Characterization of Physicochemical Properties of Cooking Oils Sold in Phnom Penh, Cambodia
Manit Say1, Punlork Heng2, Sela Kong1,2, Chin Ping Tan3, Sivchheng Phal1,2, Yukleav Nat1,2, Reasmey Tan1,2*
1Research and Innovation Center, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia
2Faculty of Chemical and Food Engineering, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia
3Department of Food Technology, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 UPM Serdang Selangor, Malaysia
*Corresponding Author: Reasmey Tan, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia.
Received: 15 August 2023; Accepted: 24 August 2023; Published: 30 January 2024
Article Information
Citation:
Manit Say, Punlork Heng, Sela Kong, Chin Ping Tan, Sivchheng Phal, Yukleav Nat, Reasmey Tan. Characterization of Physicochemical Properties of Cooking Oils Sold in Phnom Penh, Cambodia. Journal of Food Science and Nutrition Research. 7 (2024): 28-36.
DOI: 10.26502/jfsnr.2642-110000149
View / Download Pdf Share at FacebookAbstract
Cooking oils are prone to degradation through oxidation, leading to a loss of nutritional value and the development of off-flavors causing by the formation of oxidative by-products. The process, marked by reactivity and toxicity, can potentially contribute to health risks such as cancer and inflammation. In the context of Cambodia, cooking oils are currently imported from various countries without a comprehensive assessment of their quality. To address this gap, the present study aimed to assess the physicochemical characteristics of cooking oils available in Phnom Penh’s supermarkets. A total of 48 oil samples, sourced from different raw materials and brands, were subjected to extensive analysis to determine their physicochemical attributes. The results revealed a range of findings: peroxide value between 1.69 and 14.55 meq O2/kg, acid value between 0.11 and 1.62 mg KOH/g, iodine value ranging from 54.12 to 140.00 g I2/100 g, anisidine value from 19.90 to 138.13, and specific UV extinction at 233 nm and 269 nm varying between 2.30 to 10.82 and 0.32 to 4.36, respectively. Significantly, one sunflower oil sample exhibited a peroxide value exceeding the FAO’s Codex Alimentarius Standards, while five oils samples displayed acid value surpassing the recommended FAO limit. Furthermore, the color attributes (L*, a*, and b*) of the cooking oils were measured within ranges of 32.28 to 34.84, -1.75 to -0.15, and 0.93 to 6.83, respectively. These findings underscore the concern that certain cooking oils available in Phnom Penh’s supermarkets do not meet the established FAO standards, potentially attributable to factors such as expiration dates, inappropriate transportation, and improper storage conditions.
Keywords
<p>Cambodian supermarkets, Importation, Peroxide value, Acid value, Soybean oil, Sunflower oil</p>
Article Details
1. Introduction
Oils and fats are important parts of human diet as they are rich sources of dietary energy and contain more than twice the caloric value of equivalent amount of sugar [1]. Their functional and textural characteristics contribute to the flavor and palatability of natural and prepared foods [2]. They contain certain fatty acids which play an important role in nutrition and are also carriers of fat-soluble vitamins. Vegetable oils may get rancid and hence lose its nutritional values and favor upon improper extraction process, handling and storage [3]. Moisture, microbes, air, antioxidants, and exposure to sunlight are among factors determining the rancidity or deterioration time of oils [4].
In quality control, several parameters such as iodine value (degree of unsaturation), peroxide value (formation of primary oxidation products), moisture content, specific gravity (purity), and acid value (free fatty acids formation because of rancidity) are key parameters of interest as they determine the shelf-life quality and hence the economic value of oils [5]. Rancidity of vegetable oils may pose health risks including cancer and inflammation because of the formation of toxic and reactive oxidation products [5,6]. For healthy consumption, unsaturated oils are better than saturated ones. Consumption of palmitic oil (highly saturated) is associated with an increased risk of developing cardiovascular diseases [7,8]. In contrast, edible vegetable oils such as sunflower, olive, canola and niger-seed oils contain high levels of polyunsaturated fats, which make them susceptible for rancidity [9]. FAO has outlined the quality standards for various constituents of edible vegetable oils, contaminated heavy metals, fatty acid composition, antioxidants, micronutrients, and other physicochemical parameters. The FAO guideline sets the maximum allowable limit for edible oils quality parameters including moisture (0.2%), acid value (0.6 mg potassium hydroxide/g oil) and peroxide value (10 meq O2/kg oil) [10]. In Cambodia, cooking oils with different types of raw materials and brands are imported from other countries for daily consumption. In addition, their physicochemical quality has not yet been evaluated. Therefore, the purpose of this study was to evaluate the quality of cooking oils sold in the supermarkets in Phnom Penh, Cambodia.
2. Materials and Methods
2.1 Sample preparation
In this study, 48 cooking oil samples with different raw materials and brands were collected from supermarkets in Phnom Penh city, Cambodia. Among 48 oil samples, there were 2 brands of canola oil, 2 brands of coconut oil, 3 brands of corn oil, 1 brand of groundnut oil, 2 brands of palm olein, 4 brands of rice bran oil, 1 brand of sesame oil, 11 brands of soybean oil, 16 brands of sunflower oil, and 6 brands of blended oil. All oil samples were then stored in a dark place at room temperature prior to analysis of their physicochemical quality.
