Evaluation of the efficacy of plant sterols supplement sterolip® ESI in patients with type IIA hypercholesterolemia in relation to Genetic variants modulating intestinal absorption of cholesterol
Cremonini Anna Laura1,2, Formisano Elena3, Pasta Andrea1, Borgarelli Consuelo1,2, Fresa Raffaele1, Pisciotta Livia1,2
1Department of Internal Medicine, University of Genova, Italy
2IRCCS San Martino Policlinic Hospital, Genova, Italy
3Clinical Nutrition and Dietetics Unit, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
*Corresponding Author: Livia Pisciotta, Department of Internal Medicine, University of Genoa, Viale Benedetto XV,6, 16132 Genoa, Italy
Received: 14 June 2021; Accepted: 22 May 2021; Published: 09 July 2021
Article Information
Citation: Kshipra Gautam, Ashish Waghmare, Niraja Soni, Aniket A Teredesai, Manish R Shukla, Santanu Dasgupta. Algae protein enriched nutritious snacks and their sensory evaluation. Journal of Food Science and Nutrition Research 4 (2021): 181-192.
DOI: 10.26502/jfsnr.2642-11000071
View / Download Pdf Share at FacebookAbstract
Background and aim: Phytosterols (PS) are recommended by European Guidelines for the treatment of hypercholesterolemia. This study aims to evaluate the lipid-lowering activity of a PS supplement (STEROLIP® ESI) in subjects with hypercholesterolemia and the influence of genetic variants involved in cholesterol absorption in the modulation of the individual response to PS.
Methods: 60 subjects suffering from hypercholesterolemia were randomized to PS supplement STEROLIP® 1.6g/day or to placebo for 6 weeks, and subsequently they were all switched to the supplementation with PS for another 6 weeks. At baseline and every 3 weeks, anthropometric measures and lipid profile were collected. All patients were also genotyped for three genetic polymorphisms involved in intestinal cholesterol absorption (APOE, NPC1L1 and ABCG8).
Results: After 3 weeks, TC and LDL-C were significantly lower in PS group. In the 28 patients treated for 12 weeks with PS, we obtained the highest effect after 9 weeks (-8.3% for TC and -12% for LDL-C). We observed a greater reduction of TC and LDL-C in subjects carrying the E4 allele of APOE gene, in homozygous subjects for the rare allele of NPC1L1 rs2072183 polymorphism and in heterozygous carriers of rare allele rs11887534 in ABCG8 gene than in other genotypes.
Conclusion: This study confirms the lipid-lowering effect of PS that is maintained even in the midterm. We highlighted that genetic variants influencing the intestinal cholesterol absorption can contribute to the inter-individual variability of the response of PS supplementation.
Keywords
<p>Phytosterols, Hypercholesterolemia, Intestinal cholesterol absorption, Genetic polymorphisms, Lipid-lowering nutraceuticals</p>
Article Details
1. Introduction
Plant sterols and plant stanols (5-α-saturated derivatives of plant sterols) can be found in traces in fruits, vegetables, nuts, seeds and cereals. The mean daily intake of phytosterols (PS) with the Western diet is about 300 mg per day. They are structurally like cholesterol and follow the same intestinal pathway. In the first step, PS are solubilized in the intestinal lumen by entering into the micelles; once assembled, the micelles interact with the brush border membrane of the enterocytes, and in particular with the membrane transporter protein Niemann-Pick C1 like1 (NPC1L1), which allows the transport of sterols from the intestinal lumen; finally the esterified sterols can be included within nascent chylomicrons, while the unesterified sterols are pumped back into the intestinal lumen through the ATP-binding cassette protein family (ABCG5 and ABCG8) shuttles. PS compete with cholesterol in the formation of solubilized micelles thus reducing the intestinal cholesterol absorption [1]. Several meta-analyses clarified that the cholesterol-lowering effect is dose-dependent until reaching a plateau at the dose of 3 g/day, with an average effect on Low-Density Lipoprotein Cholesterol (LDL-C) of -8 to -12% [2,3]. The effect of PS on plasma triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) is low and unclear [4]. The latest European Guidelines for the treatment of dyslipidemias take into account the consumption dietary supplements such as PS in subjects with low cardiovascular (CV) risk or in addition to drugs in high CV risk patients who fail to achieve recommended targets or in statin intolerant patients [5]. In line with these recommendations, two position papers for the use of lipid-lowering nutraceuticals in clinical practice have been recently published [6,7]. However, a certain inter-individual variability in the responsiveness to PS supplementation is well documented. It can depend on the physical-chemical characteristics of the product, the vehicle for the PS used, the posology and method of administration [8]. Moreover, some genetic polymorphisms involved in the modulation of the intestinal cholesterol absorption can affect the lipid-lowering efficacy of PS supplementation [9]. The aim of this study was to evaluate the efficacy and the safety of a 12 week supplementation with PS in mild to moderate hypercholesterolemic subjects. A secondary aim was to assess the role of the APOE genotype and two other polymorphisms (NPC1L1 rs2072183 and ABCG8 rs11887534) in the modulation of the individual response to PS supplementation.
