Process Optimization and Quality Assessment of Nem, a Traditional Cambodian Lactic Acid Fermented Fish Product
Liseany Chor2, Sengly Sroy1,2, Chanthol Peng1,2*, Seyha Doeurn1,2
1Research and Innovation Center, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia
2Faculty of Chemical and Food Engineering, Institute of Technology of Cambodia, Phnom Penh, Cambodia
*Corresponding Author: Chanthol Peng, Research and Innovation Center, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia
Received: 23 November 2023; Accepted: 04 December 2023; Published: 27 February 2024
Article Information
Citation: Liseany Chor, Sengly Sroy, Chanthol Peng, Seyha Doeurn. Process Optimization and Quality Assessment of Nem, a Traditional Cambodian Lactic Acid Fermented Fish Product. Journal of Food Science and Nutrition Research. 7 (2024): 52 - 63.
DOI: 10.26502/jfsnr.2642-110000152
View / Download Pdf Share at FacebookAbstract
Cambodia has a long-standing tradition of processing freshwater fish, and fermented foods, particularly Nem, hold significant importance among all fishery products in Cambodia. However, the traditional production methods of these fermented fish products, based on generational knowledge, pose challenges in establishing standardized quality indices and optimal production methods. This study aimed to address these challenges by conducting a survey questionnaire on local Nem production, raw materials, and finished products. Through the survey results with Nem producers and a comprehensive literature review, an acceptable standardized method was documented. The study also included a quality assessment involving physicochemical, nutritional, and microbiological analyses to evaluate the safety and overall quality index of Nem produced in laboratory. Shelflife analysis was conducted over a two-week period. The raw fish used in Nem production exhibited the following quality attributes: pH 6.76, acidity 0.37%, water activity 0.99%, moisture content 79.25%, ash content 2.29%, total fat 0.25%, and total protein 18.19%. During fermentation, the pH, acidity, and water activity ranged between 4.54-4.47, 0.81%-0.77%, and 0.97% respectively. The counts of viable bacteria count (VBC), lactic acid bacteria (LAB), and total yeast and molds (TYM) at the end of the 12-day storage period ranged from 8.08 Log CFU/g to 8.11 Log CFU/g and 8.74 Log CFU/g, respectively. These results indicate that Nem maintained high-quality parameters during the fermentation period and met acceptable standards for consumption due to its high protein content. Furthermore, after 12 days of storage at 4°C, Nem demonstrated good quality in physicochemical, nutritional, and microbiological analyses
Keywords
<p><em>Nem</em>; Standard method; Quality assessment; Sensory evaluation; Shelf-life</p>
Article Details
Introduction
Fish production through aquaculture in Cambodia is considered sustainable and the most efficient way to produce high quality proteins for human consumption (Suzuki, 2021). Beyond delivering advantages over sustainable resources, fish is the main contributor for the intake of both the micronutrients (vitamins and mineral) and macronutrients (protein, fat, water, ash) in which contributing up to 37% of total protein intake (Vilain & Baran, 2016). On the same page, fish provides easily digestible protein of high biological value that is important for the growth and development of the body. To Cambodia, freshwater fisheries are an integral part of the country’s culture, economy and food security, and are a vital source of food for rural people. To produce a variety of economically preserved fish and fishery products, Cambodians have an old tradition of processing freshwater fish such as fish paste, fermented fish, dry salted fish, smoked fish, fish sauce, and dried fish for animal feed. Fermented foods are an important part of the typical diet in Cambodia (Ly et al., 2020b). Regarding freshwater fish in Tonle Sap Lake, the giant snakehead (scientific name: Channa Micropeltes), known locally as Trey Diep, is the most significant freshwater fish species due to its abundant and sustainable supply, affordable price, and its substantial flesh, which makes it a prime choice for producing commercially viable products (Vilain & Baran, 2016). All in one, one among the most popular fermented fish product in Cambodia is Nem which is primarily made of uncooked fresh-water fish such as the giant snakehead fish (LeGrand et al., 2020).
Nem is considered as an important and traditional fish product for Cambodian people. It is specifically produced mainly in only two provinces of Cambodia such as Battambang and Kratie, yet its popularity is growing and well-known to even outside of their geographical region. On the other hand, in term of processing method, the production of this special fermented fish product is merely done by local producers. There is not yet a wide scientific and standardize method to industrialize this fermented fish product with the consistent and better quality. Basically, in Cambodia's context, fermented fish products are traditionally produced based on generational knowledge, which poses challenges in establishing a standardized quality index and determining an optimal method of production. Finally, yet importantly, Nem products are generally not labelled with an appropriate shelf-life and are usually stored in the outside environment until they are completely consumed (Ly et al., 2020a). All in one, the contaminants in fermented fish products depends on many factors, such as raw materials, good handling practices, and storage conditions that need to be executed to prevent public health concern of people across the country.
To void the gaps mentioned earlier, this study aims to standardize the method of Nem production by optimizing the processing method based on survey with Nem local producers and from the existing literature review for the purpose to document and re-formulate a new processing method that would be acceptable by consumers. Also, quality assessment in term of physicochemical, microbiological, and nutritional parameters will be conducted to assess the safety and overall quality index of Nem. Shelf-life analysis will be as well included in this study.
Materials and method
Experimental plan
This study was divided into three sections. The first section aimed to define the optimized formula and processing method for Nem. This involved conducting a field survey with local Nem producers to gather information on the raw materials, ingredients, and their processing practices. Based on the findings from the survey, Nem development was replicated with some modifications to the original formula & raw materials. The Nem samples produced under these conditions were then subjected to sensory evaluation to define the most preferred condition. In the second section of the study, the Nem samples were further analyzed for physicochemical parameters (pH, total acidity, water activity), microbiological characteristics (viable bacteria count, lactic acid bacteria, total yeast and mold), and nutritional composition (moisture, ash, lipid, and protein content). Additionally, the quality of raw fish used in this Nem processing were also analyzed to compare for the changes from turning the raw to the fermented final Nem product. The third section of the study focused on assessing the shelf-life of Nem. The Nem samples were stored at 4°C in a refrigerator for up to 12 days to observe changes in quality over time.
