Inhibition of E. coli and C. albicans with a Dietary Women’s Health Formulation
Howard Robins1*, A. Reza Kamarei2 and Eric Finkelstein3
1Department of Health Education & Communication, Doctor’s Biome, Hauppauge, New York, USA
2Department of Research & Development, Doctor’s Biome, Hauppauge, New York, USA
3Department of Operations, Doctor’s Biome, Hauppauge, New York, USA
*Corresponding Author: Howard Robins, Doctor’s Biome (NewGen 27 LLC), 80 Davids Dr., Unit 2, Hauppauge, New York, 11788, USA.
Received: 20 February 2024; Accepted: 27 February 2024; Published: 29 February 2024
Article Information
Citation: Howard Robins, Reza Kamarei A and Eric Finkelstein. Inhibition of E. coli and C. albicans with a Dietary Women’s Health Formulation. Journal of Food Science and Nutrition Research. 7 (2024): 64-73.
DOI: 10.26502/jfsnr.2642-110000153
View / Download Pdf Share at FacebookAbstract
Background: Two common infections in women are urinary tract infections (UTIs) and vaginal candidiasis (VC). The focus of this research project was to counter the root causes of these two infections, namely, Escherichia coli (E. coli) and Candida albicans (C. albicans).
Purpose: The purpose of this research project was to develop a dietary formulation for women’s health that inhibits both E. coli and C. albicans.
Materials and Methods: We designed a propriety blend of five strains of Bifidobacteria, ten strains of Lactobacilli, and organic cranberry powder. For the carriers, we chose a proprietary blend of organic red fruit and vegetable juices. The probiotics were added to a sterilized blend of juice and cranberry powder. Three strains of E. coli and two strains of C. albicans were used in this study.
Results: While E. coli in the control sample showed a typical growth curve for microorganisms, E. coli growth was completely inhibited in the test sample from days 1 to 5. Similarly, while C. albicans in the control sample showed overwhelming uncountable growth, C. albicans growth in the test sample was reduced by 70% on day 1 and was completely inhibited from day 2 to day 5.
Conclusion: The results of this in vitro study indicate that E. coli O157:H7 and C. albicans (the microorganisms responsible for UTI and VC) were completely inhibited by a healthy dietary formulation for women. These findings have advanced our knowledge of the inhibition of two pathogens responsible for two common medical problems in women.
Keywords
<p>Escherichia coli, Candida albicans, Urinary tract infection, Vaginal candidiasis, Probiotics, Juice-based probiotics</p>
Article Details
Introduction
Two common medical problems in women are urinary tract infections (UTIs) and vaginal yeast infections or vulvovaginal candidiasis (VVC).
UTI is a common infection in any part of the urinary tract, including the urethra (urethritis), bladder (cystitis), ureters, and kidneys (pyelonephritis), although it is most common in the bladder [1]. Unresolved cases of lower UTIs can spread, causing pyelonephritis, male genital infections, or progression to more severe life-threatening urosepsis [2]. The risk of UTI can be as much as 14 times greater in women than in men [3]. The disproportionate infection rates are primarily due to anatomical differences in the length of the female urethra and proximity to the rectum, making bacterial entry into the urinary tract easier [4]. Other risk factors include previous UTIs, pregnancy, age, vaginal flora changes, and menopause [4]. Common UTI symptoms include urge and urinary incontinence, burning sensation while urinating, cloudy urine, and pelvic pain, while more serious symptoms can include fever and chills [1].
The most common uropathogen responsible for UTI is Escherichia coli (E. coli), a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium that commonly resides in the lower intestine [5]. The most well-known Shiga toxin-producing E. coli (STEC) is E. coli O157:H728, which can cause “severe stomach cramps, bloody diarrhea, and vomiting [6].” Healthy adults typically recover from E. coli O157:H7 infections within a week. Young children and older adults are at greater risk of developing a life-threatening form of kidney failure [6].
While E. coli is the predominant uropathogen in UTIs, other bacteria, such as Klebsiella spp., Enterococcus spp. and Pseudomonas aeruginosa, are also common in healthcare-associated UTIs, complicated UTIs, pyelonephritis, and urosepsis [2,3]. Candida spp. are the most common organisms among fungal urinary tract infections referred to as candiduria [7]. While only accounting for 1% of positive urine cultures in hospital laboratories, candiduria represents a disproportionately large proportion of ICU-acquired positive urine cultures and catheter-associated UTIs [7].
Antibiotics are often the first-line treatment for bacteria that cause tract infections. Health status and the type of bacteria found in urine determine which medicine is used and how long a person needs to take it; however, antibiotics can cause side effects such as “rash, dizziness, nausea, diarrhea, and yeast infections [7].” The increasing prevalence of uropathogenic antimicrobial resistance poses a significant threat to public health [2,3]. As these pathogens become increasingly resistant to commonly prescribed antibiotics [8-10] and antifungals [7], the treatment of urinary tract infections (UTIs) has become more challenging. Along with novel antimicrobial developments, the exploration of nonantimicrobial prevention and treatment methods that do not increase bacterial and fungal resistant pathogens is crucial.
