the other hand, the valorisation of food by-products responds both to the concern of recovering and using noble proteins and also to better valorisation of their impact on the environment [17,189,190].
However, for collagen and its derived peptides, certain factors limit this hydrolysis process, notably the presence of cross-links and hydroxyproline as well as numerous PTMs [191].
Despite the progress made in peptidomics, certain limitations exist, such as the analysis of small peptides (di-, tri-, tetra-, or penta-peptides) because their identification requires removal of a certain number of technological constraints [192]. Indeed, they are often poorly separated by RP-HPLC and are difficult to identify by MS/MS due to the low number of fragments obtained by CID. As a result, interpretations of MS/MS spectra can be ambiguous as each spectrum often corresponds to two or more possible peptide sequences owing to a lack of informative ions. In recent work, improvement of their identification through a dimethyl labelling strategy was presented, and 843 small peptides were identified [193]. This chemical derivatisation improves the signals of a1 ions (ions resulting from loss of neutral -CO of b1 ion) in MS/MS analysis and contributes to the unambiguous identification of N-terminal residues. Nevertheless, the de novo matching scores remain low owing to the lack of informative fragment ions, which reduces the number of matched fragments. Further study to improve the identification and modification of sequencing algorithms is, therefore, still required to improve the accuracy of automated data analysis.
The analysis of modified peptides is equally challenging. Information on the localisation of some PTMs can be provided by CID, but some PTMs, such as N-glycosylation, and proline hydroxylation, for instance, are rapidly lost during the fragmentation mechanism involved in CID. As an alternative, electron-capture dissociation (ECD) and its variant, electron transfer dissociation (ETD), are better suited to solve this problem [194].
On the other hand, the identification of these modified peptides and their PTMs is limited by the phenomenon of ionisation suppression occurring in ion sources. A well-known example concerns the detection of phosphorylated peptides, which is impaired by the presence of non-phosphorylated peptides, whose positive ionisation dominates over phosphopeptides, with the negative charge of the phosphate group resulting in a lower ionisation efficiency [195]. Therefore, the scientific community have developed various analytical approaches specially dedicated to detect, identify and quantify the phosphopeptides [196].
The progress of several projects and studies on the proteome in medicine has given rise to clinical peptidomics approaches. By analogy, peptidomics has been rapidly applied by food and nutrient science researchers in the more general framework of "foodomics". Thus, food peptidomics makes a crucial contribution to safety, authenticity, sensoriality, nutritional understanding, healthy food design, and by-product valorisation.
This approach nowadays represents a robust technique to identify the food peptidome through liquid chromatographic separation techniques (SEC, IEX, and RP-HPLC) and high-resolution tandem mass spectrometry combined with bioinformatics. In addition, the coupling of different chromatographic techniques upstream of the analysis provides better separation power and resolution, which result in more complete identification of the peptides present in complex mixtures. However, further research is needed for the near-complete identification of the agro-food peptidome by filling the main gaps related to the identification of size-reduced and modified peptides. The near-complete identification of agro-food proteomes will require the development of even more powerful bioinformatics tools.
Fields of application of collagen and its derivatives
Antioxidant and antimicrobial properties
Antioxidants are molecules with the ability to metabolise free radicals and, therefore, neutralise the deleterious effects of oxidative stress at the cellular as well as tissue levels [197]. The antioxidant activity of gelatine extracted from fish by-products has been the subject of several studies. Indeed, peptides were identified from different species and exerted antioxidant activities, such as PYSFK, GFGPEL, and VGGRP from grass carp [198], DPALATEPDMPF, EGL, YGDEY, and LSGYGP from Nile Tilapia (O. niloticus) [199,201], PFGPD, PYGAKG, and YGPM from Spanish mackerel (Scomberomorous niphonius) [201], GSGGL, GPGGFI, and FIGP from Bluefin leatherjacket (Navodon septentrionalis) [202], and AVGAT from Thornback ray [133]. In a general manner, the particularly low-molecular-mass (MM) peptides (< 3 kDa) exhibited higher radical scavenging properties [203], and it was shown that Tilapia skin collagen peptides (MM < 3 kDa) had protective effects against the D-galactose-induced liver and kidney damage in mice by reducing oxidative stress, for instance [204].
As for antimicrobial activity, bioactive peptides from yellowfin tuna (Thunnus albacares) skin collagen have shown efficacy notably with minimum effective concentration (MEC) values of 1.2, 6.5, 17, 8, 3, and 3.2 μg.mL-1 against B. subtilis, M. luteus, S. iniae, A. hydrophila, E. coli, and V. parahaemolytics strains, respectively [205]; skipjack tuna (Katsuwonus pelamis), with MEC values of 3, 26, 4.8, 25, 2.7, 9, and 16 μg.mL-1 against B. subtilis, M. luteus, S. iniae, A. hydrophila, E. coli, V. parahaemolytics, and C. albicans strains, respectively [206]; and yellow catfish (Pelteobagrus fulvidraco), with minimum inhibitory concentration (MIC) values of 2, 4, 16, and 64 μg.mL-1 against B. subtilis, S. aureus, E.coli, and C. albicans, respectively [207].
Anti-inflammatory properties
Collagen peptides have also been shown to be effective in preventing and reducing inflammation. Collagen peptides extracted from sea cucumber (Acaudina molpadioides) were administered by gavage to male mice for 21 days, followed by intraperitoneal injection of 10% CCL4 (nephrotoxic molecule) to establish an acute model of kidney injury. The results highlighted the protective effects of collagen peptides that significantly decreased the levels of interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumour necrosis factor-alpha (TNF-α [208].
This anti-inflammatory effect has been applied directly in the context of titanium implants. Indeed, when the latter are inserted in the body, a pro-inflammatory reaction takes place, which is necessary for the osseointegration process. However, if this inflammation is excessive and persists over time and becomes chronic, implant failure occurs. Thus, it is important to be able to control this inflammation [209] and the ‘use’ of collagen peptides can be advantageous. Indeed, a collagen-coated titanium alloy enriched with phenolic compounds has been shown to reduce inflammatory parameters by decreasing the gene expression of IL-6 and TNF-α [210].
Collagen peptides can be used in ways other than as biomaterials. In a study analysing the oral intake of collagen peptides from monkfish (Lophius litulon) skin, researchers showed an improvement in renal inflammatory status induced by a high-fat diet in mice, in particular by regulation of nuclear factor erythroid 2-related factor (Nrf2) and nucleotide-binding and oligomerisation domain-like receptors protein 3 (NLRP3) signalling pathways, which are implicated in regulation of the responses to oxidative stress and initiation of the inflammatory cascade [211,212]. Indeed, compared with the control group, the collagen peptide-fed group had decreased levels of IL-1β, IL-6, and TNF-α in kidney homogenate [213].
Antihypertensive activities
Of the numerous pathologies associated with obesity and T2D, as part of metabolic syndrome, hypertension is a growing public health problem worldwide [214]. People suffering from hypertension have systolic blood pressure values ≥ 140 mm Hg and/or diastolic blood pressure values ≥ 90 mm Hg [215]. This condition is partially due to chronic activation of the renin-angiotensin-aldosterone system (RAAS). The presence of excess angiotensin II and aldosterone in the circulation promotes a proinflammatory, fibrotic, and hypertrophic status, particularly in the heart and kidneys [216], making it paramount to address this condition.
Current pharmacological therapies use angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers. ACE inhibitors are used as a first-line treatment even before treatment with angiotensin receptor blockers. Studies are also looking at the regulation of ACE expression directly [217]. Among the multitude of therapies, bioactive peptides are used as a preventive treatment.
Indeed, more and more studies have highlighted the potential of bioactive peptides and in particular, their ACE-inhibiting properties. The antihypertensive effects of protein hydrolysates and their peptides can be determined by measuring ACE inhibitory activity in vitro or by monitoring blood pressure in spontaneously hypertensive rats (SHRs) in vivo [218,219]. Many protein hydrolysates and peptides of marine origin have been studied using the ACE inhibitory activity test and in SHRs [219-222].
ACE inhibitory peptides have molecular masses ranging from 300 to 3000 Da, with a peptide length of 2 to 13 amino acids [223,224]. In silico modelling has revealed optimal motifs for inhibition of the enzyme by candidate peptides. Since ACE is a carboxypeptidase, the C-terminal part of these peptides has been found to be crucial, particularly the C1 amino acid. Quantitative structure-activity relationship (QSAR) studies have shown that the presence of hydrophobic aliphatic amino acids (Ala, Trp, Pro, Phe, Gly, Cys, Leu, and Ile) [225] are good predictors of the peptide effectiveness in inhibiting ACE. It should also be noted that the nature of amino acids in the C2 and C4 positions is also important for ACE inhibition potential [226,227].
Moreover, according to Wu et al., the optimal amino acid residues identified in silico for potent ACE inhibition are peptides starting with Tyr or Cys in the first position at the C-terminus; Trp, Met, and His in the second position; Leu, Ile, Val, and Met in the third position, and Trp in the fourth position [227]. Peptides with hydrophobic and aromatic residues at the N- and C-terminal ends generally have higher antihypertensive activities [228].