Formula optimization for Nem Processing
Survey implementation: The survey for Nem processing methods was conducted with local producers in two main target provinces of Battambang and Kratie which are the well-known Cambodian provinces in the production of this type of fermented fish products. In total 26 local producers (20 from Battambang province and the rest from Kratie province) were interviewed face to face with the help of the prepared questionnaire. The design of questionnaire was made based on the review on several existing literatures [6-10] and following the guidelines provided by the Food and Agriculture Organization (FAO) with some modification adopting to the Cambodian context [11]. From all the information collected in this survey, two main necessary information regarding the raw materials (fish species) and processing methods (mainly ingredient usages, packaging and fermentation period) were presented in this study.
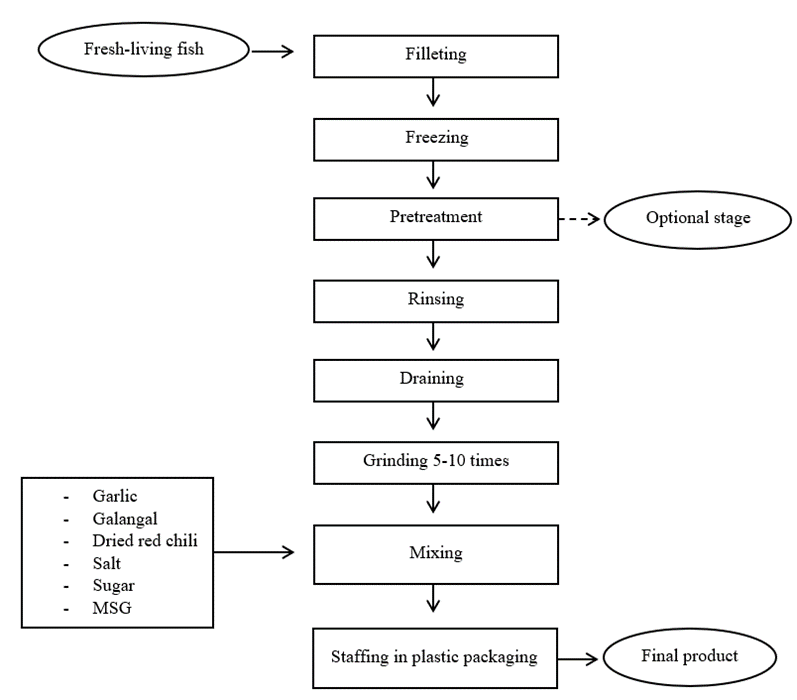
Processing of Nem: Figure 1 shows the general processing flowchart of Nem. First and foremost, the whole fish was degutted, deskinned, and deboned. Then, the filleted fish was pre-treated with salt per fish weight in order to remove the unfavorable fishy odor. The filleted fish was washed with cold water for three times to remove the blood and fat tissue. After that the fish was left to completely drained, then taken to be grounded with spices such as garlic, galangal, and dried red chili, using a meat grinder machine (Meat mincer 750W, New Zealand). The grinding process need to be repeated for several times to ensure that the filleted fish and spices were completely minced. Next is the mixing stage. All the minced fish was mixed with seasonings like sugar, salt, and MSG for 30 min. After that the mixed minced fish was stuffed into plastic packaging or banana leaves, then fermented at room temperature for 2-3 days. After the fermentation is completed, Nem was served for sensory evaluation.
Sensory evaluation: Sensory evaluation test was performed when Nem reached a favorable pH value (fermentation was completed). The purpose of the test was to define the acceptability of consumers on the new Nem formulation made. Nine-point hedonic rating scale (Wichchukit & O’Mahony, 2015) was used to assess consumer acceptability (1. dislike extremely; 2. dislike very much; 3. dislike moderately; 4 dislike slightly; 5. neither like nor dislike; 6. like slightly; 7. like moderately; 8. like very much; and 9. like extremely). In total, about 20 untrained panelists were participated in this sensory evaluation test. Most were staffs and students of the Institute of Technology of Cambodia. Samples of Nem with different formula were coded with random three-digit numbers and were presented in a small plastic container given to each panelist. During the evaluation process, the panelists were not allowed to talk to each other, and a cup of water was given to wash their mouth before and after testing each sample (Sharif et al., 2017). In total, eight attributes ranging from color, texture, flavor, sourness, sweetness, odor, and overall liking of each Nem samples were evaluated.
Quality assessment of Nem samples
Physicochemical analysis: In physicochemical analysis, samples were tested on parameters such as pH, total acidity and water activity. The pH value was determined using a pH meter (LAQUA F-72-HORIBA, USA). Five grams of samples were diluted 10 times with distilled water, then homogenized for 1 hour. The pH meter electrode was then placed into the blended sample, and the pH values of each sample were recorded at room temperature (Tsighe et al., 2018). Total acidity was determined using the titration method described in AOAC manual 935.57, which was titrated with 0.1 N NaOH using phenolphthalein as the indicator and expressed in g lactic acid per 100 g of fish (Safety et al., 2012). Water activity (aw) value was measured using the digital water activity meter (Aqualab 4TE DUO Dew Point Water Analyzer, Decagon Devices Inc, USA) after equilibration at room temperature (~25°C).
Nutritional analysis: Moisture, ash, lipid and protein content were determined using the methods of AOAC (1990) (AOAC 1990, 1990). In summary, moisture content of the samples was measured by drying 5 g of samples at 105°C for 24 h in a hot air dry oven (Memmert, Germany) (Sroy et al., 2021). While for ash content, 5 g of samples were placed in a porcelain crucible, introduced to oven at 105 °C for 24 hours before transferring to furnace at 550 °C for 3 hours until the gray content was obtained (Sroy et al., 2021). For lipid content, 5 g of sample was placed in an oven for 24 h at 105°C in order to reduce the moisture content, packed with filter paper and placed directly inside the cellulose thimble. It was then introduced into the Soxhlet extraction unit (VELP SCIENTIFICA, Italy) by using hexane as a solvent extractor. Total nitrogen or protein content was determined using the semi-micro Kjeldahl method. The crude protein content was calculated using a conversion factor of 6.25.