Candida albicans (C. albicans), a gram-positive fungus that can take on a unicellular (yeast) or multicellular form, is an opportunistic pathogenic yeast that is a common member of the human gut flora. It is detected in the gastrointestinal tract and mouth of 40–60% of healthy adults [11,12]. C. albicans is the most common Candida species that causes candidiasis in humans, resulting from overgrowth of the fungus [13,14].
Candidiasis is a broad term that clinically refers to a spectrum ranging from nonlife-threatening superficial mucocutaneous infections to life-threatening deep-seated organ infections caused by the fungi Candida genus [15]. Invasive candidiasis (IC) occurs when superficial infections invade the bloodstream and disseminate to different organs in the body, often causing severe complications [15,16]. Risk factors for IC include but are not limited to immunosuppressive agents, surgery, prolonged stay in an intensive care unit, prior administration of broad-spectrum antibiotics, and catheter use [15,16].
The most common term for candidiasis in the vagina is vaginal yeast infection [15]. Other names for this infection include vaginal candidiasis, vulvovaginal candidiasis (VVC), and candida vaginitis [17]. According to the CDC, “Candida normally lives on skin and inside the body such as in the mouth, throat, gut, and vagina, without causing any problems. Scientists have estimated that approximately 20% of women normally have Candida in the vagina without any symptoms. Candida can cause infection if the conditions inside the vagina change to encourage growth. Factors such as hormones, medicines, or changes in the immune system can increase the likelihood of infection [17].”
It is estimated that approximately 75% of women will experience an episode of VVC in their lifetime, and over 50% will experience two or more episodes in their lifetime [12]. The estimated prevalence of recurrent vulvovaginal candidiasis (RVVC) (characterized by four or more VVC episodes per year) is 5-10% of all women [12,18].
Symptoms of VVC are often mild and include but are not limited to itching, burning, pain, vaginal discharge, and bad odor [18]. Some of these symptoms overlap with other common vaginal infections, such as bacterial vaginosis (which results from an overgrowth of the bacteria naturally found in the vagina) and trichomoniasis (which is caused by a parasite and is often sexually transmitted) [12,18,19].
The treatment of candidiasis varies based on the location and severity of the infection as well as the patient’s previous exposure to antifungal agents and other comorbidities [15,16]. Of the three major classes of antifungal agents generally recognized for use against Candida spp., echinocandins are typically considered the first line of defense for the treatment of adult patients with IC [15,16,20]. Azoles such as fluconazole and certain polyenes such as nystatin can be administered orally or topically and are the most common antifungal drugs used to treat and prevent VVC, RVVC, and oral candidiasis [12,16,18,21].
Recent studies suggest that the increased prevalence of Candida spp. antifungal drug resistance will likely persist because of the limited number of antifungal treatment options, diversification of Candida spp. infections, increased reported cases of candidiasis, and the emergence of intrinsically resistant Candida spp [20,22,23].
The currently accepted definition of probiotics is “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [24].” This means that if the microorganisms are not “live,” they cannot be considered probiotic, and if their amount is not adequate to confer a health benefit, they cannot be considered probiotic either.
Probiotics have remained an area of interest for the nonantimicrobial prevention and treatment of UTIs and VVC. A healthy microbiome imbalance, such as decreased levels of Lactobacillus, has been associated with an increased risk of recurrent UTIs [3,25]. Mechanistically, probiotics, especially those belonging to the Lactobacillus genus, may help prevent and treat UTIs and VVC by inhibiting pathogen biofilm formation, preventing hyphae formation, and interfering with virulence gene expression [25-27]. However, the efficacy of probiotics in the prevention and treatment of UTIs [3,28-31] and VVC [32-35] has shown mixed results and varies depending on the type of pathogen, specific strains of probiotics used, dosage, duration of treatment, and individual patient factors. Probiotics have shown no concern in developing resistant pathogenic strains of bacteria and yeasts [34].