Currently, pharmacological ACE inhibitors comprise enalapril, fosinopril, and captopril, with favourable half maximal inhibitory concentration (IC50) values such as 6 nM for the captopril [229]. Despite the very high inhibitory potential of this captopril, many side effects are associated with its use, including hypotension, cough, and hyperkalaemia, which are the most frequently reported adverse events for the entire drug class [230]. Therefore, alternative preventive measures such as reliance on functional food solutions derived from the agri-food industry, considered safe and affordable to produce, are envisaged.
The majority of antihypertensive peptides reported from marine proteins hydrolysates have ACE IC50 values ranging from 0.3 to 1 500 μM [231]. Crustacean peptides (shrimp, krill) exhibit IC50 values ranging between 0.9-24.1 μM [232,233], between 1.2-51 μM for mollusc peptides [234,235], and finally between 2.4-23.4 μM for coelenterates and echinoderms peptides [236,237]. It should be kept in mind that pharmacological ACE inhibitors are 1 000 times more efficient than antihypertensive peptides, but without harmful side effects. Numerous studies have also focused on the ACE-inhibitory properties of peptides derived from gelatine extracted from various fish species. These peptides were generated using various commercial enzymes. Indeed, peptides derived from gelatine extracted from fish skins have proven to be very good candidates, e.g., following the use of commercial Alcalase, the peptide Gly-Pro-Leu (GPL) from Alaska pollock had an IC50 value of 2.6 μM, and Nile Tilapia hydrolysate had an IC50 value of 62.2 μM [238,239]. For gelatine extracted from the scales of triplecross lizardfish (Synodus macrops) and milkfish (Chanos chanos), the peptides exhibited IC50 values of 420 μM and 472 μM, respectively [240,241]. For collagen extracted from Theragra chalcogramma skins, the isolated peptides GPL and Gly-Pro-Met (GPM) exhibited IC50 values of 2.6 and 17.1 μM, respectively [238]. Previous work also demonstrated that a tripeptide Leu-Gly-Trp (LGW) was able to reduce blood pressure in SHRs after oral administration [242]. Altogether these results demonstrate the efficiency of food-derived peptides and more specifically gelatine- and collagen-derived peptides in the management of hypertension.
Glucose metabolism regulation
Several benefits of collagen hydrolysates are slowly emerging, demonstrating their positive effects in preventing and treating T2D, although the illustrations of their beneficial effects in the current literature are not as abundant as those for ACE inhibition. Multiple studies have highlighted the beneficial effects of protein-rich diets from animal and plant sources in the management of T2D [243]. As depicted below, recent studies have investigated the potential of gelatine and gelatine hydrolysates to prevent obesity and diabetes.
Hormone secretion modulation
In streptozotocin (STZ)-induced diabetic rats, oral intake of fish (Salmo salar) skin gelatine hydrolysate (FSGH) resulted in a significant increase in plasma GLP-1 levels relative to diabetic control. Furthermore, the total GLP-1 levels of the FSGH-treated diabetic rats were at the same level as that of the group of normal rats [244]. Closer inspection of the clinical studies revealed that collagen peptides have positive effects after ingestion. Indeed, the incretin-stimulated concentrations of GLP-1 and glucose-dependent insulinotropic peptide (GIP) were increased in a double-blind randomised clinical study in subjects with T2D after 12 weeks of fish collagen peptide ingestion [245]. This study showed an improvement in the biomarkers for unfavourable prognosis of the disease, such as a decrease in fasting blood glycemia, insulin resistance, and glycated haemoglobin compared with the control group (resistant dextrin).
Another study, also involving the ingestion of hydrolysed gelatine (the origin of which was not specified) in healthy obese patients, showed an increase in circulating GLP-1 30 min after ingestion, with a peak at 120 min [246].
In another study, fish gelatine was administered by gavage to Wistar rats and was also tested after simulated GID in a cell model using Caco-2/TC7 co-culture. The results of this study demonstrated the efficacy of fish gelatine hydrolysates in decreasing intestinal glucose absorption in vitro and ex vivo concomitantly with improvement of glucose tolerance in Wistar rats [247].
A study of a Tilapia by-product hydrolysate after simulated GID demonstrated its effects in stimulating the secretion of the gut hormones, cholecystokinin (CCK) and GLP-1 as well, using the STC-1 enteroendocrine cell model [132]. Blue whiting hydrolysate containing collagen has also been shown to decrease insulin secretion in addition to increasing GLP-1 secretion along with proglucagon production [135,248].
Sasaoka et al. demonstrated that sturgeon collagen peptides (SCPs) improved glucose tolerance in a normal mouse model [249]. Following this work, a new study on the mechanisms that ensure these phenomena allowed the authors to hypothesise that SCPs decrease the transport rate of glucose from the stomach to the duodenum, resulting in a delay in its absorption. Thus, SCPs inhibit DPP-IV and maintain a high concentration of plasma GLP-1, which in turn stimulates insulin secretion [250]. Salmon by-products such as skin and trimmings have shown beneficial effects in improving insulin secretion as well as stimulating GLP-1 secretion in BRIN-BD11 and GLUTag cell models [251].
Hydrolysed proteins from chicken feet, which are rich in cartilage and, therefore, collagen, showed an improvement in plasma glucose levels and an increase in GLP-1 secretion in glucose-intolerant rats [252]. Still using a chicken source, a study was conducted on the by-products obtained after mechanical deboning, and the peptides isolated from them proved effective in increasing glucose uptake ex vivo [253].
Inhibition of DPP-IV activity
Often going hand in hand with studies on the stimulation of the secretion of intestinal hormones, the study of the inhibition of DPP-IV by protein hydrolysates, and hence those derived from collagen, are unavoidable, all the more so as the methods of evaluation are often not very restrictive and quick to set up in vitro or ex vivo or even to some extent on serum.
The previous study also showed that in STZ-induced diabetic rats, oral intake of fish (Salmo salar) skin gelatine hydrolysate (FSGH) inhibited plasmatic DPP-IV activity, demonstrating the antihyperglycemic power of a salmon collagen hydrolysate [244].
Concomitantly, a study assessing oral intake of Tilapia (Oreochromis niloticus) collagen hydrolysate has led to establishing, this time in normal mice, the inhibitory activity for DPP-IV, with an IC50 value of 0.77 mg.mL-1 [254]. Theysgeur et al. also studied the effect of Tilapia hydrolysates containing collagen and tested their ability to inhibit DPP-IV both in vitro and in situ. They were able to identify four sequences of bioactive peptides after SGID and in vitro intestinal barrier passage [132].
In addition to marine sources, a hydrolysate of camel gelatine skin has shown beneficial effects on DPP-IV inhibition as well [255]. Recently, Jin and colleagues [127] used Atlantic salmon skin, several peptidases, and fractionation methods of hydrolysates to evaluate DPP-IV inhibition activity, and they identified novel DPP-IV inhibitory peptides from the generated subfractions; the highest IC50 value was 0.79 ± 0.13 mg.mL-1.
The literature shows, for instance, that after SGID of Alaska pollock skin collagen, the DPP-IV IC50 value was 1.39 ± 0.08 mg.mL-1 [133]. Investigation of DPP-IV inhibition of barbel skin gelatine-derived peptides [256] led, after performing hydrolysis using Esperase®, the lowest IC50 value of 2.21 mg.mL-1.
Overall, the enzymatic hydrolysates of fish by-products appear to have very promising DPP-IV inhibitor activities compared with other protein hydrolysate-derived peptides such as Japanese rice bran, milk protein, and Gouda cheese [257-259].
Conclusion
The body of literature presented in this review has shed light on the use of gelatine throughout history, its physicochemical and structural properties, and its endogenous production. The various contemporary methods for its extraction as well as the different sources from which it can be extracted are also discussed. Nevertheless, certain technological barriers do not yet allow for exhaustive identification of their bioactive peptide compositions, but thanks to the optimisation of computing tools and a better understanding of preparation methods, this should no longer be a limiting factor in this regard. In the contemporary worldwide trend, the sustainable use of agro-food waste and/or by-products to produce value-added products for potential applications in the cosmetics, pharmaceutical, or food industry can offer considerable additional income opportunities for the dependent industry. In addition, valorisation of agro-food wastes and by-products can guarantee regional food safety and thus ensure sustainable food production at a time when societal and indeed economic pressures can put food-dependent countries or regions at a harmful disadvantage. Until now, most agro-food by-products have been used as a source of fuel, animal feed, or organic fertiliser. Presently, with the availability of modern technologies, new concepts have been established that enable efficient use of by-products from the agro-food sector to produce value-added products and also to accurately identify the high-interest entities, while limiting waste generation and, therefore, the carbon footprint.
The growing interest in these sources stems in particular from the versatility of these applications both structurally and in terms of bioactivities. Indeed, for the latter (the theme of our research group), as a result of their antioxidant, anti-inflammatory, and anti-microbial properties, as well as their role in the regulation of glucose metabolism, they are options of choice in the prevention and potential treatment of numerous pathologies, making it possible to imagine natural, inexpensive, effective, and most importantly custom-made therapeutic options in the future. Thus, in order to evaluate their beneficial effects on the prevention and management of chronic diseases such as diabetes and obesity, it is necessary to set up robust and well-set-out clinical trial protocols on humans in order to determine the physiological effects of these by-products with their multiple virtues and thus to allow a large section of people to benefit from them on a broader scale.