2.2 Peroxide value of cooking oils
In this experiment, 5 g of each oil sample were dissolved in 50 mL of acetic acid/chloroform with the ratio of 3:2. The mixture solution was immediately reacted with 0.5 mL of saturated potassium iodide. A volume of 30 mL of distilled water was added into the mixture. The liberated iodine was then titrated with 0.1 M sodium-thiosulphate using 0.5 mL of starch solution as an indicator until the color disappeared. And the blank sample was performed without the sample [11-13]. The peroxide value was then calculated by using the following formula:
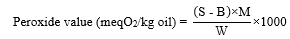
Where B is the volume of sodium-thiosulphate used for the blank, S is the volume of sodium-thiosulphate consumed by the oil sample, M is the molarity of sodium-thiosulphate, and W is the weight of oil sample.
2.3 Acid value (AV) of cooking oils
Five grams of oil sample were mixed with 75 mL of freshly neutralized hot ethyl alcohol. And phenolphthalein was used as an endpoint indicator. The solution was then titrated against 0.1 N of sodium hydroxide until the first pink color appeared which persisted at least 30s [12,13]. The acid value was then calculated as the equation below:
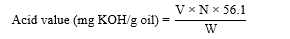
Where V is the volume of standard KOH solution (mL), N is the normality of standard KOH solution, W is the weight of oil sample in grams, and molecular weight of KOH = 56.1 g/mol.
2.4 Iodine value (IV) of cooking oils
A specific amount of oil sample was weighed and put in 500 mL. Then, 15 mL of mixture of cyclohexane and acetic acid (1:1 v/v) and 25 mL of Wijs solution were added. And the blank test was determined without the oil sample. The solution was then shaken and put in a dark place for an hour and 2 hours for the sample obtaining IV<150 and IV>150, respectively. A volume of 20 mL of 10 % KI was added, followed by 100 mL of distilled water. The final content was titrated with 0.1 mol/L sodium-thiosulphate solution using starch as an indicator [13,14]. The iodine value was then calculated as follow:
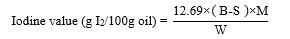
Where B is the volume in mL of standard sodium thiosulphate solution required for the blank, S is the volume in mL of standard sodium thiosulphate solution required for sample, M is the molarity of the standard sodium thiosulphate, and W is the weight in g of the sample.
2.5 Anisidine value
A gram of oil sample was dissolved and adjusted the volume to 25 mL using 2,2,4-trimethylpentance. The unreacted test solution was prepared by transferring 5 mL of previous solution into the test tube and 1 mL of glacial acetic acid was added. The solution was incubated in a dark place at 23 °C for 10 min. The solution was transferred to a clear and dry spectrophotometer cell. Absorbance of the unreacted test solution (A0) was determined at 350 nm. The absorbance of reacted test solution (A1) was analysed following the unreacted test solution. But the glacial acetic acid was substituted by anisidine reagent. The absorbance of blank (A2) was observed according to the reacted test solution method by replacing the sample to 5 mL of iso-octane [12]. The anisidine value of the vegetable oil sample was calculated as following formula:
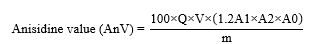
Where A0 is absorbance of the unreacted test solution, A1 is absorbance of reacted test solution, A2 is absorbance of the blank, Q is sample content (gram/mL, Q is 0.1 g/mL), V equal to 25 mL, the volume in which the test sample is dissolved, 1.2 is the correction factor for the dilution of the 5 mL of test solution with 1 mL of the reagent, and m is mass (g) of the test portion.
2.6 Specific UV extinction
The oil sample was weighted 0.12 g to the nearest 0.0001 g and dissolved in 25 mL volumetric flask using iso-octan. The absorbances of the solution were analysed using spectrophotometer at 233, 269, and 446 nm to determine the UV extinction at 233 nm, UV extinction at 269 nm, and carotene content, respectively [12]. The specific UV extinction at 233 nm and 269 nm were calculated as equation below:
Where E is absorbance at 446 nm, C is concentration of the solution (g per 100 mL), and I is path length of the cell (cm)
2.7 Color
The color of cooking oil samples was determined using Chroma Meter (CR-400, KONICA MINOLTA, Japan). The color parameters were presented as L* (lightness extends from 0 to 100 for black to white, respectively), a* (redness to greenness), and b* (yellowness to blueness). The instrument was standardized using white calibration plate. And the experiments were conducted in triplicate.
2.5 Statistical analysis
For all parameters, the experiments were done in triplicate and the values of each parameter were reported in term of mean ± standard deviation (SD). The comparison of mean value was made using analysis of variance ANOVA followed by SPSS with (p<0.05) significant level.