Cranberry (Vaccinium macrocarpon) is an evergreen shrub that has historically been used for bladder, stomach, and liver disorders [36]. Today, cranberry is often promoted for urinary tract infections (UTIs) [36]. Some research has shown that cranberry use reduces the risk of UTIs by approximately one-third in people who are at increased risk for UTIs or have had recurrent UTIs [36]. However, according to the U.S. National Center for Complementary and Integrative Health, there is still some uncertainty about the effectiveness of cranberry because some of the research has not been of high quality.” [36] It is noteworthy that cranberry alone has not been shown to be effective as a treatment for an existing UTI [37-39] as far as safety is concerned, and “cranberry products are generally thought to be safe” [36]. However, cranberries consumed in large amounts can cause upset stomach and diarrhea [36]. Little is known about the safety of cranberry consumption for health reasons during pregnancy or while breastfeeding. There is conflicting evidence about cranberry interactions with the anticoagulant warfarin [36]. It is not recommended to use cranberry products in place of proven treatment for a UTI [36]. On July 21, 2020, the U.S. Food and Drug Administration announced in a letter of enforcement discretion that it “does not intend to object to the use of certain qualified health claims regarding consuming certain cranberry products and a reduced risk of recurrent urinary tract infection (UTI) in healthy women”[40]. Specifically, “the FDA intends to exercise its enforcement discretion regarding claims for the association between the consumption of cranberry juice beverages containing at least 27 percent cranberry juice (most commercially available cranberry cocktails contain this amount) and cranberry dietary supplements containing at least 500 milligrams (mg) of cranberry fruit powder (100% fruit) and a reduced risk of recurrent UTI.
The claims do not include other conventional foods or food products made from or containing cranberries, such as dried cranberries or cranberry sauce.” [40]
The following qualified health claims for cranberry dietary supplements are included in the FDA’s letter of enforcement discretion.
- “Limited scientific evidence shows that by consuming 500 mg each day of cranberry dietary supplement, healthy women who have had a urinary tract infection (UTI) may reduce their risk of recurrent UTI.” [40]
- “Consuming 500 mg of cranberry dietary supplement may help reduce the risk of recurrent urinary tract infection (UTI) in healthy women. The FDA concluded that there is limited scientific evidence to support this claim.”40
- “Consuming 500 mg [X capsules/tablets/soft gels] each day of [this identified cranberry dietary supplement] may help reduce the risk of recurrent urinary tract infection (UTI) in healthy women. The FDA concluded that there is limited scientific evidence to support this claim.” [40]
On the one hand, the diverse observations and inconsistent results of various probiotic strains on E. coli and C. albicans, as well as the National Center for Complementary and Integrative Health, as well as the FDA’s cautious approach for the use of cranberry powder for UTI, suggest that neither probiotics alone nor cranberry powder alone can effectively inhibit both E. coli and C. albicans.
Consequently, we envisioned that the combination of an optimum probiotic blend and organic cranberry powder in an optimum carrier (liquid organic fruit/vegetable juice) should effectively inhibit both E. coli and C. albicans (as the microorganisms responsible for urinary tract infection and vulvovaginal candidiasis).
Therefore, the specific objective of this research project was to prove that our dietary women’s health formulation (containing probiotics and organic cranberry powder in a mixture of organic juices) can effectively inhibit E. coli and C. albicans, thus reducing the need for antimicrobial usage, which in turn would contribute to the reduction of overall antimicrobial resistance by these pathogens.
Materials and Methods
Active ingredients
Bifidobacteria and Lactobacilli are broadly recognized for their key roles in human intestinal microflora. Considering the results of published literature mentioned above and reviewing commercially available probiotic bacteria from credible global suppliers, we designed a propriety blend of five strains of Bifidobacteria and ten strains of Lactobacilli (“Doctor’s Biome Signature Probiotics Blend”) from the reputable company Cultures Supporting Life, CSL-USA (Table 1). Species identification of these probiotics was performed by sequence analysis of the 16S rRNA gene, whereas strain identification was performed by pulse field gel electrophoresis (PFGE) analysis. These probiotics have been shown to be sensitive to antibiotics and have passed microbiological assays and heavy metal analyses. They are not genetically modified (GMO), are free from allergens, are considered safe for bovine spongiform encephalopathy (BSE), and do not contain colorants. These probiotics have also been subjected to a series of in vitro tests to assess their gastrointestinal survival (tolerance to hydrochloric acid, HCl), tolerance to bile salts, and resistance to gastrointestinal tract enzymes pepsin and pancreatin).
Organic cranberry powder was prepared from 100% cranberries. No sugar, preservative, flavor, or color was added. These ingredients were subjected to physicochemical and microbiological analyses.
|
Doctor's Biome Signature Probiotics Blend (DBSPB) |
|
|
Genus / Species |
Strain |
|
Bifidobacterium bifidum |
SP 9 |
|
Bifidobacterium breve |
BBR8 |
|
Bifidobacterium infantis |
SP 37 |
|
Bifidobacterium longum |
SP 54 |
|
Bifidobacterium animalis subsp. lactis |
BLC1 |
|
Lactobacillus acidophilus |
LA1 |
|
Lactobacillus brevis |
SP 48 |
|
Lactobacillus bulgaricus |
LB2 |
|
Lactobacillus casei |
BGP 93 |
|
Lactobacillus gasseri |
LG050 |
|
Lactobacillus paracasei |
101/37 |
|
Lactobacillus plantarum |
14D |
|
Lactobacillus reuteri |
LR92 |
|
Lactobacillus rhamnosus |
SP 1 |
|
Lactobacillus salivarius |
SP2 |
Table 1: Doctor's Biome Signature Probiotics Blend (DBSPB) of Bifidobacteria and Lactobacilli Used in production of Dietary Women’s Health Formulation.