Author contributions
Mouna Ambli, Benoit Cudennec, Barbara Deracinois and Christophe Flahaut: Conceptualization, visualization and writing the original draft. Benoit Cudennec and Christophe Flahaut: Review and editing the manuscript. Benoit Cudennec, Christophe Flahaut and Rozenn Ravallec: Supervision, funding acquisition and project administration. All authors read and approved the manuscript.
Acknowledgment
We would like to thank ROUSSELOT BV for its contribution to this review.
Funding
This work has been carried out in the frame work of UMR-t BioEcoAgro-INRAe 1158 (University of Lille and University of Artois) through the ALIBIOTECH and BIHAUTSECO de France programs funded by the European Union, the French State, and the French Region of Hauts-de-France.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Henchion M, Hayes M, Mullen AM, et al. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 6 (2017): 53.
- Shigemura Y, Suzuki A, Kurokawa M, et al. Changes in composition and content of food-derived peptide in human blood after daily ingestion of collagen hydrolysate for 4 weeks: Hyp-peptide in blood after daily ingestion of collagen hydrolysate. Journal of the Science of Food and Agriculture 98 (2018): 1944-1950.
- IDF DAN. edition. IDF Diabetes Atlas (2019).
- Mutalipassi M, Esposito R, Ruocco N, et al. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods (Basel, Switzerland), 10 (2021): 1495.
- Shekhter A.B, Fayzullin A.L, Vukolova M.N, et al. Medical Applications of Collagen and Collagen-Based Materials. Current Medicinal Chemistry 26 (2019): 506-516.
- Verified market research. Bioactive peptides market size, share, trends, opportunities; forecast. Verified Market Research (2022).
- Mazurek J, Svoboda M, Maish J, et al. Characterization of binding media in Egyptian Romano portraits using enzyme-linked immunosorbant assay and mass spectrometry 8 (2014): 63-79.
- Adams RD. Adhesive Bonding: Science, Technology and Applications. Elsevier Science (2021).
- Walker S. Ancient faces: Mummy portraits from Roman Egypt (Routledge, 2020).
- Rouhi L. Review: Annals of the Caliphs’ Kitchens: Ibn Sayyar al-Warraq’s Tenth-Century Baghdadi Cookbook , by Nawal Nasrallah. Gastronomica 10 (2010): 110-111.
- Rao H, Yang Y, Abuduresule I, et al. Proteomic identification of adhesive on a bone sculpture-inlaid wooden artifact from the Xiaohe Cemetery, Xinjiang, China. Journal of Archaeological Science 53 (2015): 148-155.
- Kubo T, Zhao ZZ. History of the Chinese Medicinal Gelatin 5 (2022): 7.
- Viel C, Fournier J. Histoire des procédés d’extraction de la gélatine et débats des commissions académiques (XIXe siècle). Revue d’histoire de la pharmacie 94 (2006): 7-28.
- Frogerais A. La fabrication industrielle des capsules molles 31 (2021): 6681
- Tarafdar A, Gaur V.K, Rawat N, et al. Advances in biomaterial production from animal derived waste. Bioengineered 12 (2021): 8247-8258.
- Caruso G, Floris R, Serangeli C, et al. Fishery Wastes as a Yet Undiscovered Treasure from the Sea: Biomolecules Sources, Extraction Methods and Valorization. Marine Drugs 18 (2020): 622.
- Mokrejš P, Gál R, Pavlacková J, et al. Valorization of a By-Product from the Production of Mechanically Deboned Chicken Meat for Preparation of Gelatins. Molecules 26 (2021): 349.
- Debeaufort F. Active biopackaging produced from by-products and waste from food and marine industries. FEBS Open Bio 11 (2021): 984-998.
- Ben-Othman S, Jõudu I, Bhat R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 25 (2020): 510.
- Bloomfield SE, Miyata T, Dunn MW, et al. Soluble Gentamicin Ophthalmic Inserts as a Drug Delivery System. Archives of Ophthalmology 96 (1978): 885-887.
- Timorshina S, Popova E, Osmolovskiy A. (2022). Sustainable Applications of Animal Waste Proteins. Polymers 14 (2022): 1601.
- Chattopadhyay S. Raines RT. Collagen-based biomaterials for wound healing. Biopolymers 101 (2014): 821-833.
- Grabska-Zielinska S, Pin JM, Kaczmarek-Szczepanska B, et al. Scaffolds Loaded with Dialdehyde Chitosan and Collagen-Their Physico-Chemical Properties and Biological Assessment. Polymers 14 (2022): 1818.
- Lee CH, Singla A, Lee Y. Biomedical applications of collagen. International Journal of Pharmaceutics 221 (2001): 1-22.
- Banerjee P, Shanthi C. Cryptic Peptides from Collagen: A Critical Review. Protein & Peptide Letters 23 (2016): 664-672.
- Liu D, Nikoo M, Boran G, et al. Collagen and Gelatin. Annual Review of Food Science and Technology 6 (2015): 527-557.
- Lin K, Zhang D, Macedo MH, et al. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Advanced Functional Materials 29 (2019): 1804943.
- Gelse K. Collagens-Structure, function, and biosynthesis. Advanced Drug Delivery Reviews 55 (2003): 1531-1546.
- Brodsky B, Ramshaw JAM. The collagen triple-helix structure. Matrix Biology 15 (1997): 545-554
- Muyonga JH, Cole CGB, Duodu KG. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chemistry 85 (2004): 81-89.
- Haug IJ, Draget KI. Gelatin. In GO Phillips & PA. Williams (Eds.), Handbook of Food Proteins. Woodhead Publishing (2011): 92-115
- Akita M, Nishikawa Y, Shigenobu Y, et al. Correlation of proline, hydroxyproline and serine content, denaturation temperature and circular dichroism analysis of type I collagen with the physiological temperature of marine teleosts. Food Chemistry 329 (2020): 126775.
- Haug IJ, Draget KI, Smidsrød O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocolloids 18 (2004): 203-213.
- Karim AA, Bhat R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids 23(2009): 563-576.
- Krane SM. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids 35(2008): 703-710.
- Cho SM, Kwak KS, Park DC, et al. Processing optimization and functional properties of gelatin from shark (Isurus oxyrinchus) cartilage. Food Hydrocolloids 18 (2004): 573-579.
- Liu H, Li D, Guo S. Rheological properties of channel catfish (Ictalurus punctaus) gelatine from fish skins preserved by different methods. LWT - Food Science and Technology 41 (2008): 1425-1430.
- Schrieber R, Gareis H. Gelatine handbook: Theory and industrial practice. Wiley-VCH (2010).
- Holzer D. Gelatin production (Patent No. 5,484,888) (1996).
- Gudmundsson M, Hafsteinsson H. Gelatin from cod skins as affected by chemical treatments. Journal of Food Science 62 (1997): 37-39.
- Grossman S, Bergman M. Process for the production of gelatin from fish skins. (Patent No. 5,093,474.) (1992).
- Jamilah B, Harvinder KG. Properties of gelatins from skins of fish-Black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chemistry 77 (2002): 81-84.
- Zhou P, Mulvaney S.J, Regenstein J.M. Properties of Alaska pollock skin gelatin: A comparison with Tilapia and Pork Skin Gelatins. Journal of Food Science 71 (2006): C313-C321.
- Kasankala LM, Xue Y, Weilong Y, et al. Optimization of gelatine extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Bioresource Technology 98 (2007): 3338-3343.
- Badii F, Howell N. Fish gelatin: Structure, gelling properties and interaction with egg albumen proteins. Food Hydrocolloids 20 (2006): 630-640.
- Silvipriya K, Kumar K, Bhat A, et al. Collagen: Animal Sources and Biomedical Application. Journal of Applied Pharmaceutical Science 5 (2015): 123-127.
- Du J, Cullen JJ, Buettner GR. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1826 (2012): 443-457.
- Kivirikko KI, Ryhanen L, Anttinen H, et al. Further Hydroxylation of Lysyl Residues in Collagen by Protocollagen Lysyl Hydroxylase in VitroI 6 (1973): 635.
- Hudson DM, Eyre DR. Collagen prolyl 3-hydroxylation: A major role for a minor post-translational modification? Connective Tissue Research 54 (2013): 245-251.
- Clarke EP, Cates GA, Ball EH, et al. A collagen-binding protein in the endoplasmic reticulum of myoblasts exhibits relationship with serine protease inhibitors. Journal of Biological Chemistry 266 (1991): 17230-17235.
- Lang K, Schmid FX, Fischer G. (1987). Catalysis of protein folding by prolyl isomerase. Nature 329 (1987): 268-270.
- Kadler KE, Holmes DF, Trotter JA, et al. Collagen fibril formation. Biochemical Journal 316 (1996): 1-11.
- Ferreira AM, Gentile P, Chiono V, et al. Collagen for bone tissue regeneration. Acta Biomaterialia 8 (2012): 3191-3200.
- Chakka AK, Muhammed A, Sakhare PZ, et al. Poultry processing waste as an alternative source for mammalian gelatin: Extraction and characterization of gelatin from chicken feet using food grade acids. Waste and Biomass Valorization 8 (2017): 2583-2593.
- Shu Y, Ren H, Ao R, et al. Comparison of physical and chemical characteristics of collagen from the skin of cod (Gadus macrocephaius). Genetics and Molecular Research 16 (2017): 1-8.
- Rabinowitz JL, Shapiro IM. The lipids of bovine skin collagen. Archives of Oral Biology 17 (1976): 547-553.