3. Results and discussion
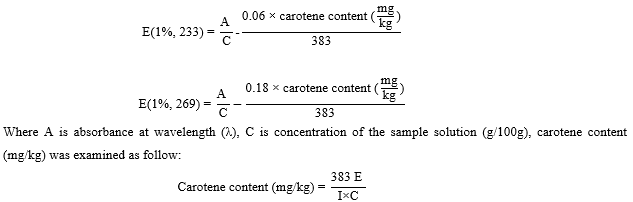
Peroxide values of cooking oils sold in the supermarkets
Peroxides are the precursors of breakdown products that cause rancid flavors in fat. The concentration of peroxides is indicative of oxidation during the early stages of lipid deterioration. This index becomes less reliable during the later stage of deterioration due to the degradation increase of peroxide [15]. When the peroxide value is between 20 and 40 meq oxygen/kg, the rancid taste begins to be noticeable. Peroxide value is defined as reactive oxygen contents expressed in terms of milliequivalents (meq) of oxygen per kilogram of fat/oil [16]. The peroxide values of cooking oils sold in the supermarkets in Phnom Penh were insignificant differences (p<0.05) among various oil brands and oil types. According to figure 1, the peroxide values of blended oil, canola oil, coconut oil, corn oil, groundnut oil, palm olein, rice bran oil, sesame oil, soybean oil, and sunflower oil ranged from 2.57 to 6.19, 3.68 to 5.51, 1,56 to 1.62, 3.38 to 5.99, 5.76, 2.69 to 4.01, 2.61 to 4.71, 3.41, 1.69 to 5.18, and 3.01 to 14.55 meq O2/Kg oil, respectively. In addition, there was only one sunflower oil sample that contained peroxide value (14.55 meq O2/Kg oil) higher than FAO’s Codex Alimentarius Standards. This sample was already expired but it was still available in the supermarket. The results showed a wide range of peroxide values of cooking oils sold in the supermarkets in Phnom Penh due to the unequal production and expiration date. The high peroxide values were found in soybean oils and sunflower oils may be due to the high unsaturated fatty acid containing [17] while the high peroxide value found in high saturated fatty acid oils close to the expiration date. The lower peroxide value of cooking oil is negatively correlated to saturation of fatty acids. Previous research mentioned that PV should not be above 10 to 20 meq O2/kg fat to avoid rancidity flavor [18].
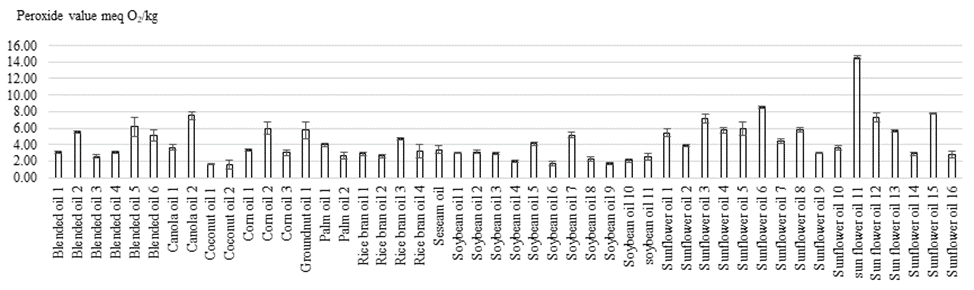
Acid values of cooking oils sold in the supermarkets
The acid value is a measurement of the extent to which the glycerides in the oil have been hydrolyzed by lipase action [19]. The glycerides are also hydrolyzed with water in the presence of air possibly bacteria. The decomposition is accelerated by heat and light [20]. The acid values of cooking oil sold in the supermarkets in Phnom Penh are shown in figure 2. There were significant differences (p<0.05) of acid values of cooking oils between different brands and types. Acid values of blended oil, canola oil, coconut oil, corn oil, groundnut oil, palm olein, rice bran oil, sesame oil, soybean oil, and sunflower oil ranged from 0.10 to 1.62, 0.12 to 0.14, 0.07 to 0.08, 0.15 to 0.22, 0.22, 0.14 to 0.26, 0.48 to 0.68, 0.56, 0.08 to 0.27, 0.10, 0.93 mg KOH/g oil, respectively. There were 5 out of 48 cooking oils samples which contained acid values higher than FAO’s Codex Alimentarius Standard (0.6 mg KOH/g oil). They were sunflower oil 8 (0.93 mg KOH/g oil), rice bran oil 2 (0.68 mg KOH/g oil), rice bran oil 4 (0.68 mg KOH/g oil), blended oil 2 (1.62 mg KOH/g oil), and sesame oil (0.79 mg KOH/g oil). In addition, high acid values were observed in all rice bran oil samples. The samples with higher acid value than the FAO standard may be caused by the inappropriate conditions during transportation, storage, and distribution which accelerated the hydrolysis of triglycerides. This high acid value can affect the peroxide value due to the peroxides that are the oxidation products of the free fatty acids [21].