2. Patients and Methods
2.1 Study design
This study was carried out in 60 moderately hypercholesterolemic subjects in primary prevention, enrolled into the Lipid Clinic of IRCCS Policlinic San Martino in Genoa.
Inclusion criteria were age>18 years, a risk score<5% and a diagnosis of primary hypercholesterolemia, defined by LDL-C between 115 and 189 mg/dL excluding secondary causes.
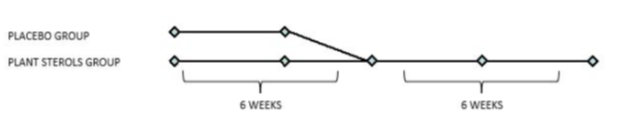
Exclusion criteria were assumption of lipid-lowering drugs, pregnancy and breastfeeding, The CV risk was calculated with the SCORE Risk Charts (www.escardio.org). Patients were randomized at 1:1 ratio to the PS supplementation STEROLIP® at the dose of 1.6 g per day or to placebo for 6 weeks, then they were all switched to the PS group for another 6 weeks (Figure 1).
At the enrollment, all patients were given dietary advice by a trained dietitian, who also assessed diet adherence at week 6 and 12 through a food frequency questionnaire.
The study was conducted in accordance with the Declaration of Helsinki for experiments involving humans and was approved by the Ethical Committee of the San Martino Hospital.
2.2 Biochemical analysis
Blood samples were collected at the enrollment and then every three weeks. Blood was drawn in the morning after a 12-hour fast, and the following parameters were measured: total cholesterol (TC), TG, HDL-C, apolipoproteinB100 (ApoB100, only at baseline). Lipids were dosed with automatic BPC analyzer and ApoB100 with nephelometric method (Siemens). LDL-C was calculated with the Friedewald Formula.
2.3 Genetic screenings methods
Patients were genetically characterized for three genetic variants involved in intestinal cholesterol absorption, in order to assess the genetic influence on PS efficacy. Genetic test was performed for the following polymorphisms: APOE genotype (E2, E3, E4), NPC1L1 c.816C>G p.L272L (rs2072183) and ABCG8 c.55G>C p.D19H (rs11887534). The analysis was performed by genomic DNA PCR amplification using allele-specific primers and subsequent digestion with restriction enzymes as previously described elsewhere (figure 2) [10,11].
2.4 Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 25. Statistical hypothesis tests and values at baseline were compared by using the two-tailed Student’s t-test for paired samples, while the comparison between patients belonging to different groups were performed using the two-tailed Student’s t-test for unpaired samples. The univariate and multivariate analyses were used to value the effects of treatments and polymorphisms studied.
3. Results
3.1 Baseline
Enrolled patients were age- and sex- matched. Twenty-eight subjects in the PS group and twentyseven subjects in the control group completed the study. Table 1 shows the baseline clinical characteristics of the enrolled patients, which were similar in both groups and no significant differences were observed regarding the lipid profile. Five subjects were excluded from the analysis: one withdrew consent, two were not compliant with the treatment and two were lost to follow-up. Diet compliance was tested at week 6 and 12 and was good in both groups. The CV risk was calculated for all subjects, and the average score was 3.5%. Table 2 summarizes the distribution of genotypes in the population studied for the APOE, NPC1L1 and ABCG8 genes.
|
Control Group |
PS Group |
p |
|
|
N |
28 |
27 |
|
|
AGE (y) |
53.1±8.9 |
53.0±8.9 |
NS |
|
BMI (kg/m²) |
24.6±3.3 |
24.6±2.8 |
NS |
|
TC (mg/dl) |
254.4±28.4 |
254.4±21.5 |
NS |
|
HDL-C (mg/dl) |
64.5±10.5 |
65.7±10.0 |
NS |
|
LDL-C (mg/dl) |
167.0±21.7 |
164.2±16.4 |
NS |
|
TG (mg/dl) |
117.8±49.7 |
124.6±52.0 |
NS |
|
ApoB (mg/dl) |
114.5±16.0 |
113.4±16.9 |
NS |
Table 1: Characteristics of patients at baseline.