- Cansu Ü, Boran G. Optimization of a Multi-Step Procedure for Isolation of Chicken Bone Collagen. Korean Journal for Food Science of Animal Resources 35 (2015): 431-440.
- Hong H, Fan H, Chalamaiah M, et al. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chemistry 301 (2019): 125222.
- Zhou P, Regenstein J.M. Effects of alkaline and acid pretreatments on alaska pollock skin gelatin extraction. Journal of Food Science 70 (2006): c392–c396.
- Ahmad M, Benjakul S. Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocolloids 25 (2011): 381-388.
- Schmidt MM, Prestes Dornelles R, Mello R, et al. Collagen extraction process. International Food Research Journal 23 (2016): 913-922.
- Giménez B, Turnay J, Lizarbe MA, et al. Use of lactic acid for extraction of fish skin gelatin. Food Hydrocolloids 19 (2005): 941-950.
- Kaewdang O, Benjakul S, Kaewmanee T, et al. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chemistry 155 (2014): 264-270.
- Pal GK, Nidheesh T, Suresh PV. Comparative study on characteristics and in vitro fibril formation ability of acid and pepsin soluble collagen from the skin of catla (Catla catla) and rohu (Labeo rohita). Food Research International, 76 (2015): 804-812.
- Blanco M, Vázquez JA, Pérez-Martín RI, et al. Collagen Extraction Optimization from the Skin of the Small-Spotted Catshark (S. canicula) by Response Surface Methodology. Marine Drugs 17 (2019): 40.
- Chen Q, Gao X, Zhang H, Li B, Yu G, Li B. Collagen peptides administration in early enteral nutrition intervention attenuates burn-induced intestinal barrier disruption: Effects on tight junction structure. J Funct Foods 55 (2019):167–74.
- Minh Thuy LT, Okazaki E, Osako K. Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chemistry 149 (2014): 264-270.
- Le TMT, Nguyen VM, Tran TT, et al. Comparison of acid-soluble collagen characteristic from three important freshwater fish skins in Mekong Delta Region, Vietnam. Journal of Food Biochemistry 44 (2020): 365.
- Seixas MJ, Martins E, Reis RL, et al. Extraction and characterization of collagen from elasmobranch byproducts for potential biomaterial use. Marine Drugs 18 (2020): 617.
- Khong NMH, Yusoff Md, Jamilah B, et al. Improved collagen extraction from jellyfish (Acromitus hardenbergi ) with increased physical-induced solubilization processes. Food Chemistry 251 (2018): 41-50.
- Tan Y, Chang S.K.C. (2018). Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chemistry 242 (2018): 147-155.
- Sousa R.O, Alves A.L, Carvalho D.N, et al. Acid and enzymatic extraction of collagen from Atlantic cod (Gadus Morhua) swim bladders envisaging health-related applications. Journal of Biomaterials Science, Polymer Edition 31 (2020): 20-37.
- Medina-Medrano JR, Quiñones-Muñoz TA, Arce-Ortíz A, et al. Antioxidant Activity of Collagen Extracts Obtained from the Skin and Gills of Oreochromis sp. Journal of Medicinal Food 22 (2019): 722-728.
- Vallejos N, González G, Troncoso E, et al. Acid and Enzyme-Aided Collagen Extraction from the Byssus of Chilean Mussels (Mytilus Chilensis): Effect of Process Parameters on Extraction Performance. Food Biophysics 9 (2014): 322-331.
- Song Z, Liu H, Chen L, et al. Characterization and comparison of collagen extracted from the skin of the Nile tilapia by fermentation and chemical pretreatment. Food Chemistry 340 (2021): 128139.
- Chen J, Li M, Yi R, et al. Electrodialysis Extraction of Pufferfish Skin (Takifugu flavidus): A Promising Source of Collagen. Marine Drugs 17 (2019): 25.
- Bai C, Wei Q, Ren X. Selective Extraction of Collagen Peptides with High Purity from Cod Skins by Deep Eutectic Solvents. ACS Sustainable Chemistry & Engineering 5 (2017): 7220-7227.
- Ojha KS, Aznar R, O’Donnell C, et al. Ultrasound technology for the extraction of biologically active molecules from plant, animal and marine sources. TrAC Trends in Analytical Chemistry 122 (2020): 115663.
- Huang CY, Kuo JM, Wu SJ, et al. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion-hydro-extraction process. Food Chemistry 190 (2016): 997-1006.
- Salvatore L, Gallo N, Natali ML, et al. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Materials Science and Engineering 113 (2020): 110963.
- Milovanovic I, Hayes M. Marine Gelatine from Rest Raw Materials. Applied Sciences 8 (2018): 2407.
- Wahyudi H, Reynolds A.A, Li Y, et al. Targeting collagen for diagnostic imaging and therapeutic delivery. Journal of Controlled Release 240 (2016): 323-331.
- Sowjanya N. P. Versitality of the Use of Collagen Membrane in Oral Cavity. Journal of clinical and diagnostic research 10 (2016): zc30-zc33.
- Lima Júnior EM, De Moraes Filho MO, Costa BA, et al. Innovative Burn Treatment Using Tilapia Skin as a Xenograft: A Phase II Randomized Controlled Trial. Journal of Burn Care & Research 41 (2020): 585-592.
- Xu X, Sui B, Liu X, et al. Superior low-immunogenicity of tilapia type I collagen based on unique secondary structure with single calcium binding motif over terrestrial mammals by inhibiting activation of DC intracellular Ca2+-mediated STIM1-Orai1/NF-?B pathway. Materials Science and Engineering 131 (2021): 112503.
- Felician FF, Xia C, Qi W, et al. Collagen from Marine Biological Sources and Medical Applications. Chemistry & Biodiversity 15 (2018): e1700557.
- Wilesmith J, Ryan J, Atkinson, M. Bovine spongiform encephalopathy: Epidemiological studies on the origin. Veterinary Record 128 (1991): 199-203.
- Avila Rodríguez MI, Rodríguez Barroso LG, Sánchez ML. Collagen: A review on its sources and potential cosmetic applications. Journal of Cosmetic Dermatology 17 (2018): 20-26.
- Cudennec B, Balti R, Ravallec R, et al. In vitro evidence for gut hormone stimulation release and dipeptidyl-peptidase IV inhibitory activity of protein hydrolysate obtained from cuttlefish (Sepia officinalis) viscera. Food Research International 78 (2015): 238-245.
- Tziveleka L.A, Ioannou E, Tsiourvas D, et al. Collagen from the Marine Sponges Axinella cannabina and Suberites carnosus: Isolation and Morphological, Biochemical, and Biophysical Characterization. Marine Drugs 15 (2017): 152.
- Ferrario C, Leggio L, Leone R, et al. Marine-derived collagen biomaterials from echinoderm connective tissues. Marine Environmental Research 128 (2017): 46-57.
- Ferrario C, Rusconi F, Pulaj A, et al. From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine. Marine Drugs 18 (2020): 414.
- Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 3 (2010): 1863-1887.
- Minamisako K, Kimura S. Characterization of muscle collagen from fleshy prawn Penaeus chinensis. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 94 (2): 349-353.
- Martins MEO, Sousa JR, Claudino RL, et al. Thermal and Chemical Properties of Gelatin from Tilapia (Oreochromis niloticus) Scale. Journal of Aquatic Food Product Technology 27 (2018): 1120-1133.
- Arumugam GKS, Sharma D, Balakrishnan RM, et al. Extraction, optimization and characterization of collagen from sole fish skin. Sustainable Chemistry and Pharmacy 9 (2019): 19-26.
- Wang B, Xie N, Li B. Influence of peptide characteristics on their stability, intestinal transport, and in vitro bioavailability: A review. Journal of Food Biochemistry 43 (2019): e12571.
- Agyei D, Tsopmo A, Udenigwe CC. Bioinformatics and peptidomics approaches to the discovery and analysis of food-derived bioactive peptides. Analytical and Bioanalytical Chemistry 410 (2018): 3463-3472.
- Zheng C, Chen A. System Biological Research on Food Quality for Personalised Nutrition and Health Using Foodomics Techniques: A Review. Journal of Food and Nutrition Research 2 (2014): 608-616.
- Wilkins MR, Sanchez JC, Gooley AA, et al. Progress with Proteome Projects: Why all Proteins Expressed by a Genome Should be Identified and How To Do It. Biotechnology and Genetic Engineering Reviews 13 (1996): 19-50.
- Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biology 18 (2017): 83.
- Agregán R, Echegaray N, Nawaz A, et al. Foodomic-Based Approach for the Control and Quality Improvement of Dairy Products. Metabolites 11 (2021): 818.
- Subramanian I, Verma S, Kumar S, et al. Multi-omics Data Integration, Interpretation, and Its Application. Bioinformatics and Biology Insights 14 (2020): 117793221989905.
- Dirong G, Nematbakhsh S, Selamat J, et al. Omics-Based Analytical Approaches for Assessing Chicken Species and Breeds in Food Authentication. Molecules 26 (2021): 6502.
- Creydt M, Fischer M. Omics approaches for food authentication. Electrophoresis 39 (2018): 1569-1581.
- Álvarez-Rivera G, Valdés A, León C, et al. Chapter 1. Foodomics - Fundamentals, State of the Art and Future Trends. In J. Barros-Velázquez (Ed.), Food Chemistry, Function and Analysis. Royal Society of Chemistry (2021): 1-53.