Microbiological analysis: Microbiological analysis was conducted by first collecting 10 g of each sample, then transferred to a stomacher bag, and homogenized with 90 mL of buffered peptone water for one minute. Appropriate dilutions of the samples were prepared using the same diluent, and 0.1 mL aliquots of each dilution were applied on various selective media using the spread plate method (Ly et al., 2020a). The viable bacteria count was cultured on Luria-Bertani agar (Difco, BD, USA) at 37°C for 24 h. Lactic acid bacteria (LAB) was cultured in DeMan Rogosa Sharpe agar (MRS) (Himedia, India) at 37°C for 24 h. Total Yeast and Mold (TYM) were cultured in Dichloran-glycerol (DG18) agar base (Oxoid, UK) at 30°C for 3-5 days, two replicates per sample, and the number of colonies determined is expressed as Log CFU/g.
Shelf-life study of Nem Product
The shelf-life analysis of Nem involved observing the quality of the final product stored at 4°C in a refrigerator for 12 days. The assessment of Nem's shelf-life was conducted by examining various parameters, including pH, acidity, water activity, viable bacteria count, lactic acid bacteria, and total yeast and mold. Duplicate samples for the shelf-life analysis were prepared and stored separately, representing Day 0, Day 4, Day 8 and Day 12, respectively.
Statistical analysis
The primary survey data were treated from questionnaire paper into a google form. Experiments were all conducted in triplication. Result units of quantitative microbiological analyses were expressed in Log CFU/g. The physicochemical parameters and nutritional profile results were analyzed with statistical analyses using the Statistical Package for the Social Sciences (SPSS, Version 25.0.0 for Windows, 2011; IBM Co., Somers, NY, USA). All data were analyzed for the degree of variation by calculating the mean and standard deviations (SDs). The significance differences were evaluated using analysis of variance (ANOVA). Significant results were selected at p-values 0.05.
Results
Results of formula optimization for Nem processing
The results from the field survey with Nem local producers in Battambang and Kratie was presented in Table 1. About 30% of the respondents were those who have experiences with Nem processing for less than 5 years while about 70% were those who have experienced for over ten years. According to our interview, a few of our respondents were also the large producers who have been producing Nem to supply to other provinces of Cambodia such as Phnom Penh, Siem Reap, Sihanoukville, and exporting to neighboring countries, including Vietnam and Thailand. Even so, the unstandardized processing techniques were still one of the main challenges to maintain the consistent quality for them. Regarding raw materials for Nem, there were mainly fishes, additional ingredients and spices. Fish used for Nem processing in Cambodia was mainly the farmed fish, responded by 77% of the respondents (Table 1). Fish was mostly brought alive to the processing places. They were then killed and filleted for only fish flesh to produce into Nem. The fish flesh needs to be washed for 3 to 4 times for the purpose of removing fat, blood, and fishy odor. All of the local producers were able to access to tap water for rinsing fish as well as for the other usages in the whole processing techniques.
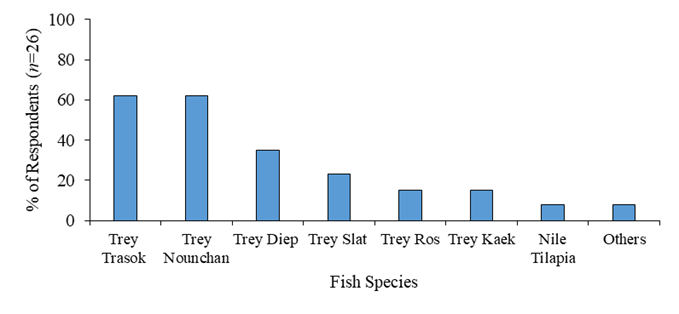
Regarding the fish species, there were many varieties used for Nem processing methods (Figure 2). The common fish species were Trey Trasok (Probarbus labeaminor) 62%, Trey Nounchan (imported from Thailand) 62%, Trey Diep (Channa micropeltes) 35%, Trey Slat (Notopterus notopterus) 23 %, Trey Ros (Channa marulius) 15%, Trey Kaek (Labeo chrysophekadion) 15%, and others such as Nile Tilapia (Oreochromis spp.), Trey Kaey (Chitala blanci), Trey Achkok (Labiobarbus siamensis) and so on. The choices of selecting these different species were mainly based on price, abundance of flesh, and availability of fish in that region. Yet, not many focuses on the fish flesh quality or its nutritional values that are also the important factors affecting to the Nem quality such as chewiness texture and flavor of Nem as the final product. In our study, Trey Diep was chosen as the main raw material in Nem processing technique. Trey Diep, a species of giant snakehead, is known for its substantial flesh, reasonable price, and accessibility for purchase. It is easy to process Nem due to its minimal bones, making it easy to fillet. Additionally, it is considered more sustainable compared to other freshwater river fish (Bich et al., 2020; Joffre et al., 2021).
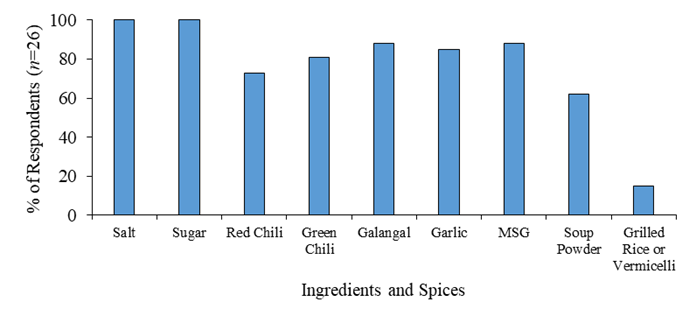
Figure 3 shows the common ingredients and spices used in Nem production by local producers. Salt and Sugar are the two must-have ingredients that were mentioned by all the interviewed local Nem producers. Besides acting as flavor enhancers, salt and sugar also plays necessary roles as a substrate and a condition to boost the fermentation of Nem processing. Other ingredients such as MSG and soup powder were also mentioned and were mainly used to enhance the flavor. Additionally, red chili, green chili, galangal and garlic were also the needed ingredients to provide unique Cambodian taste and accelerate the fermenting process of Nem. Interestingly, grilled rice and dried vermicelli was also known as additional ingredients for local preference, particularly for the Kratie Nem local producers. According to the survey, mostly in Nem processing from the producers’ experiences, ingredient for usages in processing should be in a fresh condition with high quality.