Data are mean and standard deviation.
Abbreviations: TC- Total Cholesterol; HDL-C- high-density lipoprotein cholesterol; LDL-C- lowdensity lipoprotein cholesterol; TG- triglycerides; ApoB- Apolipoprotein B100; p, p-value; PS- Plant sterols.
|
Subjects |
All |
Control Group |
PS Group |
|
|
All |
55 |
27 |
28 |
|
|
ABCG8 |
c.55G>C |
|||
|
GG |
49 (89.1%) |
24 (88.9%) |
25 (89.3%) |
|
|
GC |
6 (11.0%) |
3 (11.1%) |
3 (10.7%) |
|
|
NPC1L1 |
||||
|
c.816C>G |
||||
|
CC |
28 (50.9%) |
16 (59.3%) |
12 (42.9%) |
|
|
CG |
22 (40.0%) |
9 (33.3%) |
13 (46.4%) |
|
|
GG |
5 (9.1%) |
2 (7.4%) |
1 (3.6%) |
|
|
APOE GENOTYPE |
||||
|
ε3ε3 |
44 (80.0%) |
20 (74.1%) |
24 (85.7%) |
|
|
ε2ε2 |
1 (1.8%) |
0 (0%) |
1 (3.6%) |
|
|
ε3ε4 |
10 (18.2%) |
7 (25.9%) |
3 (1.07%) |
|
Table 2: Genetic distribution of genotypes for APOE. NPC1L1 and ABCG8 genes
Data are number and percentage of patients.
Abbreviations: ABCG8 ATP-binding cassette protein family; NPC1L1- protein Niemann-Pick C1 like1; ApoE- Apolipoprotein E; PS- plant sterols.
3.2 Intervention
|
PS Group |
Control Group |
p |
||
|
TC (mg/dl) |
Basal |
254.4±28.4 |
254.4±21.5 |
NS |
|
3 Weeks |
243.8±29.2 |
261.3±35.1 |
0.03 |
|
|
6 Weeks |
248.6±29.5 |
256.0±25.9 |
NS |
|
|
HDL-C (mg/dl) |
Basal |
64.5±10.5 |
65.7±10.0 |
NS |
|
3 Weeks |
60.7±11.0 |
65.2±11.4 |
NS |
|
|
6 Weeks |
61.0±10.4 |
62.2±10.3 |
NS |
|
|
LDL-C (mg/dl) |
Basal |
167.0±21.7 |
164.2±16.4 |
NS |
|
3 Weeks |
157.5±22.3 |
170.6±28.7 |
0.03 |
|
|
6 Weeks |
163.3±20.7 |
167.2±20.5 |
NS |
|
|
TG (mg/dl) |
Basal |
117.8±49.7 |
124.6±52.0 |
NS |
|
3 Weeks |
128.8±44.6 |
132.6±51.1 |
NS |
|
|
6 Weeks |
126.3±51.1 |
136.8±54.7 |
NS |
Table 3: Lipid profile at baseline. at 3 weeks and at 6 weeks in two groups
Data are mean and standard deviation.
Abbreviations: TC- Total Cholesterol; HDL-C- high-density lipoprotein cholesterol; LDL-C- lowdensity lipoprotein cholesterol; TG- triglycerides; p- p value; PS- plant sterols.
|
TC (mg/dl) |
HDL-C (mg/dl) |
LDL-C (mg/dl) |
TG (mg/dl) |
|
|
Basal |
254.4±28.4 |
64.5±10.5 |
167.0±21.7 |
117.8±49.7 |
|
3 Weeks |
243.8±29.2 |
60.7±11.0 |
157.5±22.3 |
128.8±44.6 |
|
p (B vs 3W) |
0.01 |
<0.01 |
0.01 |
NS |
|
6 Weeks |
248.6±29.5 |
61.0±10.4 |
163.3±20.7 |
126.3±51.1 |
|
p (B vs 6W) |
NS |
<0.01 |
NS |
NS |
|
9 Weeks |
233.7±26.9 |
60.8±9.7 |
146.5±20.0 |
130.8±46.0 |
|
p (B vs 9W) |
<0.01 |
<0.01 |
<0.01 |
NS |
|
12 Weeks |
237.9±28.8 |
62.4±9.9 |
151.3±21.1 |
123.2±50.3 |
|
p (B vs 12W) |
<0.01 |
<0.01 |
<0.01 |
NS |
Table 4: Lipid profile of those patients treated with PS for 12 weeks (N=28).
Data are mean and standard deviation.