- Cifuentes A. Food analysis and Foodomics. Journal of Chromatography A 1216 (2009): 7109.
- Ibáñez C, Simó C, García-Cañas V, et al. Metabolomics, peptidomics and proteomics applications of capillary electrophoresis-mass spectrometry in Foodomics: A review. Analytica Chimica Acta 802 (2013): 1-13.
- Schrader M, Schulz-Knappe P. Peptidomics technologies for human body fluids. Trends in Biotechnology 19 (2001): S55-S60.
- Martini S, Conte A, Tagliazucchi D. Comparative peptidomic profile and bioactivities of cooked beef, pork, chicken and turkey meat after in vitro gastro-intestinal digestion. Journal of Proteomics 208 (2019): 103500.
- Kalic T, Kamath SD, Ruethers T, et al. Collagen- An Important Fish Allergen for Improved Diagnosis. The Journal of Allergy and Clinical Immunology: In Practice 8 (2020): 3084-3092.
- Liang X, Wang Z, Yang H, et al. Evaluation of allergenicity of cow milk treated with enzymatic hydrolysis through a mouse model of allergy. Journal of Dairy Science 105 (2022): 1039-1050.
- Han S, Yan Z, Huang X, et al. Response boosting-based approach for absolute quantification of gelatin peptides using LC-MS/MS. Food Chemistry 390 (2022): 133111.
- Dalabasmaz S, Pischetsrieder M. Peptidomics in Food. In Comprehensive Foodomics 21 (2021): 651-665.
- Sha XM, Jiang WL, Hu ZZ, et al. Traceability and identification of fish gelatin from seven cyprinid fishes by high performance liquid chromatography and high-resolution mass spectrometry. Food Chemistry 400 (2023): 133961.
- Zhang G, Liu T, Wang Q, et al. Mass spectrometric detection of marker peptides in tryptic digests of gelatin: A new method to differentiate between bovine and porcine gelatin. Food Hydrocolloids 23 (2009): 2001-2007.
- Daher D, Deracinois B, Courcoux P, et al. Sensopeptidomic Kinetic Approach Combined with Decision Trees and Random Forests to Study the Bitterness during Enzymatic Hydrolysis Kinetics of Micellar Caseins. Foods 10 (2021): 1312.
- Gan R, He Y, Li Y. Structural characteristics of taste active peptides in protein hydrolysates from tilapia by-products. Journal of Food Measurement and Characterization 16 (2022): 1674-1687.
- Islam Md, Wang H, Admassu H, et al. Health benefits of bioactive peptides produced from muscle proteins: Antioxidant, anti-cancer, and anti-diabetic activities. Process Biochemistry 116 (2022): 116-125.
- Zhao W, Li J, Li Y, Chen Y, Jin H. Preventive Effect of Collagen Peptides from Acaudina molpadioides on Acute Kidney Injury through Attenuation of Oxidative Stress and Inflammation. Oxidative Medicine Cellular Longevity. 2022 (2022):8186838.
- Athira S, Mann B, Saini P, et al. Production and characterisation of whey protein hydrolysate having antioxidant activity from cheese whey: Characterisation of whey protein hydrolysate. Journal of the Science of Food and Agriculture 95 (2015): 2908-2915.
- Fan J, Hu X, Tan S, et al. Isolation and characterisation of a novel angiotensin I-converting enzyme-inhibitory peptide derived from douchi , a traditional Chinese fermented soybean food: A novel ACE-inhibitory peptide from douchi. Journal of the Science of Food and Agriculture 89 (2009): 603-608.
- Rizzetti DA, Martín Á, Corrales P, et al. Egg white-derived peptides prevent cardiovascular disorders induced by mercury in rats: Role of angiotensin-converting enzyme (ACE) and NADPH oxidase. Toxicology Letters 281 (2017): 158-174.
- Gao Y, Wu X, McClements DJ, et al. Encapsulation of bitter peptides in water-in-oil high internal phase emulsions reduces their bitterness and improves gastrointestinal stability. Food Chemistry 386 (2022): 132787.
- Wang T.Y, Hsieh C.H, Hung C.C, et al. Fish skin gelatin hydrolysates as dipeptidyl peptidase IV inhibitors and glucagon-like peptide-1 stimulators improve glycaemic control in diabetic rats: A comparison between warm- and cold-water fish. Journal of Functional Foods 19 (2015): 330-340.
- Jin R, Teng X, Shang J, et al. Identification of novel DPP–IV inhibitory peptides from Atlantic salmon (Salmo salar) skin. Food Research International 133 (2020): 109161.
- Harnedy-Rothwell PA, McLaughlin CM, O’Keeffe MB, et al. Identification and characterisation of peptides from a boarfish (Capros aper) protein hydrolysate displaying in vitro dipeptidyl peptidase-IV (DPP-IV) inhibitory and insulinotropic activity. Food Research International 131 (2020): 108989.
- Mora L, Bolumar T, Heres A, et al. Effect of cooking and simulated gastrointestinal digestion on the activity of generated bioactive peptides in aged beef meat. Food & Function 8 (2017): 4347-4355.
- Görgüç A, Gençdag E, Yilmaz FM. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments - A review. Food Research International 136 (2020): 109504.
- Caron J, Chataigné G, Gimeno JP, et al. Food peptidomics of in vitro gastrointestinal digestions of partially purified bovine hemoglobin: Low-resolution versus high-resolution LC-MS/MS analyses: General Electrophoresis 37 (2016): 1814-1822.
- Theysgeur S, Cudennec B, Deracinois B, et al. New Bioactive Peptides Identified from a Tilapia Byproduct Hydrolysate Exerting Effects on DPP-IV Activity and Intestinal Hormones Regulation after Canine Gastrointestinal Simulated Digestion. Molecules 26 (2020): 136.
- Guo L, Harnedy PA, Zhang L, et al. In vitro assessment of the multifunctional bioactive potential of Alaska pollock skin collagen following simulated gastrointestinal digestion. Journal of the Science of Food and Agriculture 95 (2015): 1514-1520.
- Harnedy PA, Parthsarathy V, McLaughlin CM, et al. Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. Journal of Functional Foods 40 (2018): 137-145.
- Harnedy-Rothwell PA, Khatib N, Sharkey S, et al. Physicochemical, Nutritional and In Vitro Antidiabetic Characterisation of Blue Whiting (Micromesistiuspoutassou) Protein Hydrolysates. Marine Drugs 19 (2021): 383.
- Honour JW. Gas chromatography-mass spectrometry. Methods in Molecular Biology 324 (2006): 53-74.
- Cserháti T. Chromatography of amino acids and short peptides. New advances. Biomedical Chromatography: BMC 21 (2007): 780-796.
- Farmerie L, Rustandi RR, Loughney JW, et al. Recent advances in isoelectric focusing of proteins and peptides. Journal of Chromatography A 1651 (2021): 462274.
- Hall M. Chapter 21-Size Exclusion Chromatography (SEC). Biopharmaceutical Processing (2018): 421-432.
- Chen J, Li L, Yi R, et al. Release kinetics of Tilapia scale collagen I peptides during tryptic hydrolysis. Food Hydrocolloids 77 (2018): 931-936.
- Khiari, Z., Ndagijimana, M., & Betti, M. (2014). Low molecular weight bioactive peptides derived from the enzymatic hydrolysis of collagen after isoelectric solubilization/precipitation process of turkey by-products. Poultry Science, 93(9), 2347–2362.
- López-Morales CA, Vázquez-Leyva S, Vallejo-Castillo L, et al. Determination of Peptide Profile Consistency and Safety of Collagen Hydrolysates as Quality Attributes: Quality of collagen hydrolysate. Journal of Food Science 84 (2019): 430-439.
- Cao L, Majura JJ, Liu L, et al. The cryoprotective activity of tilapia skin collagen hydrolysate and the structure elucidation of its antifreeze peptide. LWT -- Food Science and Technology 179 (2023): 114670.
- Li Z, Wang B, Chi C, et al. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Research International 51 (2013): 283-293.
- Nimalaratne C, Bandara N, Wu J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chemistry 188 (2015): 467-472.
- Ortiz-Martinez M, Mejia E, García-Lara S, et al. Antiproliferative effect of peptide fractions isolated from a quality protein maize, a white hybrid maize, and their derived peptides on hepatocarcinoma human HepG2 cells. Journal of Functional Foods 34 (2017): 36-48.
- Sharma N, Kukreja D, Giri T, et al. Synthetic pharmaceutical peptides characterization by chromatography principles and method development. Journal of Separation Science 45 (2022): 2200-2216.
- Huang CY, Wu CH, Yang JI, et al. Evaluation of iron-binding activity of collagen peptides prepared from the scales of four cultivated fishes in Taiwan. Journal of Food and Drug Analysis 23 (2015): 671-678.
- Sun L, Chang W, Ma Q, et al. Purification of Antioxidant Peptides by High Resolution Mass Spectrometry from Simulated Gastrointestinal Digestion Hydrolysates of Alaska Pollock (Theragra chalcogramma) Skin Collagen. Marine Drugs 14 (2016): 186.
- Wu H, Ren C, Yang F, et al. Extraction and identification of collagen-derived peptides with hematopoietic activity from Colla Corii Asini. Journal of Ethnopharmacology 182 (2016): 129-136.