Iodine values of cooking oils sold in the supermarkets
Iodine value is used to indicate the degree of unsaturation in vegetable oil/animal fat/methyl ester or average number of double bonds in fats and oils, also known as iodine number [22]. The decrease in iodine value shows the decrease in the number of double bonds and it indicates the lower oxidation of oil. The iodine value indicates the mass of iodine (I2) in grams that is necessary to completely saturate, by mean of a stoichiometric reaction, the molecules of 100g of a given oil [23]. There were significant differences (p<0.05) of iodine values between oil samples collected from the supermarkets. The iodine values by oil types are also presented in table 1. The results showed that the iodine values of blended oils, canola oils, coconut oils, corn oils, groundnut oil, palm olein oil, rice bran oil, sesame oil, soybean oils, and sunflower oils sold in the supermarkets in Phnom Penh ranged from 54.12 to 123.21, 110.09 to 113.34, 8.31 to 10.91, 111.31 to 128.54, 104.08, 66.11 to 68.61, 99.81 to 106.97, 112.47, 108.53 to 140.00, and 124.40 to 136.09, respectively. These results agreed with those described by [24,25] which presented the same range of iodine values. The high iodine values were found in soybean oil and sunflower oil due to the high unsaturated fatty acids. The iodine value refers to the saturation of fatty acids which the higher iodine value, the lower saturation of oil [26]. High unsaturated fatty acids are recommended for the healthy consumption over the high saturated fatty acids containing oils [5].
|
Oil types |
Iodine value (mg I2/100g) |
Anisidine value |
Specific UV 233 |
Specific UV 269 |
|
Blended oil 1 |
110.77 ± 1.24 |
45.34 ± 1.59 |
7.19 ± 0.02 |
1.10 ± 0.00 |
|
Blended oil 2 |
54.12 ± 2.52 |
37.93 ± 2.41 |
2.44 ± 0.00 |
0.49 ± 0.00 |
|
Blended oil 3 |
101.62 ± 3.47 |
30.64 ± 0.59 |
5.68 ± 0.00 |
1.81 ± 0.00 |
|
Blended oil 4 |
88.93 ± 2.41 |
20.78 ± 1.78 |
2.30 ± 0.00 |
0.32 ± 0.00 |
|
Blended oil 5 |
123.21 ± 14.81 |
34.12 ± 15.40 |
3.28 ± 0.00 |
0.77 ± 0.00 |
|
Blended oil 6 |
114.75 ± 6.15 |
82.06 ± 37.57 |
5.29 ± 0.01 |
1.15 ± 0.04 |
|
Canola oil 1 |
110.09 ± 8.0 |
10.60 ± 3.02 |
3.07 ± 0.00 |
0.47 ± 0.00 |
|
Canola oil 2 |
113.34 ± 3.14 |
46.73 ± 2.42 |
3.78 ± 0.01 |
2.11 ± 0.00 |
|
Coconut oil 1 |
10.91 ± 4.88 |
9.94 ± 0.88 |
1.12 ± 0.00 |
0.07 ± 0.00 |
|
Coconut oil 2 |
8.31 ± 6.20 |
7.11 ± 11.72 |
1.27 ± 0.00 |
0.11 ± 0.00 |
|
Corn oil 1 |
111.31 ± 1.57 |
28.27 ± 2.94 |
3.60 ± 0.00 |
1.45 ± 0.00 |
|
Corn oil 2 |
128.54 ± 20.43 |
39.40 ± 30.76 |
3.01 ± 0.01 |
1.37 ± 0.00 |
|
Corn oil 3 |
114.40 ± 16.47 |
19.95 ± 19.90 |
2.01 ± 0.00 |
0.81 ± 0.00 |
|
Groundnut oil 1 |
104.08 ± 6.36 |
37.58 ± 29.85 |
3.05 ± 0.00 |
1.58 ± 0.00 |
|
Palm olein 1 |
68.61 ± 3.50 |
40.26 ± 1.93 |
3.34 ± 0.00 |
0.78 ± 0.00 |
|
Palm olein 2 |
66.11 ± 11.80 |
33.50 ± 36.37 |
1.80 ± 0.00 |
0.54 ± 0.00 |
|
Rice bran oil 1 |
100.09 ± 2.01 |
39.89 ± 3.41 |
7.28 ± 0.01 |
2.76 ± 0.00 |
|
Rice bran oil 2 |
99.81 ± 1.41 |
62.79 ± 3.76 |
6.07 ± 0.00 |
3.08 ± 0.00 |
|
Rice bran oil 3 |
106.97 ± 0.83 |
51.31 ± 1.96 |
10.82 ± 0.09 |
3.24 ± 0.00 |
|
Rice bran oil 4 |
102.82 ± 2.37 |
132.95 ± 54.21 |
8.49 ± 0.01 |
2.14 ± 0.00 |
|
Sesame oil |
112.47 ± 27.43 |
32.40 ± 6.45 |
3.51 ± 0.00 |
1.02 ± 0.00 |
|
Soybean oil 1 |
120.97 ± 2.27 |
21.62 ± 2.52 |
5.69 ± 0.00 |
1.03 ± 0.00 |
|
Soybean oil 2 |
121.53 ± 2.97 |
30.77 ± 0.81 |
4.78 ± 0.00 |
1.77 ± 0.00 |
|
Soybean oil 3 |
121.34 ± 1.67 |
51.74 ± 1.60 |
5.44 ± 0.00 |