Abbreviations: TC- Total Cholesterol; HDL-C- high density lipoprotein cholesterol; LDL-C- lowdensity lipoprotein cholesterol; TG- triglycerides; p- p value; W- Weeks.
3.2.1 Effects of a 6Week Supplementation with PS: Plasma lipid parameters at 3 and 6 weeks of the study are listed in Table 3.
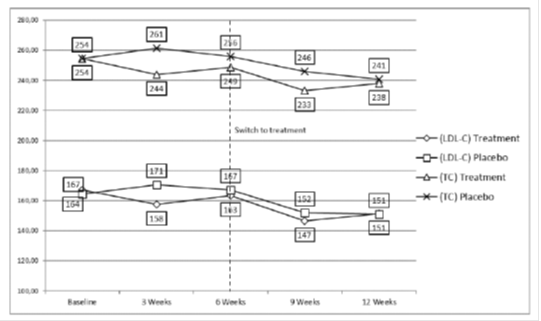
3.2.2 Effects of a 12-Week Supplementation with PS: Table 4 shows a reduction of 3.7% for TC and of 4.9% for LDL-C at week 3. After 6weeks of treatment. TC and LDL-C levels were no longer statistically different from baseline, even if there was a trend of reduction. At week 9, the maximum reduction of TC and LDL-C was obtained. -8.3% and -11.9% respectively, which was partially maintained after 12 weeks of treatment (Figure 2 and Table 4). Patients did not show any significant modification in the TG levels, while HDL-C levels were reduced by an average of 3.27 mg/dl throughout the study period.
3.3 Evaluation of therapeutic response in relation to genetic polymorphisms
3.3.1 Apo E polymorphism: The distribution of the APOE genotypes were reported in Table 2 and were equally distributed in the two groups of patients (p=0.4). Table 5 shows the effect of a 6-week supplementation with PS in the subjects with different APOE genotypes. At baseline there were no significant differences in TC and LDL-C values between ℇ3ℇ3 and ℇ 3ℇ4 subjects. At week 3, the reduction of TC and LDL-C was -4% and -7% respectively in subjects with the ℇ3ℇ3 genotype, with a slight decline at week 6. Subjects carrying the E4 allele showed a greater response to the PS (mean reduction at week 3: TC -8% and LDL-C -12%; at Week 6: TC -9% and LDL-C -10%). Anyway, the presence of the allele E4 of ApoE did not show any significant difference for any of the variables studied.
|
E3E3 |
E2E2 |
E3E4 |
||
|
TC (mg/dl) |
Basal |
255.5±28.4 |
239 |
255.4±21.8 |
|
3 Weeks |
246.4±29.8 (-4%) |
254.0 (+7.6%) |
237.4±25.7 (-8%) |
|
|
6 Weeks |
247.1±27.0 (-3%) |
238 (-0.4%) |
235.0±21.2 (-9%) |
|
|
LDL-C (mg/dl) |
Basal |
166.9±21.7 |
149.8 |
169.6±18.3 |
|
3 Weeks |
155.5±26.1 (-7%) |
158.0 (+5%) |
151.5±26.4 (-12%) |
|
|
6 Weeks |
158.3±22.0 (-5%) |
146.8 (-2%) |
153.8±20.3 (-10%) |
Table 5: TC and LDL-C reductions in relationship with APOE genotypes.
Data are mean and standard deviation and percentage of variation.
Abbreviations: TC- Total Cholesterol; LDL-C- low-density lipoprotein cholesterol.
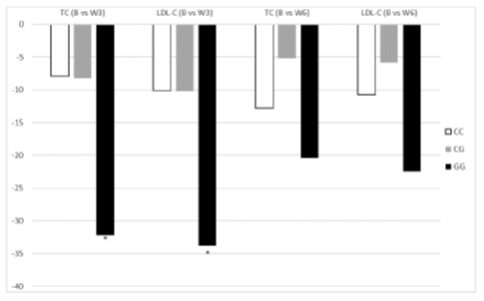
3.3.2 NPC1L1 polymorphism: The distribution of the genotypes of NPC1L1 were reported in Table 2 and the were equally distributed in the two groups of patients (p=0.5). Table 6 shows the lipid values of 55 patients divided into GG. CC and CG genotypes. The GG genotype was associated with a more pronounced reduction of TC (-14%) and LDL-C (-25%) after 3 weeks of treatment (p<0.05) compared to heterozygous (GC) or wild-type (CC) genotypes (Figure 3); at week 6, there is a trend of greater reduction of TC and LDL-C in patients with GG genotype. even if it is no longer statistically significant. We performed a multinomial logistic regression and the GG genotype was found to be more predisposing to greater reductions in TC and LDL-C levels after 3 weeks. even if with a borderline statistical significance (respectively p=0.057 and p=0.07).