- Hk M, Acharya PP, Bhat G, et al. Biophysical and in vitro wound healing assessment of collagen peptides processed from fish skin waste. Journal of Bioactive and Compatible Polymers 38 (2023): 25-40.
- Bougatef H, De la Vega-Fernández C, Sila A, et al. Identification of ACE I-Inhibitory Peptides Released by the Hydrolysis of Tub Gurnard (Chelidonichthys lucerna) Skin Proteins and the Impact of Their In Silico Gastrointestinal Digestion. Marine Drugs 21 (2023): 131.
- Han QY, Koyama T, Watabe S, et alIsolation and Characterization of Collagen and Collagen Peptides with Hyaluronidase Inhibition Activity Derived from the Skin of Marlin (Istiophoridae). Molecules 28 (2023): 889.
- Sheng Y, Wang W.Y, Wu M.F, et al. Eighteen Novel Bioactive Peptides from Monkfish (Lophius litulon) Swim Bladders: Production, Identification, Antioxidant Activity, and Stability. Marine Drugs 21 (2023): 169.
- Cao S, Cai J, Wang X, et al. Cryoprotective effect of collagen hydrolysates from squid skin on frozen shrimp and characterizations of its antifreeze peptides. LWT 174 (2023): 114443.
- He L, Wang X, Wang Y, et al. Production and identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from discarded cowhide collagen. Food Chemistry 405 (2023): 134793.
- Zhu X, Gu S, Guo D, et al. Determination of porcine derived components in gelatin and gelatin-containing foods by high performance liquid chromatography-tandem mass spectrometry. Food Hydrocolloids 134 (2023): 107978.
- Nascimento E, Anaya K, Oliveira JMC, et al. Identification of bioactive peptides released from in vitro gastrointestinal digestion of yam proteins (Dioscorea cayennensis). Food Research International 143 (2021): 110286.
- Escudero E, Mora L, Fraser PD, et al. Identification of novel antioxidant peptides generated in Spanish dry-cured ham. Food Chemistry 138 (2013): 1282-1288.
- Cui L, Li B. Enrichment of antiplatelet peptides and removal of fishy odor from silver carp skin collagen hydrolysates by macroporous resins: PH value of loading sample affects the peptides separation. Food Chemistry 411 (2023): 135481.
- Huang J, Li H, Xiong G, et al. Extraction, identification and anti-photoaging activity evaluation of collagen peptides from silver carp (Hypophthalmichthys molitrix) skin. LWT 173 (2023): 114384.
- Yang Y, Zhu L, Guo Z, et al. Yak bone collagen-derived anti-inflammatory bioactive peptides alleviate lipopolysaccharide-induced inflammatory by inhibiting the NF-κB signaling pathway and nitric oxide production. Food Bioscience 52 (2023): 102423.
- Cacciola F, Dugo P, Mondello L. Multidimensional liquid chromatography in food analysis. Trends in Analytical Chemistry 96 (2017): 116-123.
- You L, Zhao M, Regenstein JM. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry". Food Research International 43 (2010): 1167-1173.
- Ren J, Zhao M, Shi J, et al. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chemistry 108 (2008): 727-736.
- Deracinois B, Matéos A, Romelard A, et al. Partial-, Double-Enzymatic Dephosphorylation and EndoGluC Hydrolysis as an Original Approach to Enhancing Identification of Casein Phosphopeptides (CPPs) by Mass Spectrometry. Foods 10 (2021): 21-34.
- Montaudo G, Samperi F, Montaudo MS. Characterization of synthetic polymers by MALDI-MS. Progress in Polymer Science 31 (2006): 277-357.
- Kenny DJ, Brown JM, Palmer ME, et al. A Parallel Approach to Post Source Decay MALDI-TOF Analysis. Journal of the American Society for Mass Spectrometry 17 (2006): 60-66.
- Medzihradszky KF, Campbell JM, Baldwin MA, et al. The Characteristics of Peptide Collision-Induced Dissociation Using a High-Performance MALDI-TOF/TOF Tandem Mass Spectrometer. Analytical Chemistry 72 (2000): 552-558.
- Lin X, Lu Y, Zhang T, et al. Accuracy and Precision Comparison for Molecular Weight Distribution Assay of Fish Collagen Peptides: A Methodology Study Between Two Gel Permeation Chromatography Columns. Food Analytical Methods 12 (2019): 246-257.
- Hong H, Fan H, Roy BC, et al. Amylase enhances production of low molecular weight collagen peptides from the skin of spent hen, bovine, porcine, and tilapia. Food Chemistry 352 (2021): 129355.
- Robert M, Zatylny-Gaudin C, Fournier V, et al. Molecular characterization of peptide fractions of a Tilapia (Oreochromis niloticus) by-product hydrolysate and in vitro evaluation of antibacterial activity. Process Biochemistry 50 (2015): 487-492.
- Yoo HJ, Kim DH, Park SJ, et al. Analysis of Low Molecular Weight Collagen by Gel Permeation Chromatography. Mass Spectrometry Letters 12 (2021): 81-84.
- Mollé D, Jardin J, Piot M, et al. Comparison of electrospray and matrix-assisted laser desorption ionization on the same hybrid quadrupole time-of-flight tandem mass spectrometer: Application to bidimensional liquid chromatography of proteins from bovine milk fraction. Journal of Chromatography. A 1216 (2009): 2424-2432.
- Bezerra T, Estévez M, Lacerda JT, et al. (2020). Chicken Combs and Wattles as Sources of Bioactive Peptides: Optimization of Hydrolysis, Identification by LC-ESI-MS2 and Bioactivity Assessment. Molecules 25 (7): 1698.
- Liu H, Li B. Separation and identification of collagen peptides derived from enzymatic hydrolysate of Salmo salar skin and their anti-inflammatory activity in lipopolysaccharide ( LPS )-induced RAW264 .7 inflammatory model. Journal of Food Biochemistry 46 (2022): 369-401.
- Atef M, Chait YA, Ojagh SM, et al. Anti-Salmonella Activity and Peptidomic Profiling of Peptide Fractions Produced from Sturgeon Fish Skin Collagen (Huso huso) Using Commercial Enzymes. Nutrients 13 (2021): 2657.
- Pisanu S, Pagnozzi D, Pes M, et al. Differences in the peptide profile of raw and pasteurised ovine milk cheese and implications for its bioactive potential. International Dairy Journal 42 (2003) 26-33.
- Piovesana S, Capriotti AL, Cavaliere C, et al. Peptidome characterization and bioactivity analysis of donkey milk. Journal of Proteomics 119 (2015): 21-29.
- Deracinois B, Flahaut C, Duban-Deweer S, et al. Comparative and Quantitative Global Proteomics Approaches: An Overview. Proteomes 1 (2013): 180-218.
- González-García E, Puchalska P, Marina ML, et al. Fractionation and identification of antioxidant and angiotensin-converting enzyme-inhibitory peptides obtained from plum (Prunus domestica L.) stones. Journal of Functional Foods 19 (2015): 376-384.
- Peng J, Zhang H, Niu H, et al. Peptidomic analyses: The progress in enrichment and identification of endogenous peptides. Trends in Analytical Chemistry 125 (2020): 115835.
- Agyei D, Bambarandage E, Udenigwe CC. The Role of Bioinformatics in the Discovery of Bioactive Peptides. Encyclopedia of Food Chemistry. Academic Press (2019).
- Zhang J, Xin L, Shan B, et al. PEAKS DB: De Novo sequencing assisted database search for sensitive and accurate peptide identification. Molecular & Cellular Proteomics 11 (2012): M111.010587.
- Vyatkina K. Validation of De Novo Peptide Sequences with Bottom-Up Tag Convolution. Proteomes 10 (2021): 1.
- Wielsch N, Thomas H, Surendranath V, et al. Rapid Validation of Protein Identifications with the Borderline Statistical Confidence via De Novo Sequencing and MS BLAST Searches. Journal of Proteome Research 5 (2006): 2448-2456.
- Wong EHJ, Ng CG, Goh KL, et al. Metabolomic analysis of low and high biofilm-forming Helicobacter pylori strains. Scientific Reports 8 (2018): 1409.
- Bräcker J, Brockmeyer J. Characterization and Detection of Food Allergens Using High-Resolution Mass Spectrometry: Current Status and Future Perspective. Journal of Agricultural and Food Chemistry 66 (2018): 8935-8940.
- Capozzi F. Food Innovation in the Frame of Circular Economy by Designing Ultra-Processed Foods Optimized for Sustainable Nutrition. Frontiers in Nutrition 9 (2022): 886220.
- Uranga J, Etxabide A, Cabezudo S, et al. Valorization of marine-derived biowaste to develop chitin/fish gelatin products as bioactive carriers and moisture scavengers. Science of The Total Environment 706 (2020): 135747.
- Smith LE, Rogowska-Wrzesinska A. The challenge of detecting modifications on proteins. Essays in Biochemistry 64 (2020): 135-153.
- Fricker LD. Limitations of Mass Spectrometry-Based Peptidomic Approaches. Journal of the American Society for Mass Spectrometry 26 (2015): 1981-1991.
- Huang YP, Dias FFG, Moura Bell JM. A complete workflow for discovering small bioactive peptides in foods by LC-MS/MS: A case study on almonds. Food Chemistry 369 (2022): 130834.