Liquid carrier
In search of an optimum carrier (also known as an excipient or medium), we chose a proprietary blend of organic red fruit and vegetable juices consisting of 100% organic watermelon juice, organic apple juice, organic pomegranate juice, organic beet juice, and organic strawberry juice. Organic cranberry powder was added to a liquid blend of organic juices (500 mg per 2 fluid ounces) before sterilization. A Doctor’s Biome Signature Probiotic Blend was then added (27 billion CFU per 2 fluid ounces) to the sterilized blend of juices. This formulation was protected by a pending patent.
Product samples for microbiology study
Two-ounce bottles of dietary women’s health formulation (AKA Doctor’s Biome Women’s Health) were delivered to Eurofins Microbiology Laboratories for this study. The samples were stored at refrigeration temperature.
Pathogenic organisms and preparation of inoculum cocktails
Three strains of E. coli O157:H7 and two strains of ATCC C. albicans were included in this study. A list of organisms and their sources of isolation, where available, is provided in table 2.
|
Inoculum Cocktail Group |
Strain |
Identification NumberA |
Strain Information |
|
E. coli |
Escherichia coli O157:H7 |
NFPA 4203 |
Patient isolate |
|
Escherichia coli O157:H7 |
NFPA 4206 |
Human isolate, Univ. of Georgia |
|
|
Escherichia coli O157:H7 |
NFPA 4210 |
1991 MA apple cider outbreak, patient isolate |
|
|
Yeast |
Candida albicans |
ATCC 24433 |
Nail infection |
|
Candida albicans |
ATCC 14053 |
Blood |
Table 2: List of organisms used in this study
To prepare E. coli O157:H7 strains for this study, isolated colonies of each strain were individually subcultured in tryptic soy broth (TSB) and incubated for 18 ± 2 h at 35°C. Subsequent growth was transferred to fresh TSB supplemented with 1% glucose (w/w) and incubated at 35°C for 24 ± 2 h to facilitate acid adaptation. Following acid adaptation, equivalent volumes of each culture were pooled, pelleted by centrifugation, washed once using 0.1% peptone water (PW), and resuspended in PW to form the E. coli O157:H7 inoculum cocktail. The inoculum cocktail was prepared on the day of the study and stored at 4°C until further use.
ATCC Candida albicans strains were individually cultured in potato dextrose broth (PDB) and incubated on a shaker at 25°C for 2 days. Aliquots of PDB were transferred to prepoured acidified potato dextrose agar (PDA) plates and incubated at 25°C for 5 days. Following incubation, an equivalent number of plates per strain was flooded with 7 ml of PW and then gently scraped with a sterile spreader to collect the surface growth. The resulting suspensions were combined to form the Candida albicans inoculum cocktail. The Candida albicans inoculum cocktail was then pelleted by centrifugation, washed once using PW, and then resuspended in PW. The Candida albicans inoculum cocktail was prepared on the day of the study and stored at 4°C when not in immediate use.
Preparation of test samples and control samples
Individual 2-ounce bottles of Doctor’s Biome Women Health were composited and homogenized to create one sample and then centrifuged at 4,500 RPM for 15 min to prepare the “test sample”. Photos of the Doctor’s Biome Women Health product before and after the centrifugation step are provided in figure 1. The pH of each sample was measured prior to centrifugation (one replicate sample). To prepare the inoculated test samples, the Doctor’s Biome Women Health was aliquoted into two portions, and each portion was bulk inoculated with one of the inoculum cocktails (either E. coli O157:H7 or C. albicans) at a target concentration of approximately 4 log CFU/ml. Immediately following inoculation, the samples were mixed to ensure an even distribution of the inoculum.
Tryptic soy broth (TSB) for E. coli O157:H7 and potato dextrose broth (PDB) for ATCC C. albicans were used as the “control samples” and were inoculated in the same manner. The inoculated samples prepared in this study are summarized as follows.
- Test Sample: Doctor’s Biome Women’s Health centrifuged and inoculated with coli O157:H7.
- Test sample: Doctor’s Biome Women Health centrifuged inoculated with albicans
- Control sample: tryptic soy broth inoculated with coli O157:H7
- Control sample: Potato dextrose broth inoculated with ATCC albicans
Incubation of test samples and control samples
The inoculated test and control samples were incubated at 37°C for up to five days. The samples were evaluated according to the sampling scheme listed in table 3. Uninoculated samples (negative controls) were excluded from this study because of limited product availability.
|
Sample Description |
Incubation Temperature |
Sampling Times |
Replicates/Sampling Time |
|
Test Samples |
37°C |
Days 0A, 1, 2, 3, and 5 |
3 replicatesB per organism |
|
Control Samples |
37°C |
Days 0A, 1, 2, 3, and 5 |
3 replicatesB per organism |
AInitial counts of organisms in test samples or control samples prior to the incubation at 37°C.