Table 1: Survey results on Nem processing methods by local producers in Battambang and Kratie
|
Questions |
Responses |
% of respondents (n=26) |
|
Experiences in Nem producing |
5-10 years |
31 |
|
>10 years |
69 |
|
|
Fish origin |
Farm |
77 |
|
Wild |
19 |
|
|
Both |
4 |
|
|
Status of fish |
Dead |
31 |
|
Alive |
69 |
|
|
Rinsing time |
3 times |
15 |
|
4 times |
85 |
|
|
Rinsing reasons |
Remove fat |
100 |
|
Remove blood |
92 |
|
|
Remove fishy smell |
73 |
|
|
Type of water use |
Tap water |
100 |
|
Food additives |
Yes |
15 |
|
No |
85 |
Sensory evaluation result for the optimized Nem formulation
To define the optimized Nem formula, first and foremost, the early trail processing based on existing literature review on similar Nem products were conducted. Table 2 shows two processing techniques including fish species used, ingredients and fermentation period of two recipes from (Sangjindavong et al., 2000, 2005). Following these two formulations, the results were not suitable for Cambodian preferences due to the lack of ingredients and excess of spices. Next, we re-formulated another Nem processing following the survey results with several modification in term of the selection of raw materials, and carefully pay attention in each step of the processing techniques. Trey Diep was chosen as the main raw material in our new Nem processing due to its high-quality flesh content. Fresh spices such garlic, galangal, and dried red chili were also added in a well-defined ratio. From each step to another such as washing, rinsing, grinding and mixing, times were always recorded to ensure the better production and consistent practices for the Nem produced. Figure 1 shows the defined processing flowchart modifying from both the survey results and existing literature.
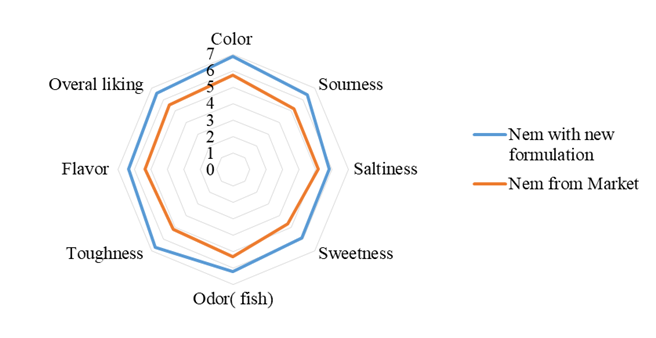
After 3 days incubation when the fermentation was complete, the new Nem formulation was tested for the sensory scores comparing to the Nem purchased from the market. Figure 4 shows the result of sensory evaluations of all the attributes for both Nem products. For the Nem purchased from market, all attributes scores were all above 5.0 yet only the sweetness attribute achieve the lowest score of only 4.7. On the other hand, for the new Nem formulation, the sensory scores for all attributes ranges from 5.8 to almost 7.0 in the nine-point hedonic scale. This indicates the consumers found the new Nem product to have more superior quality in term of color, sourness, saltiness, sweetness, odor, toughness, and flavor. It is possibly that by utilizing the standardized method with the well-defined ratio give rise in acceptable preference to the panelists.
Table 2: Detailed information from the literature review for trail processing formulation for Nem
Quality assessment of raw fish and Nem
Physicochemical analysis: Table 3 presents the values of pH, acidity and water activity (aw) of both the raw fish and Nem sample. The pH of raw fish was found to be approximately close to 7, which is considered neutral on the pH scale. The recommended pH range for freshwater fish is generally between 6.5 and 8.5 [20,21]. These findings are in good agreement with previous studies on freshwater fish conducted by other authors [22]. On the other hand, Nem sample has a significant lower pH value at only 4.43 which was due to increasing amounts of organic acids, mainly lactic acid produced by LAB during the fermentation process. Naturally occurring lactic acid bacteria (LAB) ferment hexose sugars, such as glucose, by oxidizing NADH generated during glycolysis, with pyruvate serving as the electron acceptor, to form lactic acid as the major product [23]. In a similar study on the production of fermentation of Nham, a type of fermented fish product in Thailand that generally takes 3-5 days at room temperature (30 C) for fermentation, Nham usually has a pH ranging from 4.4 to 4.8 [24,25]. Lactobacilli are the major producers of lactic acid responsible for the decrease in pH and the increase in acidity during the fermentation [25]. Lactic and acetic acids are often suggested to be major contributors to the acid aromas and tastes and the development of the Nhams texture of fermented sausage and in Nham [25].
Regarding total acidity, the value in raw fish was 0.37%, while in Nem, the fermented fish, was 0.85%. These align with the range of standard acidity of fermented fish reported in a study where the predominant acid constituent, the lactic acid, typically falls between 0.2% and 1.0% [26]. Moreover, it was observed that the total acidity values were in the inverse correlation to the pH in which the total acidity was in a rapid increase when the pH drop during the fermentation process [27]. This trend was also spotted in the study by Visessanguan et al. [10] where Nham in day 0 (or raw fish) has the pH of 6.3, with TA 1.8 and day 2 (when fermentation complete), the pH was 4.3, and TA 4.2. And this author also stated that Nham is normally uncooked, to ascertain the safety of Nham from survival and growth of some pathogenic microorganisms naturally contaminated in pork, it is recommended that Nham with pH lower than 4.6 is safe for consumption. The increase of total acidity along with the increment fermentation time could be attributed to the accumulation of some organic acid i.e. lactic acid and acetic acid that results from the activities of fermentative organisms such as lactic acid in the fermenting foods [28].
In terms of water activity, the absolute value of aw measured in raw fish was 0.99. This indicates that the raw fish meat can highly be conducive to spoilage of bacterial growth if not processed accordingly. The water activity level required for microbial growth is at or higher than 0.85 [29]. In our study, after fermentation, the aw of Nem was slightly decrease to 0.97. This result was similar to study by Ly et al. [5] where the water activity values in fermented fisheries products were between 0.75-0.97. Further reduction of aw in food would help to decrease bacterial growth rate thus prolong shelf life. The water activity can also be reduced by using three basic methods, namely dehydration, crystallization, and addition of solutes. One of those method relate to the addition of salt and sugar which are count as solutes that lead to a little bit decrease of water activity during fermentation [30].