1.89 ± 0.00 |
|
Soybean oil 4 |
125.92 ± 0.63 |
28.71 ± 2.48 |
4.20 ± 0.00 |
2.21 ± 0.00 |
|
Soybean oil 5 |
121.51 ± 0.64 |
24.86 ± 3.81 |
8.15 ± 0.01 |
2.10 ± 0.00 |
|
Soybean oil 6 |
140.00 ± 3.61 |
37.16 ± 1.94 |
5.81 ± 0.01 |
1.64 ± 0.00 |
|
Soybean oil 7 |
108.53 ± 3.03 |
27.34 ± 3.07 |
4.59 ± 0.00 |
1.12 ± 0.00 |
|
Soybean oil 8 |
108.83 ± 0.37 |
36.60 ± 2.87 |
4.42 ± 0.00 |
2.32 ± 0.00 |
|
Soybean oil 9 |
126.30 ± 1.96 |
30.47 ± 2.28 |
5.16 ± 0.00 |
2.23 ± 0.00 |
|
Soybean oil 10 |
109.18 ± 1.04 |
19.91 ± 5.28 |
5.26 ± 0.01 |
1.57 ± 0.00 |
|
Soybean oil 11 |
116.90 ± 5.35 |
33.98 ± 3.83 |
3.34 ± 0.01 |
0.58 ± 0.00 |
|
Sunflower oil 1 |
124.40 ± 0.97 |
87.17 ± 1.50 |
5.09 ± 0.00 |
3.05 ± 0.00 |
|
Sunflower oil 2 |
128.89 ± 2.94 |
62.50 ± 1.30 |
4.97 ± 0.00 |
2.83 ± 0.00 |
|
Sunflower oil 3 |
124.76 ± 1.66 |
120.06 ± 1.32 |
5.86 ± 0.01 |
3.89 ± 0.00 |
|
Sunflower oil 4 |
134.17 ± 1.51 |
91.37 ± 2.49 |
3.95 ± 0.00 |
4.36 ± 0.00 |
|
Sunflower oil 5 |
130.79 ± 1.25 |
106.33 ± 3.58 |
4.95 ± 0.00 |
1.99 ± 0.00 |
|
Sunflower oil 6 |
132.65 ± 4.27 |
49.35 ± 2.09 |
5.48 ± 0.00 |
2.02 ± 0.00 |
|
Sunflower oil 7 |
136.09 ± 1.98 |
77.51 ± 4.63 |
4.38 ± 0.00 |
2.94 ± 0.00 |
|
Sunflower oil 8 |
127.38 ± 2.02 |
22.62 ± 2.03 |
5.34 ± 0.00 |
1.62 ± 0.00 |
|
Sunflower oil 9 |
126.04 ± 3.42 |
90.44 ± 3.56 |
6.51 ± 0.01 |
2.38 ± 0.00 |
|
Sunflower oil 10 |
105.96 ± 1.53 |
79.47 ± 0.73 |
5.25 ± 0.00 |
2.54 ± 0.01 |
|
Sunflower oil 11 |
115.11 ± 1.36 |
138.13 ± 1.92 |
10.09 ± 0.04 |
1.50 ± 0.00 |
|
Sunflower oil 12 |
131.82 ± 4.63 |
43.40 ± 0.95 |
5.26 ± 0.00 |
0.66 ± 0.00 |
|
Sunflower oil 13 |
127.39 ± 3.17 |
40.29 ± 0.79 |
4.11 ± 0.00 |
1.64 ± 0.00 |
|
Sunflower oil 14 |
123.14 ± 4.52 |
103.93 ± 11.88 |
2.69 ± 0.00 |
1.38 ± 0.00 |
|
Sunflower oil 15 |
131.68 ± 5.00 |
47.07 ± 24.69 |
3.21 ± 0.02 |
0.72 ± 0.00 |
|
Sunflower oil 16 |
118.63 ± 16.56 |
93.64 ± 6.77 |
3.89 ± 0.01 |
0.63 ± 0.00 |
Table 1: Iodine value, anisidine value, specific UV extinction at 233 nm, and specific UV extinction at 269 nm of the cooking oils sold in Cambodian supermarkets.
Anisidine values of cooking oils sold in the supermarkets
Anisidine value is a parameter that indicates the secondary oxidation compounds, primarily 2-alkenals and 2,4-alkadienals generated due to hydroperoxide decomposition, and it is more sensitive to unsaturated aldehydes. Anisidine value, as a measure of second oxidation products, is used instead of, or together with peroxide value to indicate the quality of oils. Anisidine value is considered to be more reliable parameter of oxidative rancidity that peroxide value in a long-time storage stability [27]. Anisidine values of different cooking oils are presented in table 1. The means of anisidine values between oil brands showed significant difference (p<0.05). The anisidine values of blended oil, canola oil, coconut oil, corn oil, groundnut oil, palm olein, rice bran oil, sesame oil, soybean oil, and sunflower oil ranged from 20.78 to 82.06, 10.60 to 46.73, 7.11 to 9.94, 19.95 to 39.40, 37.58, 33.05 to 40.25, 39.89 to 132.95, 32.40, 19.90 to 37.16, 22.61 to 138.13, respectively. The high anisidine value of cooking oils sold in the supermarkets due to the breakdown of peroxides and hydroperoxide [28,29]. Even though the anisidine value is considered to be more reliable parameter than peroxide value, but peroxide value still show fair correlations with the sensory parameters such as aroma, flavor, aftertaste of the samples while acid value showed no correlations [30].