B1-ml sample removed from the test sample or control sample was considered to be one replicate.
Table 3: Sampling scheme
Evaluation of test samples and control samples
At each sampling time, the inoculated test samples and control samples were removed from the incubator, and 3 × 1 ml aliquots of the samples were removed from the inoculated bulk sample, serially diluted with PW, and enumerated for the target organisms. Each 1 ml sample was considered to be one replicate sample. Appropriate dilutions were then pour-plated onto the appropriate media. and incubated under the appropriate conditions, and colonies were counted as the target organism based on characteristic colony morphology. No additional confirmation was obtained. The bulk samples were placed in the incubator.
Results
All microbiological data were reported as log CFU/ml. The limit of detection for this plating method was 1.0 log CFU/ml. The pH of the precentrifuged Doctor’s Biome Women Health sample was 3.27. The population levels of E. coli O157:H7 and ATCC C. albicans recovered from artificially inoculated test samples and control samples are reported in tables 4 and 5 and figures 2 and 3.
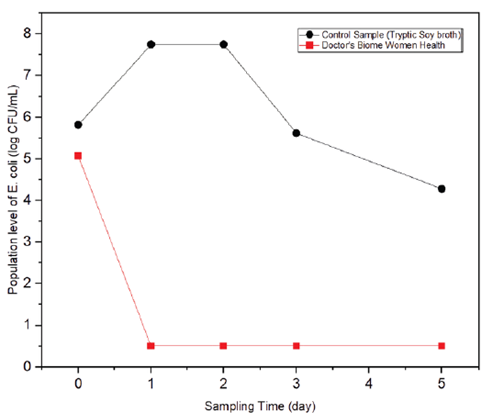
As shown in table 4 and figure 2, while on Day 0, the E. coli population in the test sample was almost equal to that in the control sample, E. coli growth was completely inhibited in the test sample from days 1 to 5. In contrast, E. coli in the control sample showed a typical growth curve for microorganisms (a drastic population increase peaked on day 1, followed by a gradual decrease in population).
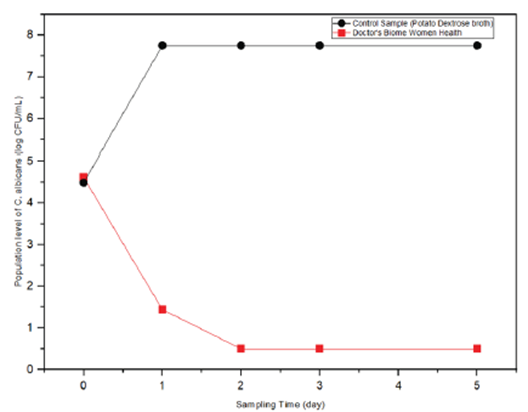
As shown in table 5 and figure 3, while on day 0, the C. albicans population in the test sample was almost equal to its population in the control sample, C. albicans growth in the test sample was reduced by 70% on day 1, and then its growth was completely inhibited from day 2 to day 5. C. albicans in the control sample showed such overwhelming growth that counts were outside the countable range on the highest dilution plated.
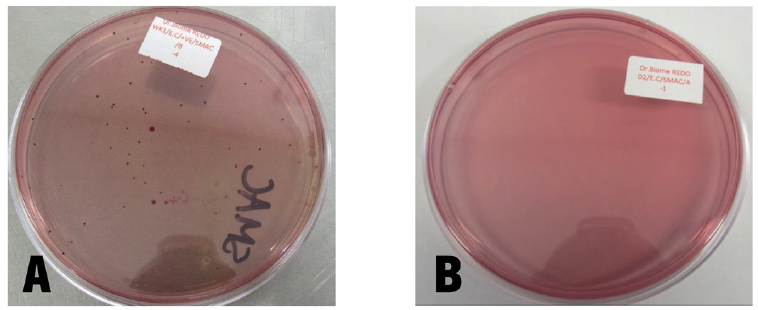
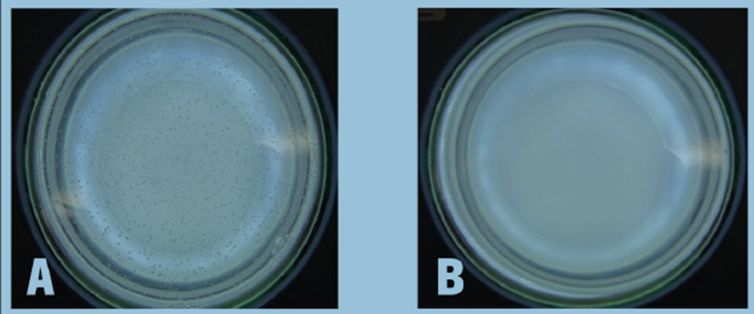
Photographs of one agar plate per sample are shown in figures 4 and 5, where subfigures A and B show control and test samples, respectively. In figure 4A, colonies of E. coli recovered from the control sample at a 1:10,000 dilution can be easily seen, while figure 4B shows that no E. coli colonies could be recovered from the test sample even at a 1:10 dilution. This shows the total inhibition of E. coli by dietary formulations for women’s health. Similarly, in figure 5A, an overwhelming number of colonies of C. albicans recovered from the control sample at a 1:10,000 dilution can be seen, whereas figure 5B shows that no C. albicans colonies could be recovered from the test sample even at a 1:10 dilution. Likewise, this shows the total inhibition of C. albicans by dietary women’s health formulations.