- Creese AJ, Cooper HJ. Liquid Chromatography Electron Capture Dissociation Tandem Mass Spectrometry (LC-ECD-MS/MS) versus Liquid Chromatography Collision-induced Dissociation Tandem Mass Spectrometry (LC-CID-MS/MS) for the Identification of Proteins. Journal of the American Society for Mass Spectrometry 18 (2007): 891-897.
- Savastano ML, Liu Y, Mels J, et al. Profiling of multiphosphorylated peptides in kefir and their release during simulated gastrointestinal digestion. ACS Omega 4 (2019): 7963-7970.
- Tenenbaum M, Deracinois B, Dugardin C, et al. Identification, production and bioactivity of casein phosphopeptides - A review. Food Research International 157 (2022): 111360.
- López-Alarcón C, Denicola A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Analytica Chimica Acta 763 (2013): 1-10.
- Cai L, Wu X, Zhang Y, et al. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. Journal of Functional Foods 16 (2015): 234-242.
- Ngo DH, Qian ZJ, Ryu B, et al. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. Journal of Functional Foods, 2 (2010): 107-117.
- Sun L, Zhang Y, Zhuang Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. Journal of Functional Foods 5 (2013): 154-162.
- Zhang Y, Duan X, Zhuang Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 38 (2012): 13-21.
- Chi CF, Wang B, Hu FY, et al. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Research International 73 (2015): 124-129.
- Chen Y, Jin H, Yang F, et al. Physicochemical, antioxidant properties of giant croaker (Nibea japonica) swim bladders collagen and wound healing evaluation. International Journal of Biological Macromolecules 138 (2019): 483-491.
- Li DD, Li WJ, Kong SZ, et al. Protective effects of collagen polypeptide from tilapia skin against injuries to the liver and kidneys of mice induced by d-galactose. Biomedicine & Pharmacotherapy 117 (2019): 109-204.
- Seo JK, Lee MJ, Go HJ, et al. Purification and characterization of YFGAP, a GAPDH-related novel antimicrobial peptide, from the skin of yellowfin tuna, Thunnus albacares. Fish & Shellfish Immunology 33 (2012): 743-752. 206. Seo JK, Lee MJ, Go HJ, et al. Antimicrobial function of the GAPDH-related antimicrobial peptide in the skin of skipjack tuna, Katsuwonus pelamis. Fish & Shellfish Immunology 36 (2014): 571-581.
- Su, Y. Isolation and identification of pelteobagrin, a novel antimicrobial peptide from the skin mucus of yellow catfish (Pelteobagrus fulvidraco). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 158 (2011): 149-154.
- Zhao W, Li J, Li Y, et al. Preventive Effect of collagen peptides from acaudina molpadioides on acute kidney injury through attenuation of oxidative stress and inflammation. Oxidative Medicine and Cellular Longevity (2022): 8186838.
- Amengual-Peñafiel L, Brañes-Aroca M, Marchesani-Carrasco, et al. Coupling between Osseointegration and Mechanotransduction to Maintain Foreign Body Equilibrium in the Long-Term: A Comprehensive Overview. Journal of Clinical Medicine 8 (2019): 139.
- Mieszkowska A, Beaumont H, Martocq L, et al. Phenolic-Enriched Collagen Fibrillar Coatings on Titanium Alloy to Promote Osteogenic Differentiation and Reduce Inflammation. International Journal of Molecular Sciences 21 (2020): E6406.
- Geyikoglu F, Emir M, Colak S, et al. Effect of oleuropein against chemotherapy drug-induced histological changes, oxidative stress, and DNA damages in rat kidney injury. Journal of Food and Drug Analysis 25 (2017): 447-459.
- Shao BZ, Xu ZQ, Han BZ, et al. NLRP3 inflammasome and its inhibitors: A review. Frontiers in Pharmacology 6 (2015).
- Miao B, Zheng J, Zheng G, et al. Using Collagen Peptides From the Skin of Monkfish (Lophius litulon) to Ameliorate Kidney Damage in High-Fat Diet Fed Mice by Regulating the Nrf2 Pathway and NLRP3 Signaling. Frontiers in Nutrition 9 (2022): 798708.
- Elliott WJ. Systemic hypertension. Current Problems in Cardiology 32 (2007): 201-259.
- Begic E, Mandzuka M, Begic Z, et al. Antihypertensive therapy dosage calculator. In A. Badnjevic (Ed.), CMBEBIH 62 (2017): 660-665.
- Ames MK, Atkins CE, Pitt B. The renin-angiotensin-aldosterone system and its suppression. Journal of Veterinary Internal Medicine 33 (2019): 363-382.
- Patel S, Rauf A, Khan H, et al. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 94 (2017): 317-325.
- Ko SC, Kang N, Kim EA, et al. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochemistry 47 (2012): 2005-2011.
- Lee SH, Qian ZJ, Kim SK. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chemistry 118 (2010): 96-102.
- Du L, Fang M, Wu H, et al. A novel angiotensin I-converting enzyme inhibitory peptide from Phascolosoma esculenta water-soluble protein hydrolysate. Journal of Functional Foods 5 (2013): 475-483.
- He H, Chen X, Wu H, et al. High throughput and rapid screening of marine protein hydrolysates enriched in peptides with angiotensin-I-converting enzyme inhibitory activity by capillary electrophoresis. Bioresource Technology 98 (2007): 3499-3505.
- Qian ZJ, Heo SJ, Oh CH, et al. Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptide Isolated from Biodiesel Byproducts of Marine Microalgae, Nannochloropsis Oculata. Journal of Biobased Materials and Bioenergy 7 (2013): 135-142.
- Ishak NH, Sarbon NM. A Review of Protein Hydrolysates and Bioactive Peptides Deriving from Wastes Generated by Fish Processing. Food and Bioprocess Technology 11 (2018): 2-16.
- Pujiastuti DY, Ghoyatul Amin MN, Alamsjah MA, et al. Marine Organisms as Potential Sources of Bioactive Peptides that Inhibit the Activity of Angiotensin I-Converting Enzyme: A Review. Molecules 24 (2019): E2541.
- Nongonierma AB, FitzGerald RJ. Learnings from quantitative structure activity relationship (QSAR) studies with respect to food protein-derived bioactive peptides: A review. RSC Advances 16 (2016): 56-72.
- Sagardia I, Roa-Ureta RH, Bald C. A new QSAR model, for angiotensin I-converting enzyme inhibitory oligopeptides. Food Chemistry 136 (2013): 1370-1376.
- Wu J, Aluko RE, Nakai S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure- Activity Relationship Study of Di- and Tripeptides. Journal of Agricultural and Food Chemistry 54 (2006): 732-738.
- Wang Q. Preparation of Functional Peanut Oligopeptide and Its Biological Activity. In Q. Wang, Peanut Processing Characteristics and Quality Evaluation. Springer Singapore (2018): 461-537.
- FitzGerald RJ, Meisel H. Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. The British Journal of Nutrition 84 (2000): S33-37.
- Piepho RW. Overview of the angiotensin-converting-enzyme inhibitors. American Journal of Health-System Pharmacy: AJHP: Official Journal of the American Society of Health-System Pharmacists 57 (2000): S3- S 7.
- He HL, Liu D, Ma CB. Review on the Angiotensin-I-Converting Enzyme (ACE) Inhibitor Peptides from Marine Proteins. Applied Biochemistry and Biotechnology 169 (2013): 738-749.
- Hai-Lun H, Xiu-Lan C, Cai-Yun S, et al. Analysis of novel angiotensin-I-converting enzyme inhibitory peptides from protease-hydrolyzed marine shrimpAcetes chinensis. Journal of Peptide Science 12 (2006): 726-733.
- Park SY, Je JY, Ahn CB. Protein Hydrolysates and Ultrafiltration Fractions Obtained from Krill (Euphausia superba): Nutritional, Functional, Antioxidant, and ACE-Inhibitory Characterization. Journal of Aquatic Food Product Technology 25 (2016): 1266-1277.
- Noorani KPM, Nazeer RA. Enzymatic Production of Two Tri-peptides on ACE-I Inhibition and Antioxidant Activities. International Journal of Peptide Research and Therapeutics 26 (2020): 2365-2377.
- Sasaki C, Tamura S, Tohse R, et al. Isolation and identification of an angiotensin I-converting enzyme inhibitory peptide from pearl oyster (Pinctada fucata) shell protein hydrolysate. Process Biochemistry 77 (2019): 137-142.
- Liu X, Zhang M, Shi Y, et al. Production of the angiotensin I converting enzyme inhibitory peptides and isolation of four novel peptides from jellyfish (Rhopilema esculentum) protein hydrolysate: ACE inhibitory peptides from jellyfish protein hydrolysate. Journal of the Science of Food and Agriculture 96 (9): 3240-3248.
- Zhao Y, Li B, Dong S, et al. A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate. Peptides 30 (2009): 1028-1033.
- Byun HG, Kim SK. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process Biochemistry 36 (2001): 1155-1162.
- Vo T.S, Ngo D.H, Kim J.A, et al. An Antihypertensive Peptide from Tilapia Gelatin Diminishes Free Radical Formation in Murine Microglial Cells. Journal of Agricultural and Food Chemistry 59 (2011): 12193-12197.
- Chen J, Liu Y, Wang G, et al. Processing Optimization and Characterization of Angiotensin-Ι-Converting Enzyme Inhibitory Peptides from Lizardfish (Synodus macrops) Scale Gelatin. Marine Drugs 16 (2018): 228.