|
CC |
CG |
GG |
||
|
TC (mg/dl) |
Basal |
253.8±31.2 |
254.7±21.0 |
264.8±28.4 |
|
3 Weeks |
245.9±32.3 (-3%) |
246.5±26.7 (-3%) |
232.6±15.6 (-14%) |
|
|
6 Weeks |
241.0±26.3 (-5%) |
249.5±25.8 (-2%) |
244.4±28.3 (-8%) |
|
|
LDL-C (mg/dl) |
Basal |
166.8±25.4 |
166.4±15.7 |
171.8±14.7 |
|
3 Weeks |
156.7±26.6 (-6%) |
156.2±25.2 (-7%) |
137.9±20.2 (-25%) |
|
|
6 Weeks |
156.1±19.6 (-7%) |
160.6±21.4 (-4%) |
149.3±32.0 (-15%) |
|
Table 6: TC and LDL-C reductions in relationship with NPC1L1 genotypes.
Abbreviations: TC- Total Cholesterol; LDL-C- low-density lipoprotein cholesterol.
3.3.3 ABCG8 polymorphism: Six subjects were heterozygous for the p.D19H variant of ABCG8 gene (Table 2). The ABCG8 genotypes were equally distributed in the two groups of patients (p=0.96). Table 7 shows the lipid profile of patients divided by the presence of D19H variant: TC and LDL-C were lower at 3 and 6 weeks in patients who carried the minor allele (C) compared to the subjects without this polymorphism. reaching a statistically significant difference only after 6 weeks (p<0.05).
|
GG |
GC |
||
|
TC (mg/dl) |
Basal |
257.0±26.5 |
240.3±28.2 |
|
3 Weeks |
246.7±29.2 (-4%) |
230.2±22.9 (-4%) |
|
|
6 Weeks |
246.9±26.0 (-4%) |
226.7±20.1 (-6%)* |
|
|
LDL-C (mg/dl) |
Basal |
168.1±20.6 |
158.9±23.7 |
|
3 Weeks |
155.7±26.5 (-8%) |
147.9±17.5 (-7%) |
|
|
6 Weeks |
158.4±22.1 (-6%) |
147.7±11.6 (-8%)* |
|
Table 7: TC and LDL-C reductions in relationship with ABCG8 genotypes.
Data are mean and standard deviation and percentage of variation.
Abbreviations: TC- Total Cholesterol; LDL-C- low-density lipoprotein cholesterol.
*p<0.05
4. Discussion
In this study we investigated the effect of a 12-week treatment with PS at a daily dose of 1.6 g and how the response to this supplementation can be modulated by the presence of some variants in genes involved in the intestinal cholesterol absorption/excretion pathway. We documented a mean reduction of TC and LDL-C by 6.2 and 8.7% respectively after 12weeks of treatment, with the best effect achieved at week 9 (-8.3% and -11.9% for TC and LDL-C. respectively). Several RCTs and metaanalyzes published in the last 15 years show that the lipid-lowering effect of PS is dose dependent and proportional up to a daily dose of 3g, which can be considered as a plateau, due to saturation of the uptake of cholesterol and transport process; the intake of 2-3g/day of PS is associated with significant lowering of TC and LDL-C (between 8 and 12%) [6,7]. As shown in Figure 2, the lipid-lowering effect after 6-weeks treatment is attenuated, with lower levels of TC and LDL-C in the PS group but not significantly different compared to the placebo group. This partial and temporary loss of efficacy, already documented in other studies. can be explained by compensatory mechanisms activated by the liver in response to the alteration of cholesterol homeostasis, which is the result of a complex balance between intestinal absorption and hepatic synthesis of cholesterol. In fact. the consumption of PS at high dosages determines a reduction in intestinal cholesterol absorption and a relative lack of intrahepatic cholesterol. which represents a strong signal for the LDL-Receptor (LDL-R)-mediated reuptake of LDLs. thus resulting in initial reduction of LDL-C; moreover. the liver increases the synthesis of endogenous cholesterol and decreases the production and excretion of bile acids [12,13]. These changes in the regulatory mechanisms of cholesterol metabolism may explain the attenuation of PS efficacy after a few weeks of treatment [13]. Furthermore. there is an inter-individual difference in the response to PS supplementation. In our study, 67% of patients in the treatment group showed a good response to the PS supplementation. We further divided them into Low-Responders (LR, LDL-C variation <5% at 12 weeks) and in High- Responders (HR. LDL-C variation >5%) depending on the magnitude of the response. The other 33% of patients did not respond to supplementation (Non-Responders. NR) or even showed worse lipid profiles compare to baseline. These results are in line with the data present in the literature. in which the percentage of NR varies from 20 to 38% in different trials [9,14,15]. Many authors have already identified some factors that could explain the great inter-individual variability in the response to PS