Specific UV extinction of cooking oils sold in the supermarkets
The absorbance at ultra-violet wavelengths of the cooking oil products provides an indication of deterioration and purity of the oil product. In the ultra-violet region, the autoxidation products of fats and oils present the spectra characteristics: hydroperoxide and conjugated dienes from decomposition were detected at 233 nm, while the secondary products of autoxidation and ethylenic diketone were detected at 268 nm. The determination of absorbance in cooking oils can provide the indication of the state of autoxidation [12,31]. The specific UV extinctions of cooking oils at 233 nm and 269 nm are presented in table 1. There were significant differences (p<0.05) of specific UV extinction at 233 nm and 269 nm between cooking oils sold in the supermarkets in Phnom Penh. This high content of hydroperoxide, conjugated dienes, secondary products of autoxidation, and ethylenic diketone may be caused by the oil’s decomposition and autoxidation [32].
Color of cooking oils sold in the supermarkets
Color is an important parameter of edible oil, both in the refining process and in the marketplace. Oil appearance might be an indicator of the problems having occurred during processing and storage. The color of the oil samples is shown in table 2. The color (L*, a*, and b*) of cooking oils sold in the supermarkets in Phnom Penh was significant difference (p<0.05) between oil brands and oil types. The lightness and a* (green – red) of all cooking oils were in a narrow range while b* (blue – yellow) was in a wide range. The color of the cooking oil is due to carotenoids and other pigments while the formation of polymers and combination of peroxide and aldehyde will affect the redness and yellowness, respectively [33]. The difference of color in cooking oil may be due to the oil types, degree of oil refinery, and storage conditions [34,35].
|
Oil types |
L* |
a* |
b* |
|
Blended oil 1 |
32.83 ± 0.67 |
-0.31 ± 0.05 |
1.53 ± 0.23 |
|
Blended oil 2 |
32.50 ± 0.86 |
-1.56 ± 0.09 |
5.69 ± 0.21 |
|
Blended oil 3 |
33.56 ± 1.09 |
-0.71 ± 0.02 |
2.46 ± 0.23 |
|
Blended oil 4 |
32.74 ± 0.99 |
-1.37 ± 0.02 |
4.94 ± 0.14 |
|
Blended oil 5 |
33.17 ± 0.53 |
-0.01 ± 0.05 |
1.44 ± 0.03 |
|
Blended oil 6 |
33.14 ± 0.79 |
-0.42 ± 0.06 |
1.77 ± 0.14 |
|
Canola oil 1 |
32.71 ± 0.52 |
-0.44 ± 0.11 |
2.08 ± 0.15 |
|
Canola oil 2 |
33.40 ± 0.66 |
-0.51 ± 0.05 |
2.29 ± 0.13 |
|
Coconut oil 1 |
32.26 ± 0.39 |
-0.24 ± 0.07 |
1.58 ± 0.05 |
|
Coconut oil 2 |
30.60 ± 5.87 |
-0.03 ± 0.02 |
1.36 ± 0.15 |
|
Corn oil 1 |
33.61 ± 0.91 |
-1.31 ± 0.09 |
4.62 ± 0.18 |
|
Corn oil 2 |
31.94 ± 0.64 |
-0.49 ± 0.05 |
3.18 ± 0.23 |
|
Corn oil 3 |
31.91 ± 0.58 |
-0.85 ± 0.03 |
3.83 ± 0.23 |
|
Groundnut oil 1 |
31.00 ± 0.11 |
-0.55 ± 0.02 |
3.03 ± 0.10 |
|
Palm olein 1 |
2.65 ± 0.83 |
-1.49 ± 0.17 |
5.25 ± 0.51 |
|
Palm olein 2 |
32.57 ± 0.44 |
-1.28 ± 0.06 |
5.15 ± 0.12 |
|
Rice bran oil 1 |
32.38 ± 0.82 |
-1.17 ± 0.09 |
4.84 ± 0.18 |
|
Rice bran oil 2 |
32.89 ± 0.83 |
-1.69 ± 0.07 |
6.84 ± 0.35 |
|
Rice bran oil 3 |
33.39 ± 1.08 |
-1.76 ± 0.09 |
6.27 ± 0.15 |
|
Rice bran oil 4 |
33.08 ± 0.32 |
-2.10 ± 0.01 |
8.20 ± 0.15 |
|
Sesame oil |
30.98 ± 0.43 |
-0.73 ± 0.06 |
13.11 ± 0.44 |
|
Soybean oil 1 |
33.06 ± 0.14 |
-0.36 ± 0.06 |
1.77 ± 0.25 |
|
Soybean oil 2 |