Table 3: pH, acidity and water activity of raw fish and Nem
|
Parameters |
Raw fish |
Nem |
|
pH |
6.76 0.14b |
4.43±0.04a |
|
Acidity (%) |
0.37 0.04a |
0.85±0.01b |
|
aw?(%) |
0.99 0.001b |
0.97±0.002a |
a-b-c indicates the significant differences among value within rows; aw: water activity
Nutritional analysis: Nutritional components such as moisture, ash, fat and protein content of the raw fish and Nem were compared in table 4. In term of moisture content, the values were approximately 80% in raw fish and was decreased to around 66% for Nem, after the 3 days fermentation. Similar to water activity, moisture content in a food product is important as it may affect to the growth of some microbes in the food. The decrease of moisture content in Nem might be attributed to the addition of spices in the Nem formulation thus increase dry matter contents during the fermentation process[28]. The moisture content of Nham-Pla, the sour fish cake from Thailand, on the other hand, found to be higher than our study which may be due to shorter fermentation period which took only two days fermentation at room temperature [9].
For ash content, the values in raw fish were 2.2% while in Nem was 4.06%. Generally, ash is an inorganic residue from the combustion process of organic compounds showing the total minerals in the food ingredients [31]. The significant increase of ash content after the fermentation process may be attributed to the raw materials used in the Nem processing techniques. Those include the species of fish as well as the addition of mineral substances of the spices such as galangal, red chili, garlic, salt, sugar, monosodium glutamate (MSG) [32]. Among those spices, galangal is used in Nem processing as the flavoring agent and to reduce undesirable order in meat products [33], and the ash content of edible portion fish is varied due to the weight and length and the season and habitats of those fishes [11,34].
For fat content, there was no significant different observed in both the raw fish and Nem samples, yet a slight increase was found from 0.25% in raw fish to 0.34% in Nem (Table 4). The fat content of freshwater fish usually ranged from 2.09%-7.17% [35]. However, these values may be varied due to species, seasons, life cycles, food availability in the environment and the reproduction activities of fish species, and mostly 70% from the nature of fish species [36,37-39]. As for instance, in our study, the fat content was lower because Trey Diep contains variable fat as mentioned in the article of Zugarramurdi [35], the variations in the ω-fatty acid content in C. micropeltes might be due to the difference in locations and feeding habitats. This is because C. micropeltes is classified as a carnivorous fish and depends on other fish for its diet [35]. Plus, the fillet was rinse thoroughly prior to processing several times and the reason behind the cleansing is to remove fat, blood and fishy odor. After fermentation, a slight increase of fat content was observed in Nem could be attributed to extensive breakdown of large fat molecule to simpler fatty acid units due to the high activity of lipolytic enzymes. Also, the dead microflora or the fact that fermenting microflora do not use fat from these foods (substrate) as a source of energy may also be another reason of this increment [40].
Regarding protein content, the result shown in approximately of 18 % for the raw fish which was similar to other studies where protein content was mostly varied from 11.8% - 20.9% [41,17]. A significant increase was also observed after the fermentation in which the protein content increased up to 29.27% for Nem samples. This increment may be attributed partly to the degradation of dry matter throughout fermentation, particularly the bacterial fermentation has been shown to improve the lysine content in fermented foods [42]. Nem fermentation process causes the breakdown of complex compounds in the form of proteins into simpler compounds or derivatives of proteins, such as: proteose, peptones, peptides and amino acids [43]. Another study also stated that moisture content of Nam Pla-ra decreased with fermentation time which correlated with an increase in protein and ash [44].
Table 4: Nutritional analysis of raw fish compares to Nem
|
Parameters |
Raw fish |
Nem |
|
Moisture content (g/100g) |
79.25 1.56b |
66.31 1.24a |
|
Ash content (g/100g) |
2.29 0.41a |
4.06 0.17b |
|
Total fats (g/100g) |
0.25 0.12a |
0.34 0.06a |
|
Total proteins (g/100g) |
18.19 1.87a |
29.27 1.07b |
a-b indicates the significant differences among value within rows.
Microbiological analysis: Microbiological results of Viable Bacterial Count (VBC), Lactic Acid Bacteria (LAB), and Total Yeasts and Molds (TYM) for both raw fish and Nem were listed in Table 5. In this study, the VBC of giant snakehead fish was found to be 5.32± 0.3 Log CFU/g, while the TYM was at 3.46 ± 0.02 Log CFU/g. Yet, the proposed limit for aerobic plate count shall be at 5 Log CFU/g for fresh fish, and TYM count shall be 2.22 ± 0.02 Log CFU/g [45,46]. The higher concentrations of each bacterial result of raw fish in our study could be attributed to factors such as seasonal variations, life cycles, food availability in the environment, as well as the hygienic conditions during fish filleting processes. Furthermore, the differences in the origin of the fish, such as being sourced as fresh fish in comparison to other studies, might also contribute to the observed variations.
Overall, comparing raw fish to Nem samples, the concentration of VBC, TYM, and LAB were all rapidly increased. This is possibly because of fermentation mechanisms in which naturally fermentation was allowing all the microorganisms the favorable environment to growth. And it also responsible of water activity of 0.97 that enhance the most satisfactory condition for bacteria, yeast and mold to tend to absorb nutrient in the early stage of fermentation.
Table 5: Microbiological analysis of raw fish compares to Nem
|
Parameters |
Raw fish |
Nem |
|
VBC (Log CFU/g) |
5.32 0.3a |
8.08 0.03b |
|
LAB (Log CFU/g) |
4.21 0.08a |
8.74 0.24b |
|
TYM (Log CFU/g) |
3.46 0.02a |
5.6 0.05a |
a-b indicates the significant differences among value within rows; VBC: Viable bacteria count; LAB: Lactic acid bacteria; TYM: Total Yeasts and Mold.