supplementation. Some of these factors relate to the characteristics of the product used: the content and type of fat of foods enriched with PS. the form of the supplement (capsules or tablets), the use of free or esterified PS, the type of fatty acid used for esterification, the frequency and timing of administration [9,14-19]. Despite the limited sample sizes of some of these studies. PS seem to be more effective when they are added to spreadable fats [17] and consumed during a main meal [18], while the once-daily frequency of administration seems suboptimal [19]. In our study the supplementation consisted in taking PS in the form of tablets twice a day. at the daily dose of 1.6 g. Other factors that can explain the interindividual variability in the response to treatment may be the subject characteristics: the blood cholesterol levels at baseline. the balance between cholesterol liver synthesis and intestinal absorption and excretion and finally the genetic background [20]. We documented a significant association between the baseline LDL-C levels and the magnitude of LDLC reduction after treatment. In fact, dividing patients in R and NR we noted that R patients have significantly higher baseline LDL-C levels compared to NR patients (173.33±18.92 vs 154.32±19.33; p<0.01), and this is also demonstrable for HR (175.15±20.34 vs 154.32±19.33; p<0.01) and LR patients (169.02±15.03 vs 154.32±19.33; p<0.05) (Table 8 and Figure 4). It has also been described that the cholesterol-lowering effect of PS is greater in subjects with higher plasma levels of PS (campesterol and sitosterol). which are an indirect index of intestinal absorption capacity of PS. Conversely, in subjects with a more pronounced cholesterol synthesis, as shown by higher plasma levels of the cholesterol precursors (latosterol and desmosterol). the cholesterollowering effect of PS is lower [20,21]. Our study participants were all affected by primary hypercholesterolemia, which is a condition associated with increased intestinal absorption efficiency [22,23]. Although we did not measure the blood levels of sterols or cholesterol synthesis precursors, we found that ApoB100 was higher in the NR group of patients compared to the R group. ApoB100 is the main apoprotein of lipoproteins originating in the liver and so it can be considered a surrogate marker of the hepatic synthesis of cholesterol [24]. Finally, another important factor of interindividual variability is the genetic background. Many studies have highlighted the role of some polymorphisms of genes involved in the intestinal cholesterol absorption (ABCG5. ABCG8. APOE. CYP7A1 and NPC1L1). ABCG5/ABCG8 transporters play a fundamental role in the regulation of intestinal absorption of sterols. Rare loss of function mutations in one of these two genes causes sitosterolemia, characterized by elevated plasma levels of PS and cholesterol and premature atherosclerosis [25]. The p.D19H variant of the ABCG8 gene is a “gain of function” sequence variation that has been associated with an increased risk of gallstones disease and with a reduction in intestinal cholesterol absorption capacity [26,27]. However, no study has clearly demonstrated the association between the presence of this gene variant and the cholesterol-lowering effect of PS supplementation. In our study, patients carrying the 19H allele showed a trend of lower TC and LDL-C at Week 3 and Week 6. achieving a statistically significant difference only after 6 weeks (p<0.05). This can be explained by the fact that the p.D19H polymorphism results in a gain of function. so the reduction in cholesterol/sterol absorption associated with the presence of the rare allele is due to the increased extrusion of sterols. which is further enhanced by PS supplementation. NPC1L1 plays a key role in intestinal absorption of cholesterol and phytosterols. In recent years. studies have investigated how NPC1L1 variations may alter cholesterol absorption. Particularly, polymorphism c.816 C>G is associated with an increase in response to ezetimibe. a drug that interferes with the function of NPC1L1. inhibiting intestinal cholesterol absorption [28]. Lupattelli et al. showed an increase in cholesterol absorption in hypercholesterolemic patients carrying the G allele, as highlighted by a significant greater blood concentration of campesterol and sitosterol [22].