32.71 ± 0.24 |
-0.66 ± 0.05 |
2.54 ± 0.20 |
|
Soybean oil 3 |
33.40 ± 0.49 |
-0.51 ± 0.03 |
1.80 ± 0.23 |
|
Soybean oil 4 |
33.22 ± 0.49 |
-0.45 ± 0.06 |
1.64 ± 0.11 |
|
Soybean oil 5 |
33.23 ± 0.65 |
-0.37 ± 0.03 |
1.76 ± 0.03 |
|
Soybean oil 6 |
33.36 ± 0.89 |
-0.59 ± 0.08 |
2.02 ± 0.16 |
|
Soybean oil 7 |
33.19 ± 0.62 |
-0.41 ± 0.09 |
1.58 ± 0.10 |
|
Soybean oil 8 |
32.98 ± 0.54 |
-0.38 ± 0.06 |
1.90 ± 0.15 |
|
Soybean oil 9 |
32.95 ± 0.41 |
-0.69 ± 0.02 |
2.15 ± 0.21 |
|
Soybean oil 10 |
33.61 ± 0.80 |
-0.61 ± 0.08 |
2.11 ± 0.19 |
|
Soybean oil 11 |
32.44 ± 0.81 |
-0.26 ±0.07 |
2.08 ± 0.12 |
|
Sunflower oil 1 |
33.64 ± 0.10 |
-0.91 ± 0.03 |
4.47 ± 0.03 |
|
Sunflower oil 2 |
34.42 ± 0.18 |
-0.34 ± 0.05 |
1.50 ± 0.23 |
|
Sunflower oil 3 |
34.70 ± 1.03 |
-0.28 ± 0.07 |
1.28 ± 0.23 |
|
Sunflower oil 4 |
34.85 ± 1.01 |
-0.42 ± 0.04 |
1.68 ± 0.22 |
|
Sunflower oil 5 |
34.80 ± 0.98 |
-0.36 ± 0.07 |
1.37 ± 0.29 |
|
Sunflower oil 6 |
33.37 ± 0.61 |
-0.16 ± 0.01 |
0.93 ± 0.07 |
|
Sunflower oil 7 |
33.20 ± 0.73 |
-0.42 ± 0.03 |
1.40 ± 0.12 |
|
Sunflower oil 8 |
33.44 ± 0.62 |
-0.34 ± 0.05 |
1.63 ± 0.17 |
|
Sunflower oil 9 |
33.27 ± 0.82 |
-0.27 ± 0.05 |
1.25 ± 0.01 |
|
Sunflower oil 10 |
33.53 ± 0.70 |
-0.44 ± 0.05 |
1.51 ± 0.15 |
|
Sunflower oil 11 |
33.94 ± 1.15 |
-0.21 ± 0.07 |
1.56 ± 0.16 |
|
Sunflower oil 12 |
33.48 ± 0.94 |
-0.29 ± 0.06 |
1.06 ± 0.16 |
|
Sunflower oil 13 |
33.33 ± 0.34 |
-0.42 ± 0.04 |
1.73 ± 0.09 |
|
Sunflower oil 14 |
33.18 ± 0.55 |
-0.30 ± 0.04 |
1.82 ± 0.08 |
|
Sunflower oil 15 |
33.03 ± 0.68 |
-0.15 ± 0.04 |
1.59 ± 0.16 |
|
Sunflower oil 16 |
31.93 ± 0.30 |
-0.59 ± 0.01 |
2.48 ± 0.15 |
Table 2: Color of cooking oils sold in Cambodian supermarkets.
4. Conclusion
In the context of cooking oils available in Phnom Penh’s supermarkets, a noteworthy proportion displayed elevated peroxide values, indicative of advanced rancidity. Among the forty-eight samples evaluated, fifteen exhibited rancidity levels surpassing half of the FAO standard. In addition, five samples surpassed the FAO standard for acid values. Intriguingly, these deviations were not inherently tied to oil types, suggesting that production and expiration date disparities played a role. The observed high anisidine values were likely influenced by the high peroxide value. Subtle variations in color were possibly attributed to oil types, pigments, and refining processes. The broad spectrum of physicochemical qualities observed can be attributed to inappropriate transportation, storage, and on-shelf conditions. For consumers, seeking optimal cooking oils, prioritizing a substantial period before the expiration date emerges as a pivotal factor in making informed purchasing decision.
Acknowledgement
The work was funded by Cambodia Higher Education Improvement Project (Credit No. 6221-KH).
References
- Van D, Gerrit. Food safety management: Oils and fats 12 (2014): 325-345.
- Atkinson G. Manley’s technology of biscuits, crackers and cookies: Fats and oils as biscuit ingredients 18 (2011): 160-180.
- Okparanta S. Assessment of rancidity and other physicochemical properties of edible oils (mustard and corn oils) stored at room temperature. Journal of Food and Nutrition Sciences 6 (2018): 70.
- Rossell JB. Frying: Factors affecting the quality of frying oils and fats 21 (2001): 115-164.