Shelf life of Nem product
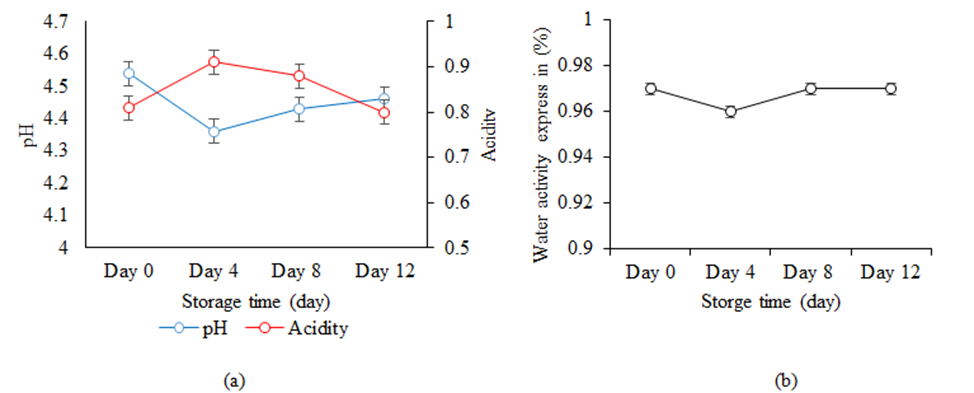
Physicochemical changes of Nem during storage: Figure 5 shows the physicochemical changes such as pH, acidity and aw of Nem during the storing period of 12 days in the refrigerated condition. It was observed that from day 0 to day 4, the pH was slightly decreased from 4.54 to 4.36 which may be due to lactic acid accumulation by LAB. Usually, the lower pH is preferred in fermented products as it would provide high acidity content that creates the antibacterial effects thus prolonging the spoilage of food products [46]. From day 4 onward, it was observed that the pH was slightly increase to 4.46 which may be due the production of total volatile nitrogenous compounds [50] and their accumulation in the fish during the fermentation process. Overall, the pH values within these 12 days were in the range of 4.54 to 4.46. which were within acceptable condition similar to other types of fermented fish products in Asia ranging from 4.3 to 7.8 [51].
For acidity, the rapid rise and drop was observed for Nem samples from day 0 to day 12 at 0.81 % to 0.80, respectively. This increase was due to the increasing amounts of organic acids (natural acid), mainly lactic acid produced by LAB during the storing period [52,53]. Other studies of traditional fermented meat, Naem in Thailand was also found to have the acidity values from 0.4-1.4% during the period of 3 days [54]. It also be noted that the increase of total acidity during this storing period corresponding to the decrease of pH values of Nem within this same period.
Changes of water activity, aw, of Nem was also depicted in figure 5b. This study found that aw of Nem was almost at a constant value of 0.97 from day 0 to day 12. These high water activity content may be caused by the addition of fresh spices such as garlic and galangal that during the storing process the water content remains the same from day 0 to day 15 [55,56]. However, this result was still in similar range to some of fermented fish products studied by Ly et al. [5] in which the aw was between 0.75 – 0.97. The high aw content can sustain the necessary microbes in fermentation and inhibit the growth of unwanted bacteria in the fermented fish products [57]. Yet, in general, to prolong shelf life of a food product, it is recommended for the aw to be at or lower than 0.85 [29]. Thus, further processing techniques is recommended to study to reduce the water activity of Nem to this preferred value of aw.
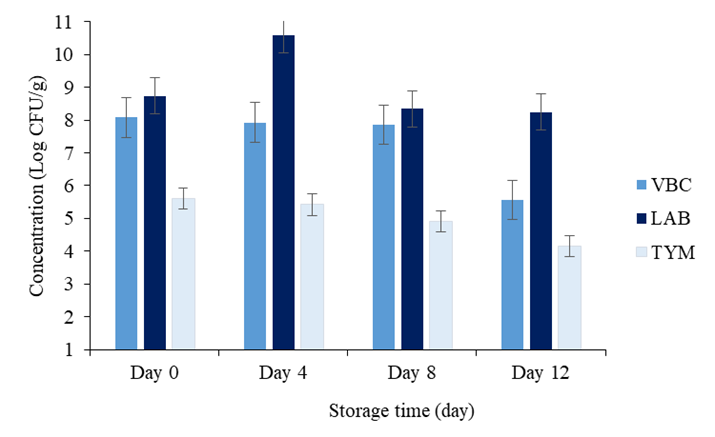
Microbiological trends of Nem during storage time: The microflora of sour naturally fermented fish consisted of various species of microorganisms such as aerobic, anaerobic, facultative anaerobic bacteria, yeast and mold. Figure 6 shows the microbial contents changes such as VBC, LAB, TYM of Nem during storing period of 12 days in the refrigerated conditions.
From day 0 to day 12, the VBC was decreased slowly from 8.08 Log CFU/g to 5.56 Log CFU/g (Figure 6). This could be caused by lack of nutrients, log phase of LAB growth curve, pH increases during storage time [58], declination of total acidity, lower of water activity, temperature below the tolerance and or other injurious conditions. The microscale of microorganism numbers of storage time are possibly due to the number of microorganisms in fresh fish, quality of salt used, fermentation period, storage temperature sanitation and hygiene during processing [22]. These result was in an agreement of author Gassem which reported that the microbial load range from 5.51-8.14 Log CFU/g for the total aerobic bacterial count. While many others author reported lower range than this study [60,61]. In contrast, when VBC of less than 6 Log CFU/g is usually associated with a mixed flora, like aerobic, facultative and anaerobic bacteria present in the product [62]. As the duration of storage increases the aerobic colony count also increases; this will also occur if refrigeration temperatures are poorly controlled or if the food is frequently taken in and out of refrigeration prior to analysis.
The graph of lactic acid bacteria was statically increased from 8.74 Log CFU/g to 8.25 Log CFU/g in line from day 0 to day 12 as shown in figure 6. It might be due to the fermentation mechanisms activity and the decreasing of pH during storage time. Organic acid profiles revealed an increasing trend of lactic acid in the maturation stage. These metabolites are important contributors to the flavor, aroma, and texture developments of fermented food products [10,63]. In addition, some of these metabolites can serve as antimicrobial agent [10]. And theses result are similarity in the agreement to the other author [54,62,65]. Corresponded to the microbial counts, lactic acid could be further metabolized by LAB. Oxidative actions of LAB, related to NADH oxidase, on lactate, sugar, or related compounds were associated with hydrogen peroxide production [66]. Lastly, the cell density of LAB is 9 ± 1.3 Log CFU/g by at the end of day 12 of study period, which is quite high and lowers the pH of the final product to 4.64–4.77, which was required to keep the product at the acceptable microbiological safety.