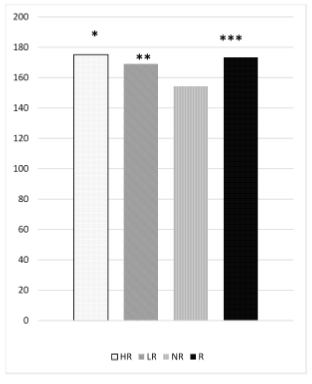
In another study the haplotype involving two polymorphisms c.872 C>G and c.3929 G>A was associated with greater reduction in LDL-C concentrations in the carriers of the minor allele [29]. In our study. subjects with the GG genotype showed a higher and statistically significant reduction in TC and LDL-C after 3 weeks compared to both GC and CC genotypes. In this context, the presence of the allele G (gain of function variation) leads to high intestinal concentrations of PS. so the NPC1L1 transporter absorbs less cholesterol. The APOE gene has 3 different alleles in the population that can be combined into six possible phenotypes. The ℇ4 allele has been associated with higher TC and LDL-C and higher cholesterol absorption. Anyway. some different studies led to conflicting results. Vanhanen et al. showed that the LDL-C reduction in hypercholesterolemic subjects carrying at least one ℇ4 allele was greater in response to PS supplementation (LDL-C -11.8%) compared to ε3ε3 subjects (LDL-C -6%); moreover, ε3ε4 or ε4ε4 subjects showed a reduction in plasma concentrations of sitosterol and campesterol and an increase in cholesterols precursors after treatment. indicating a decrease in intestinal cholesterol absorption followed by a compensatory increase in its synthesis. Conversely. other studies did not find any difference in the response to PS supplementation according to the APOE genotype. perhaps because these studies did not have sufficient statistical power to detect a significant difference [30,31]. In our study, there was a trend of a greater cholesterol-lowering effect in ℇ4 allele carriers compared to ε3ε3 subjects (Table 5), but without achieving the statistical significance. The two main limitations of this study are represented by the small sample size and the lack of data concerning the plasma modifications of PS and precursors of cholesterol levels as a response to treatment. These limits did not allow us to fully understand why the effect of PS supplementation varied over time. Therefore. further studies are needed to evaluate the multiple genetic and nongenetic variables that can influence the response to PS supplementation. in order to identify the most suitable patients for long-term treatment.
|
LDL-C (mg/dl) at baseline |
P value (vs Non-responders) |
|
|
Responders |
173.33±18.92 |
< 0.01 |
|
High-responders |
175.15±20.34 |
< 0.01 |
|
Low-responders (0-5% reduction) |
169.02±15.03 |
< 0.05 |
|
Non-responders |
154.32±19.33 |
Table 8: LDL-C values at baseline in various response-based subgroups.
Data are mean and standard deviation.
Abbreviations: LDL-C low-density lipoprotein cholesterol.
5. Conclusion
PS are an effective cholesterol-lowering nonpharmacological therapy. and recently the dietary supplementation of phytosterols is suggested by international guidelines and statements of some scientific societies [5-7]. The only documented side effect with their use, when the recommended doses are exceeded (3g/day) [32], is a modest reduction in blood carotenoid levels, which can be easily corrected by adequate consumption of fruits and vegetables. It should be emphasized that there is a great inter-individual variability in the response to PS treatment. The identification of predisposing genetic factors to a greater response to PS supplementation could allow the customization of cholesterol-lowering therapy. in order to obtain the best result with minimum effort.
References
- Gylling H, Plat J, Turley S, et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 232 (2014): 346-360.
- Ras T, Hiemstra H, Lin Y, et al. Consumption of plant sterol-enriched foods and effects on plasma plant sterol concentrations- a meta-analysis of randomized controlled studies. Atherosclerosis 230 (2013): 336-346.
- Ferguson JJA, Stojanovski E, MacDonald-Wicks L, et al. Fat type in phytosterol products influence their cholesterol-lowering potential: A systematic review and meta-analysis of RCTs. Lipid Res 64 (2016): 16-29.
- Wu T, Fu J, Yang Y, et al. The effects of phytosterols/stanols on blood lipid profiles: a systematic review with meta-analysis. Asia Pac J Clin Nutr 18 (2009): 179-186.
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 41 (2020): 111-188.
- Cicero AFG, Colletti A, Bajraktari G, et al. Lipid-lowering nutraceuticals in clinical practice: position paper from an International Lipid Expert Panel Nutr Rev 75 (2017): 731-767.
- Pirro M, Vetrani C, Bianchi C, et al. Joint position statement on “Nutraceuticals for the treatment of hypercholesterolemia” of the Italian Society of Diabetology (SID) and of the Italian Society for the Study of Arteriosclerosis (SISA). Nutr Metab Cardiovasc Dis 27 (2017): 2-17.
- Trautwein EA, Vermeer MA, Hiemstra H, et al. LDL-Cholesterol Lowering of Plant Sterols and Stanols-which factors influence their efficacy? Nutrients 10 (2018).