|
Target Organisms |
Sampling Times |
Replicates |
Population Level of Target Organism (log CFU/ml) |
|
|
Doctor’s Biome Women Health |
Control Samples (Tryptic Soy broth) |
|||
|
E. coli O157:H7 |
Day 0 |
A |
5.45 |
5.86 |
|
B |
5.25 |
5.79 |
||
|
C |
4.52 |
5.81 |
||
|
Avg ± SDA |
5.07 ± 0.49 |
5.82 ± 0.04 |
||
|
Day 1 |
A |
<1.00B |
>7.75C |
|
|
B |
<1.00B |
>7.75C |
||
|
C |
<1.00B |
>7.75C |
||
|
Avg ± SDA |
<1.00B |
>7.75C |
||
|
Day 2 |
A |
<1.00B |
>7.75C |
|
|
B |
<1.00B |
>7.75C |
||
|
C |
<1.00B |
>7.75C |
||
|
Avg ± SDA |
<1.00B |
>7.75C |
||
|
Day 3 |
A |
<1.00B |
5.6 |
|
|
B |
<1.00B |
5.59 |
||
|
C |
<1.00B |
5.66 |
||
|
Avg ± SDA |
<1.00B |
5.62 ± 0.04 |
||
|
Day 5 |
A |
<1.00B |
4.11 |
|
|
B |
<1.00B |
4.49 |
||
|
C |
<1.00B |
4.23 |
||
|
Avg ± SDA |
<1.00B |
4.28 ± 0.19 |
||
AAvg ± SD: average ± standard deviation
BBelow the limit of detection of 1.00 log CFU/ml
CEstimated count, counts were outside countable range on highest dilution plated.
Table 4: Population of E. coli O157:H7 recovered from Doctor’s Biome Women Health product (test sample) and Tryptic Soy Broth (control samples) incubated at 37°C
|
Target Organisms |
Sampling Times |
Replications |
Target Organism (log CFU/ml) |
|
|
Doctor’s Biome Women Health |
Control Samples (Potato Dextrose Broth) |
|||
|
C. albicans |
Day 0 |
A |
4.61 |
4.46 |
|
B |
4.59 |
4.45 |
||
|
C |
4.66 |
4.56 |
||
|
Avg ± SDA |
4.62 ± 0.04 |
4.49 ± 0.06 |
||
|
Day 1 |
A |
1.48 |
>7.75D |
|
|
B |
1.85 |
>7.75D |
||
|
C |
<1.00B |
>7.75D |
||
|
Avg ± SDA |
1.44 ± 0.42C |
>7.75D |
||
|
Day 2 |
A |
<1.00B |
>7.75D |
|
|
B |
<1.00B |
>7.75D |
||
|
C |
<1.00B |
>7.75D |
||
|
Avg ± SDA |
<1.00B |
>7.75D |
||
|
Day 3 |
A |
<1.00B |
>7.75D |
|
|
B |
<1.00B |
>7.75D |
||
|
C |
<1.00B |
>7.75D |
||
|
Avg ± SDA |
<1.00B |
>7.75D |
||
|
Day 5 |
A |
<1.00B |
>7.75D |
|
|
B |
<1.00B |
>7.75D |
||
|
C |
<1.00B |
>7.75D |
||
|
Avg ± SDA |
<1.00B |
>7.75D |
||
AAvg ± SD: average ± standard deviation
BBelow the limit of detection (1.00 log CFU/ml)
CFor the purpose of calculation, the value <1.00 was treated as 1.00 to calculate average and standard deviation
DEstimated count, counts were outside countable range on highest dilution plated.
Table 5: Population of ATCC C. albicans recovered from Doctor’s Biome Women Health product (test sample) and Tryptic Soy Broth (control samples) incubated at 37°C
The total inhibition of E. coli and C. albicans by dietary formulations for women’s health should be considered from both quantitative and qualitative perspectives.
From a quantitative point of view, the general mechanism of microbial inhibition is the “competitive exclusion principle.” This means that when two species compete in a limited environment (e.g., colon) and compete for the same limited amount of nutrients, the species that have advantages over the others (e.g., larger numbers) will dominate the environment and cause exclusion of the weaker competitor.