According to figure 6, the load of TYM was drastically declined from 5.61 Log CFU/g to 4.15 Log CFU/g in all storage time condition from early to the end of storage time. The unfavorable conditions for bacterial growth (high salt content, a low pH or aw) may result in higher yeasts and mold numbers. As recommended by the European Food Safety Authority (EFSA), the accepted limit for TYM in foods is <106 CFU/g. Considering that, it is likely that some interactions happened between bacteria and yeasts that coexist in fermented fish product. The data pointed out that LAB, VBC, and TYM showed a high positive correlation, so it could be speculated that there was not an inhibitory or competitive exclusion between bacteria and yeasts in fermented fish product’s ecosystem.
further processing techniques is recommended to study to reduce the water activity of Nem to this preferred value of aw.Conclusions
In conclusion, by refocusing on the formulation process using high-quality raw materials and ingredients, as well as adhering to hygienic and proper processing techniques at every step, the new Nem product achieved superior sensory scores compared to existing Nem products in the market. This study also defined the nutritional components, physicochemical properties, and microbiological aspects that serve as quality indicators for Nem. Furthermore, a shelf-life analysis revealed that after 12 days of storage at 4°C, the physicochemical, nutritional, and microbiological characteristics remained in optimal condition, making it suitable for consumers. Overall, this paper provides valuable data for local producers to enhance the quality of their fishery products, gain insights into processing techniques and on-site hygiene practices, and facilitate the inclusion of this valuable fermented fish product in general trade supplies. Additionally, it serves as a reference source to support Small and Medium Enterprises in the fermented fish business and enlighten low-income families about its potential.
Author contributions
Liseany Chor: Data curation; investigation and experiment; data interpretation, writing.
Sengly Sroy: Methodology, project administration, supervision, writing-review, and editing.
Chanthol Peng: Concept design; methodology; analysis; writing-review and editing; supervision
Seyha Doeurn: Project coordination and administration; writing-review and editing.
Acknowledgements
This work was supported by Cambodia Higher Education Improvement Project (Credit No. 6221-KH).
Conflict of interest
The authors declare that there are no conflicts of interest in this article.
Ethical statement
This study involved no humans or animals.
References
- Suzuki A. Rising Importance of Aquaculture in Asia: Current Status, Issues, and Recommendations. Adb.Org 12 (2021): 1-43.
- Vilain C, Baran E, Nutritional and Health Value of Fish: the Case of Cambodia 7 (2016): 2.
- Ly SD. Mayrhofer J, Schmidt U, et al. Characteristics of Cambodian Fermented Foods. Food 9 (2020): 1-19.
- LeGrand K, LeGrand K, LeGrand K, et al. Tradition and fermentation science of prohok, an ethnic fermented fish product of Cambodia. J. Ethn. Foods 7 (2020): 1-19.
- Ly D, Mayrhofer S, Schmidt JM, et al. Biogenic amine contents and microbial characteristics of Cambodian fermented foods. Foods 9 (2020): 1-19.
- Anh N. Health-promoting microbes in traditional Vietnamese fermented foods: A review. Food Sci. Hum. Wellness 4 (2015): 147-161.
- Riebroy S, Benjakul S, Visessanguan W, et al. Physical properties and microstructure of commercial Som-fug, a fermented fish sausage. Eur. Food Res. Technol 220 (2005): 520-525.
- Sangjindavong M, Chuapoehuk P, Raksakulthai N. Quality characteristics of fermented sour fish cake (Nham-Pla). Int. J. Food Prop 3 (2000): 399-406.
- Sangjindavong M, Chuapoehuk P, Vareevanich D. Studies on Nham-Pla’s processing by using rock salt and solar salt. Kasetsart Journal Nat. Sci 39 (2005): 294-299.
- Visessanguan W, Benjakul S, Smitinont T, et al. Changes in microbiological, biochemical and physico-chemical properties of Nham inoculated with different inoculum levels of Lactobacillus curvatus. LWT - Food Sci. Technol 39 (2006): 814-826.
- Rittenschober D, Nowak V, Charrondiere UR. Review of availability of food composition data for fish and shellfish. Food Chem 141 (2013): 4303-4310.
- Wichchukit S, O’Mahony M. The 9-point hedonic scale and hedonic ranking in food science: Some reappraisals and alternatives. J. Sci. Food Agric 95 (2015): 2167-2178.
- Sharif MK, Sharif HR, Nasir M. Sensory evaluation and consumer acceptability. Handb. Food Sci. Technol 10 (2017): 361-386.
- Tsighe N, Wawire M, Bereket A, et al. Physicochemical and microbiological characteristics of fresh Indian mackerel, spotted sardine and yellowtail scad, from Eritrea Red Sea waters. J. Food Compos. Anal. 70 (2018): 98-104.
- Manual of Methods of Analysis of Foods Food Safety and Standards Authority of India Ministry of Health and Family Welfare Government of India New Delhi (2012).
- Asociation Of Official Analytical Chemists- 75 Years Report. Anal. Sci. 1915-1990 73 (1990): 1-192.
- Sroy S, Arnaud E, Servent A, et al. Nutritional benefits and heavy metal contents of freshwater fish species from Tonle Sap Lake with SAIN and LIM nutritional score. J. Food Compos. Anal 96 (2020): 103731.
- Bich TTN, Tri DQ, Yi-Ching C, et al Productivity and economic viability of snakehead Channa striata culture using an aquaponics approach. Aquac. Eng 89 (2020): 89.
- Joffre OM, Freed S, Bernhardt J, et al. Assessing the Potential for Sustainable Aquaculture Development in Cambodia. Front. Sustain. Food Syst 5 (2021): 1-17.
- Anter esmail D, Hassan M, Elbahy E. Bacterial evaluation of the quality of farm ED FISH of Kafr El- Sheikh city in EGYPT. Benha Vet. Med. J 41 (2022): 16-21.
- Gerhard A. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis 21 (2006): 404-412.
- Ruíz-Osorio YL, Amorocho-Cruz CM, Gutiérrez-Guzmán N. Physicochemical and microbiological changes in gutted and ungutted red tilapia (Oreochromis ssp) stored in ice. Cienc. e Investig. Agrar 42 (2015): 263-272.
- Kopermsub P, Yunchalard S. Safety control indices for plaa-som, a Thai fermented fish product. African J. Microbiol. Res 2 (2008): 18-25.