- Fumeron F, Bard JM, Lecerf JM, Interindividual variability in the cholesterol-lowering effect of supplementation with plant sterols or stanols. Nutr Rev 75 (2017): 134-145.
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 31 (1990): 545-548.
- Pisciotta L, Fasano T, Bellocchio A, et al. Effect of ezetimibe coadministered with statins in genotype-confirmed heterozygous FH patients. Atherosclerosis 194 (2007): e116-122.
- Jones PJ, Raeini-Sarjaz M, Ntanios FY, et al. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J Lipid Res 41 (2000): 697-705.
- O’Neill FH, Sanders TAB, Thompson GR. Comparison of efficacy of plant stanol ester and sterol ester: short-term and longer-term studies. Am J Cardiol 96 (2005): 29D-36D.
- Sierksma A, Weststrate JA, Meijer GW. Spreads enriched with plant sterols. either esterified 4,4-dimethylsterols or free 4-desmethylsterols. and plasma total and LDL-cholesterol concentrations. Br J Nutr 82 (1999): 273-282.
- Thomsen AB, Hansen HB, Christiansen C, et al. Effect of free plant sterols in low-fat milk on serum lipid profile in hypercholesterolemic subjects. Eur J Clin Nutr 58 (2004): 860-870.
- Jones PJH, Shamloo M, MacKay DS, et al. Progress and perspectives in plant sterol and plant stanol research. Nutr Rev 76 (2018): 725-746.
- Ras RT, Geleijnse JM, Trautwein EA. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomised controlled studies. Br J Nutr 112 (2014): 214-219.
- Doornbos AME, Meynen EM, Duchateau GSMJE, et al. Intake occasion affects the serum cholesterol lowering of a plant sterol-enriched single-dose yoghurt drink in mildly hypercholesterolaemic subjects. Eur J Clin Nutr 60 (2006): 325-333.
- Demonty I, Ras RT, Van der Knaap HCM, et al. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J Nutr 139 (2009): 271-284
- Rideout TC, Harding SV, Mackay D, et al. High basal fractional cholesterol synthesis is associated with nonresponse of plasma LDL cholesterol to plant sterol therapy. Am J Clin Nutr 92 (2010): 41-46.
- Mackay DS, Gebauer SK, Eck PK, et al. Lathosterol-to-cholesterol ratio in serum predicts cholesterol-lowering response to plant sterol consumption in a dual-center. randomized. singleblind placebo-controlled trial. Am J Clin Nutr 101 (2015): 432-439.
- Lupattelli G, Pisciotta L, De Vuono S, et al. A silent mutation of Niemann-Pick C1-like 1 and apolipoprotein E4 modulate cholesterol absorption in primary hyperlipidemias. J Clin Lipidol 7 (2013): 147-152.
- Tada H, Nohara A, Inazu A, et al. Sitosterolemia. Hypercholesterolemia. and Coronary Artery Disease. J Atheroscler Thromb 25 (2018): 783-789.
- Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem 277 (2002): 17377-17380.
- Buch S, Schafmayer C, Volzke H, et al. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat Genet 39 (2007): 995-999.
- Berge KE, Von Bergmann K, Lutjohann D, et al. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J Lipid Res 43 (2002) 486-494.
- Gylling H, Hallikainen M, Pihlajamaki J, et al. Polymorphisms in the ABCG5 and ABCG8 genes associate with cholesterol absorption and insulin sensitivity. J Lipid Res 45 (2004): 1660-1665.
- Hegele RA, Guy J, Ban MR, et al. NPC1L1 haplotype is associated with inter-individual variation in plasma low-density lipoprotein response to ezetimibe. Lipids Health Dis 4 (2005): 16.
- Zhao HL, Houweling AH, Vanstone CA, et al. Genetic variation in ABC G5/G8 and NPC1L1 impact cholesterol response to plant sterols in hypercholesterolemic men. Lipids 43 (2008): 1155-1164.
- Ishiwata K, Homma Y, Ishikawa T, et al. Influence of apolipoprotein E phenotype on metabolism of lipids and apolipoproteins after plant stanol ester ingestion in Japanese subjects. Nutrition 18 (2002): 561-565.
- MacKay D, Eck PK, Gebauer SK, et al. CYP7A1-rs3808607 and APOE isoform associate with LDL cholesterol lowering after plant sterol consumption in a randomized clinical trial. Am J Clin Nutr 102 (2015): 951-957.
- Plant Sterols and Stanols and Blood Cholesterol Available online: http://www.efsa.europa.eu/en/efsajournal/pub/2693 (accessed on Jun 28. 2020).


 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 