- Huang CY, Tsai YH, Hong YH, et al. Characterization and Antioxidant and Angiotensin I-Converting Enzyme (ACE)-Inhibitory Activities of Gelatin Hydrolysates Prepared from Extrusion-Pretreated Milkfish (Chanos chanos) Scale. Marine Drugs 16 (2018): 346.
- Yamamoto N. Antihypertensive peptides derived from food proteins. Biopolymers 43 (1997): 129-134.
- Lombardo M, Bellia C, Moletto C, et al. Effects of Quality and Quantity of Protein Intake for Type 2 Diabetes Mellitus Prevention and Metabolic Control. Current Nutrition Reports 9 (2020): 329-337.
- Hsieh CH, Wang TY, Hung CC, et al. Improvement of glycemic control in streptozotocin-induced diabetic rats by Atlantic salmon skin gelatin hydrolysate as the dipeptidyl-peptidase IV inhibitor. Food & Function 6 (2015): 1887-1892.
- Devasia S, Kumar P, Stephena S, et al. Double Blind, Randomized Clinical Study to Evaluate Efficacy of Collagen Peptide as Add on Nutritional Supplement in Type 2 Diabetes. Journal of Clinical Nutrition and Food Science 12 (2018) : 56-61.
- Rubio IG, Castro G, Zanini AC, et al. Oral ingestion of a hydrolyzed gelatin meal in subjects with normal weight and in obese patients: Postprandial effect on circulating gut peptides, glucose and insulin. Eating and Weight Disorders 13 (2008): 48-53.
- Dugardin C, Cudennec B. An Exploratory Study of the Role of Dietary Proteins in the Regulation of Intestinal Glucose Absorption. Frontiers in Nutrition 8 (2022): 10.
- Heffernan S, Nunn L, Harnedy-Rothwell PA, et al. Blue Whiting (Micromesistius poutassou) Protein Hydrolysates Increase GLP-1 Secretion and Proglucagon Production in STC-1 Cells Whilst Maintaining Caco-2/HT29-MTX Co-Culture Integrity. Marine Drugs 20 (2022): 112.
- Sasaoka Y, Kishimura H, Adachi S, et al. Collagen peptides derived from the triple helical region of sturgeon collagen improve glucose tolerance in normal mice. Journal of Food Biochemistry 42 (2018): e12478.
- Sasaoka Y, Takagi T, Michiba S, et al. Study on the Mechanism of the Blood-Glucose-Lowering Effect of Collagen Peptides from Sturgeon By-Products. Marine Drugs 19 (2021): 584.
- Harnedy PA, Parthsarathy V, McLaughlin CM, et al. Atlantic salmon (Salmo salar) co-product-derived protein hydrolysates: A source of antidiabetic peptides. Food Research International 106 (2018b): 598-606.
- Casanova-Martí À, Bravo FI, Serrano J, et al. Antihyperglycemic effect of a chicken feet hydrolysate via the incretin system: DPP-IV-inhibitory activity and GLP-1 release stimulation. Food & Function 10 (2019): 4062-4070.
- Lima R, Berg RS, Rønning SB, et al. Peptides from chicken processing by-product inhibit DPP-IV and promote cellular glucose uptake: Potential ingredients for T2D management. Food & Function 10 (2019): 1619-1628.
- Iba Y, Yokoi K, Eitoku I, et al. Oral Administration of Collagen Hydrolysates Improves Glucose Tolerance in Normal Mice Through GLP-1-Dependent and GLP-1-Independent Mechanisms. Journal of Medicinal Food 19 (2016): 836-843.
- Mudgil P, Jobe B, Kamal H, et al. Dipeptidyl peptidase-IV, α-amylase, and angiotensin I converting enzyme inhibitory properties of novel camel skin gelatin hydrolysates. LWT 101 (2019): 251-258.
- Sila A, Martinez-Alvarez O, Haddar A, et al. Recovery, viscoelastic and functional properties of Barbel skin gelatine: Investigation of anti-DPP-IV and anti-prolyl endopeptidase activities of generated gelatine polypeptides. Food Chemistry 168 (2015): 478-486.
- Hatanaka T, Inoue Y, Arima J, et al. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chemistry 134 (2012): 797-802.
- Nongonierma AB, FitzGerald RJ. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 39 (2013): 157-163.
- Uenishi H, Kabuki T, Seto Y, et al. Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-4)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. International Dairy Journal 22 (2012): 24-30.

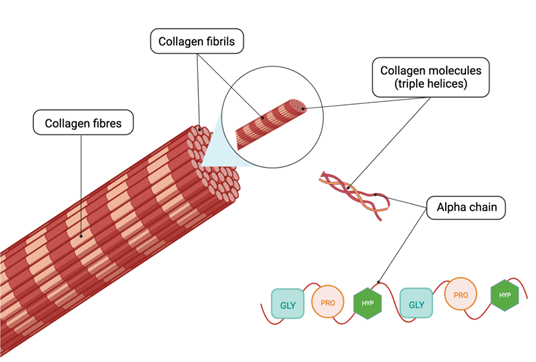
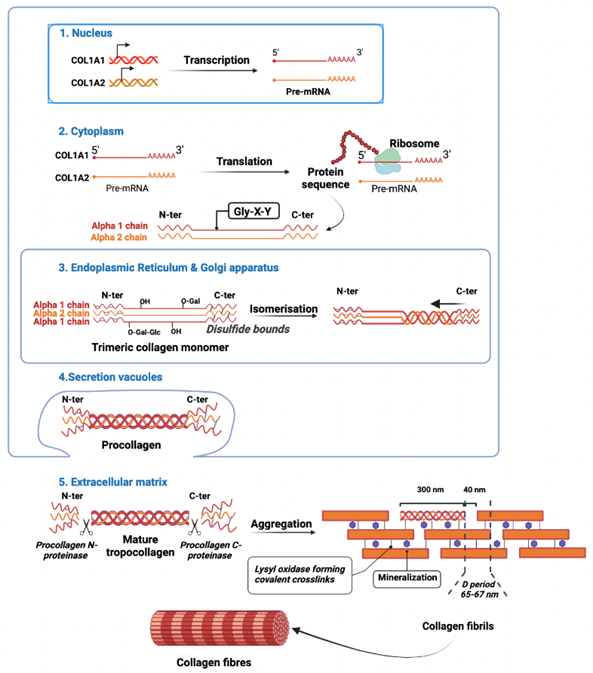
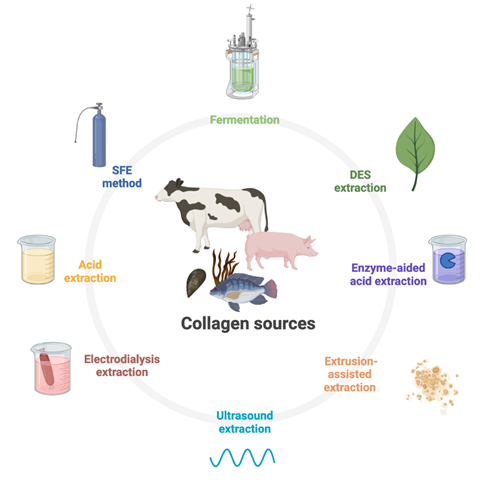
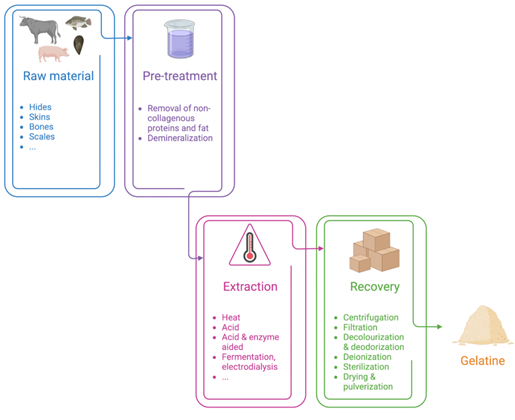
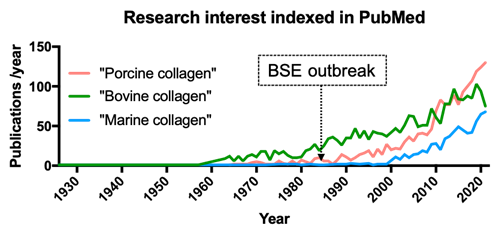
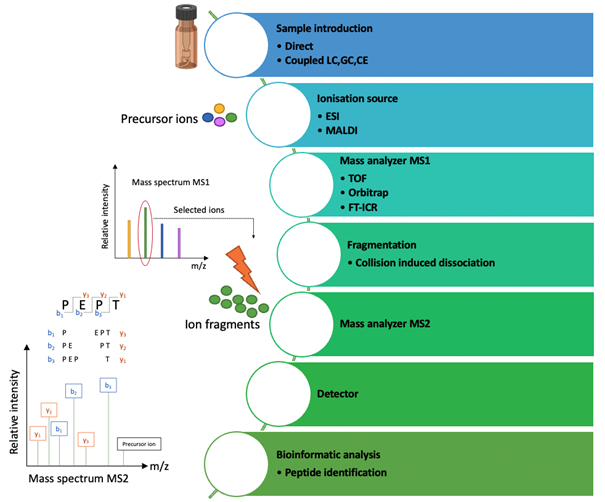

 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 