From a qualitative point of view, the chosen blend of Bifidobacteria and Lactobacilli and cranberry powder functioned in a complementary, additive, and possibly synergistic manner to completely inhibit E. coli and C. albicans. It is logical to infer that the chosen probiotics released some bioactive compounds (i.e., postbiotics) into the juice, which had an inhibitory effect on these pathogens. One such compound could be lactic acid (and possibly other organic acids), which can reduce the pH of the environment to an acidic range unfavorable for these microorganisms. We can conclude that the bioactive compounds secreted from the 15 chosen probiotic strains into the organic fruit and vegetable juices, together with bioactive compounds of cranberry powder, such as proanthocyanidins (PACs), played an effective inhibitory role against E. coli and C. albicans.
Discussion
Most probiotics on the market are in the form of capsules (loose powder) or tablets (compressed powder). In the dry form, probiotic cells are in a state of “suspended animation,” which means that most of their vital functions temporarily cease. In other words, probiotic cells in the dry form, while alive at the time of manufacturing and after freeze-drying, are not living (not physiologically active). To make dry, live probiotic cells become physiologically active, and they need to be fully hydrated and surrounded in an aqueous environment with sufficient available water and nutrients. When probiotics are consumed in the form of a dry powder, they must be fully hydrated in the highly acidic environment of the stomach (pH ~ 1.5). It is not clear how many percent of dry probiotics can successfully become fully hydrated, as the highly acidic environment of the stomach is not the best condition for the rehydration of cells. This has led to mixed results in studies that have tested the benefits of probiotics under various conditions.
However, when probiotics are consumed in liquid form (e.g., in a blend of organic fruit and vegetable juices), they are fully hydrated and suspended in an aqueous environment. In other words, prehydrated probiotics are physiologically active and functional when consumed.
To our knowledge, this study is the first to show an inhibitory effect in vitro on the pathogens E. coli and C. albicans with a dietary formulation containing a blend of prehydrated probiotics in a liquid juice medium.
The inconsistency in research results using other forms of probiotics and cranberries as a method of prevention or management of UTIs [21-23,29] and VVC [24-27] warrants further exploration of the form used in this study, especially as interest has increased in potential nonantimicrobial therapeutic options owing to recent concerns over uropathogen and Candida spp. drug resistance [6,12,28].
Conclusion
The results of this in vitro study indicate that E. coli O157:H7 and C. albicans (the microorganisms responsible for UTI and vaginal candidiasis) were completely inhibited by the dietary women’s health formulation to below the limit of detection of 1.0 log CFU/ml after 1 and 2 days of incubation (37°C), respectively. In comparison, the populations of these organisms grew normally in tryptic soy and potato dextrose broths under the same incubation conditions. Based on these results, a prospective, randomized, double-blind clinical trial (based on Good Clinical Practice) on the safety and efficacy of dietary women’s health formulations in patients with UTI and vaginal candidiasis is warranted.
Acknowledgment
The authors acknowledge the valuable contributions of Ms. Shirin Abd, M.S. (Senior Technical Manager), and Fei Wang, Ph.D. (Project Microbiologist) from the Eurofins Microbiology Laboratories (www.eurofins.com) in performing this inoculation challenge study.
Disclosure and Conflict of interest statement
Dr. Robins, Dr. Kamarei, and Mr. Finkelstein are partners in the Doctor’s Biome Company (Newgen 27 LLC). Dr. Kamarei was paid for consulting services, and Eurofins Microbiology Laboratories was paid for microbiological services.
Funding statement
This research was entirely funded by Doctor’s Biome Company (NewGen 27 LLC).
Supplementary files
This is a list of supplementary files associated with this preprint. Click to download.
EurofinsFinalReportInoculationStudy.pdf
References
- Cleveland Clinic. Urinary Tract Infections. In: Cleveland Clinic (2023)
- Wagenlehner FME, Bjerklund JTE, Cai T, et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nature Reviews Urology 17 (2020): 586-600.
- Wawrysiuk S, Naber K, Rechberger T, et al. Prevention and treatment of uncomplicated lower urinary tract infections in the era of increasing antimicrobial resistance-non-antibiotic approaches: a systemic review. Archives of Gynecology and Obstetrics 300 (2019): 821-828
- Centers for Disease Control and Prevention Urinary Tract Infection. In: Centers for Disease Control and Prevention (2021).
- World Health Organization E. coli. In: WHO (2018).
- Mayo Clinic E. coli - Symptoms and causes. In: Mayo Clinic (2022).
- Odabasi Z, Mert A. Candida urinary tract infections in adults. World Journal of Urology 38 (2020): 2699-2702.
- Stapleton AE, Wagenlehner FME, Mulgirigama A, et al. Escherichia coli Resistance to Fluoroquinolones in Community-Acquired Uncomplicated Urinary Tract Infection in Women: a Systematic Review. Antimicrobial Agents and Chemotherapy 64 (2020): 112.