- Negash YA, Amare DE, Bitew BD, et al. Assessment of quality of edible vegetable oils accessed in Gondar City, Northwest Ethiopia. BMC Research Notes 12 (2019): 1-5.
- Banshi S, Singh RB, Bhardwaj K, et al. The role of functional food security in global health: Fats and oils for health promotion and disease prevention 8 (2019): 273-285.
- Mukherjee S, Mitra A. Health effects of palm oil. Journal of Human Ecology 26 (2009): 197-203.
- Marcus JB. Culinary Nutrition: Lipids basics: Fats and oils in foods and health 18 (2013): 231-277.
- Tao L. Oxidation of polyunsaturated fatty acids and its impact on food qualities and human health. Advance Food Technology and Nutritional Science 1 (2015): 135-142.
- Contoh B, Issa J, Tabares I, et al. Codex Stan 21-1999. Standard for named vegetable oils. Codex Alimentarius 44 (2011).
- AOCS Official Method Cd 8b-90. Peroxide value acetic acid-isooctane method. official methods and recommended practices of the AOCS, Sixth edition. Firestone, D. (edtn); AOCS Press, Champaign, IL, USA (2009): 1-3.
- MPOB Test Method. Methods of test for palm and palm oil products. Palm Oil Research Institute of Malaysia, Ministry of Primary Industries, Malaysia (2004): 1-416.
- Kim L, Siang C. Analysis of oils: Determination of peroxide value (2021).
- AOCS Official Method Cd 1d-92. Iodine value of fats and oils cyclohexane-acetic acid method. official methods and recommended practices of the AOCS, Sixth edition. Firestone, D. (edtn); AOCS Press, Champaign, IL, USA (2009): 1-3.
- Echegaray N, Pateiro M, Nieto G, et al. Chapter 7: Lipid oxidation of vegetable oils. In Food Lipids; Press: San Diego, CA, USA (2023): 127-152.
- Laguerre M, Bily A, Birtic S. Lipid oxidation in food. InC.M. Galanakis (Ed.), Lipids and edible oils; Academic Press (2020): 243-287.
- Sahin S. Evaluation of stability against oxidation in edible fats and oils. Journal of Food Science and Nutrition Research 2 (2019): 283-297.
- Kong F. Food and beverage stability and shelf life: Advances in instrumental methods to determine food quality deterioration (2011): 381-404.
- Zamuz S, Pateiro M, Conte-Junior CA, et al. Food Lipids: Fat and fatty acids, Academic Press (2022): 155-172.
- Agregán R, Popova T, López-Pedrouso M, et al. Chapter 12: Fatty acids: In Food Lipids. Academic Press: San Diego, CA, USA (2022): 257-286.
- Ainsa A, Marquina PL, Roncales P, et al. Enriched fresh pasta with a sea bass by-product, a novel food: fatty acid stability and sensory properties throughout shelf life. Foods 15 (2021): 10.255.
- Przybylski R, Eskin NAM. Oil composition and properties: Canola (2011): 189-227.
- Cognat C, Shepherd T, Verrall SR, et al. Relationship between volatile profile and sensory development of an oat-based biscuit. Food Chem 160 (2014): 72-81.
- Della Porta RA. Edible oils manual. AOCS Press (2006).
- Seneviratne K, Jayathilaka N. Coconut oil: Chemistry and nutrition. In Lakva Publisher (2016): 1-130.
- Yunsheng L. Quality changes in chicken nuggets fried in oils with different degrees of hydrogenation, McGill University, Canada (2005): 1-103.
- Rahman A, Indrayanto D. Food qualities analysis: Fourier transform infrared spectroscopy combined with multivariate analysis for quality analysis of fats and oils (2023): 49-70.
- Yang X, Boyle RA. Sensory evaluation of oils/fats and oil/fat–based foods. Oxidative stability and shelf life of foods containing oils and fats (2016): 157-185.
- Talbot G. The stability and shelf life of fats and oils. The stability and shelf-life of food: 2nd edtn, (2016): 461-503.
- Majchrzak T, Wojnowski W, Dymersk T, et al. Electronic nose in classification and quality control of edible oils: A review. Food Chem 246 (2018): 192-201.
- Geng L, Zhou W, Qu X, et al. Iodine value, peroxide value, and acid value of Bohai algae oil compared with other oil during cooking. Heliyon 9 (2023): e15088.
- Chebet J, Kingyanjui T, Cheplogoi PK. Impact of frying on iodine value of vegetable oils before and after deep frying in different types of food in Kenya. Journal of Scientific and Innovative Research 5 (2016): 193-196.
- Bhatnagar AS, Hemavathy J, Gopala Krishna AG. Development of a rapid method for determination of lignans content in sesame oil. Journal of Food Science and Technology 52 (2013): 521-527.
- Hutchings JB. Color in food. In Best, J. (Ed.), Color design: Theories and applications. (2nd edtn), USA: Woodhead Publishing (2017).
- Kiliç K, Onal-Ulusoy B, Boyaci IH. A novel method for color determination of edible oils in L*a*b* format. European Journal of Lipid Science and Technology 109(2007): 157–164.


 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 