- Mancuso G, Midiri A, Gerace E, et al. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 12 (2023): 623
- Al-Zahrani J, Al Dossari K, Gabr AH, et al. Antimicrobial resistance patterns of Uropathogens isolated from adult women with acute uncomplicated cystitis. BMC Microbiology 30 (2019): 56.
- Wikipedia Contributors Candida albicans. In: Wikipedia (2019).
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Critical reviews in microbiology 42 (2016): 905-927
- Candidiasis. In: Centres for Disease Control and Prevention (2019).
- Cleveland Clinic. Candida Albicans: Infections, Symptoms & Treatments. In: Cleveland Clinic (2022).
- Pappas PG, Lionakis MS, Arendrup MC, et al. Invasive candidiasis. Nature Reviews Disease Primers 4 (2018): 18026.
- Uppuluri P, Khan A, Edwards JE. Current Trends in Candidiasis. Candida albicans: Cellular and Molecular Biology 12 (2017): 5-23
- Centers for Disease Control and Prevention (2019) Vaginal Candidiasis. In: CDC.
- Blostein F, Levin-Sparenberg E, Wagner J, et al. Recurrent vulvovaginal candidiasis. Annals of Epidemiology 27 (2017): 575-582.e3
- Brusselmans J, An De Sutter, Brecht D, et al. Scoping review of the association between bacterial vaginosis and emotional, sexual and social health. BMC Women’s Health 23 (2023): 168.
- Arendrup MC, Patterson TF. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. The Journal of Infectious Diseases 216 (2017): S445-S451
- Bhattacharya S, Sae-Tia S, Fries BC. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 9 (2020): 312
- Prasad R, Nair R, Banerjee A. Multidrug transporters of Candida species in clinical azole resistance. Fungal Genetics and Biology 132 (2019): 103252
- Ksiezopolska E, Gabaldón T. Evolutionary Emergence of Drug Resistance in Candida Opportunistic Pathogens. Genes 9 (2018): 461
- Martín R, Langella P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Frontiers in Microbiology 21 (2019): 124.
- Chen YC, Lee WC, Chuang YC. Emerging Non-Antibiotic Options Targeting Uropathogenic Mechanisms for Recurrent Uncomplicated Urinary Tract Infection. International Journal of Molecular Sciences 24 (2023): 7055
- Boahen A, Than LTL, Loke YL, et al. The Antibiofilm Role of Biotics Family in Vaginal Fungal Infections. Frontiers in Microbiology 13 (2022): 218.
- Andrade JC, Kumar S, Kumar A, et al. Application of probiotics in candidiasis management. Critical Reviews in Food Science and Nutrition 62 (2021): 8249-8264.
- Abdullatif VA, Sur RL, Eshaghian E, et al. Efficacy of Probiotics as Prophylaxis for Urinary Tract Infections in Premenopausal Women: A Systematic Review and Meta-Analysis. Cureus 13 (2021): e18843
- Ng QX, Peters C, Venkatanarayanan N, et al. Use of Lactobacillus to prevent recurrent urinary tract infections in females. Medical Hypotheses 114 (2018): 49-54.
- New F, Shenthiuiyan T, Julliebø JP, et al. Role of Probiotics for Recurrent UTIs in the Twenty-First Century: a Systematic Review of Literature. Medical Hypotheses 23 (2022): 19-28.
- González de Llano D, Moreno AMV, Bartolomé B. Cranberry Polyphenols and Prevention against Urinary Tract Infections: Relevant Considerations. Molecules 25 (2020): 3523
- Mizgier M, Jarzabek-Bielecka G, Mruczyk K, et al. The role of diet and probiotics in prevention and treatment of bacterial vaginosis and vulvovaginal candidiasis in adolescent girls and non-pregnant women. Ginekologia Polska 91 (2020): 412-416.
- Buggio L, Somigliana E, Borghi A, et al. Probiotics and vaginal microecology: fact or fancy? BMC Women’s Health 25 (2019): 631.
- Shenoy A, Gottlieb A. Probiotics for oral and vulvovaginal candidiasis: A review. Dermatologic Therapy 32 (2019): e12970
- Wijgert J, Verwijs M. Lactobacilli?containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG: An International Journal of Obstetrics & Gynaecology 127 (2019): 287-299.
- In: NCCIH (2023).
- Fu Z, Liska D, Talan D, et al. Cranberry Reduces the Risk of Urinary Tract Infection Recurrence in Otherwise Healthy Women: A Systematic Review and Meta-Analysis. The Journal of Nutrition 147 (2017): 2282-2288.
- Luís Â, Domingues F, Pereira L. Can Cranberries Contribute to Reduce the Incidence of Urinary Tract Infections? A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Clinical Trials. Journal of Urology 198 (2017): 614-621.
- Nicolle LE. Cranberry for Prevention of Urinary Tract Infection? JAMA 316 (2016): 1873.
- Nutrition CFSAN. FDA Announces Qualified Health Claim for Certain Cranberry Products and Urinary Tract Infections. FDA (2020).



 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 