- Malik IA, Elgasim EA, Adiamo OQ, et al. Effect of frozen storage on the biochemical composition of five commercial freshwater fish species from River Nile, Sudan. Food Sci. Nutr 9 (2021): 3758-3767.
- Visessanguan W, Benjakul W, Riebroy S, et al. Changes in composition and functional properties of proteins and their contributions to Nham characteristics. Meat Sci 66 (2004): 579-588.
- Nazzaro F, Fratianni F, De Martino L, et al. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 6 (2013): 1451-1474.
- Cabello-Olmo M. Influence of storage temperature and packaging on bacteria and yeast viability in a plant-based fermented food. Foods 9 (2020): 56.
- Desta DT. Influence of fermentation time on proximate composition and microbial loads of Enset, (Ensete ventricosum), sampled from two different agroecological districts. Food Sci. Nutr 9 (2021): 5641-5647.
- Da Silva VM, Silva LA, De Andrade JB, et al. Determination of moisture content and water activity in algae and fish by thermoanalytical techniques. Quim. Nova 31 (2008): 901-905.
- Tapía MS, Alzamora SM, Chirife J. Effects of Water Activity (aw) on Microbial Stability as a Hurdle in Food Preservation. Water Act. Foods Fundam. Appl 11 (2020): 323-355.
- El Hosry L, Sok N, Richa R, et al. Sample Preparation and Analytical Techniques in the Determination of Trace Elements in Food: A Review. Foods 12 (2023): 631.
- Bogard JR. Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. J. Food Compos. Anal 42 (2015): 120-133.
- Das G. Galangal, the multipotent super spices: A comprehensive review. Trends Food Sci. Technol 101 (2020): 50-62.
- Patrick Saoud I, Batal M, Ghanawi J, et al. Seasonal evaluation of nutritional benefits of two fish species in the eastern Mediterranean Sea. Int. J. Food Sci. Technol 43 (2008): 538-542.
- Zugarramurdi A. Seasonal Variation in Condition Factor, Gonadosomatic Index and Processing Yield of Carp (Cyprinus carpio). J. Aquat. Food Prod. Technol 12 (2003): 33-45.
- Skare M, Chan SS, Handeland SO, et al. A comparative study on quality, shelf life and sensory attributes of Atlantic salmon slaughtered on board slaughter vessels against traditional land-based facilities. Aquaculture 540 (2020): 20-29.
- Ozogul Y, Duysak O, Ozogul F, et al. Seasonal effects in the nutritional quality of the body structural tissue of cephalopods. Food Chem 108 (2008): 847-852.
- Wang S. Microplastics in wild freshwater fish of different feeding habits from Beijiang and Pearl River Delta regions, south China. Chemosphere 258 (2020): 127345.
- Petenuci ME. Fatty acid composition and nutritional profiles of Brycon spp. from central Amazonia by different methods of quantification. J. Food Sci. Technol 56 (2019): 1551-1558.
- Felix AE, Francis AK. Effect of Traditional Fermentation Process on the Nutrient and Anti-nutrient Content of Maize and African Locust Beans. J. Food Sci. Nutr. Res 2 (2019): 65-75.
- Steffens W, Anglers G, Berlin A. Freshwater fish - wholesome foodstuffs. Bulg. J. Agric. Sci 12 (2006): 67-68.
- Senanayake D, Torley PJ, Chandrapala J, et al. Microbial Fermentation for Improving the Sensory, Nutritional and Functional Attributes of Legumes. Fermentation 9 (2023): 238.
- Yani AV, Idealistuti D, Savitri N, et al. Analysis of Protein Levels of Fermented Sarden Fish Pempek. Indones. J. Agric. Res 5 (2023): 172-178.
- Khongla C, Musika S, Sangsawad P. Changes in antioxidant activity of fermented fish sauce (nam pla-ra) from osteochilus hasseltii during fermentation. Suranaree J. Sci. Technol 27 (2020): 1-8.
- Omemu AM, Obadina OA, Taiwo GJ, et al. Microbiological Assessment and Prevalence of Food Borne Pathogens in Street Vended Wara - Nigerian White Cheese. Am. J. Food Nutr 2 (2014): 59-62.
- Nguyen TTH, Adhikari A, Bhattacharya D, et al. Microbial Food Safety Risks Associated with Fresh and Thawed Catfish Fillets during Refrigerated Storage. Food Nutr. Sci 9 (2018): 1261-1272.
- Agency HP Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods Placed on the Market. Heal. Prot. Agency. London 11 (2009): 37.
- Koesoemawardani D, Afifah LU, Herdiana N, et al. Microbiological, physical and chemical properties of joruk (Fermented fish product) with different levels of salt concentration. Biodiversitas 22 (2021): 132-136.
- An Y. Quality Improvement of Zhayu, a Fermented Fish Product in China: Effects of Inoculated Fermentation with Three Kinds of Lactic Acid Bacteria. Foods 11 (2022): 18.
- Zhou Q, Li P, Fang S, et al. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass (Lateolabrax maculatus) during superchilling (-0.9 °C) storage. Coatings 9 (2019): 8.
- Narzary Y, Das S, Goyal AK, et al. Fermented fish products in South and Southeast Asian cuisine: indigenous technology processes, nutrient composition, and cultural significance. J. Ethn. Foods 8 (2021): 192.
- Yuliana N, Koesoemawardani D, Kurniati Y. Lactic acid bacteria during fish fermentation (rusip). MOJ Food Process. Technol 6 (2018): 211-216.
- Ringø E, Hoseinifar SH, Ghosh K, et al. Lactic acid bacteria in finfish-An update. Front. Microbiol 9 (2018): 1-37.
- Deesanam N, Chomsri N, Dechthummarong C, et al. Effect of fermentation temperatures on quality of naem made from raw materials treated with plasma. Int. J. Plasma Environ. Sci. Technol 12 (2019): 59-63.
- Zhang Q. Changes of Physicochemical Characteristics and Flavor during Suanyu Fermentation with Lactiplantibacillus plantarum and Saccharomyces cerevisiae. Foods 11 (2022): 24.
- Lee YC, Kung HF, Huang CY, et al. Reduction of histamine and biogenic amines during salted fish fermentation by Bacillus polymyxa as a starter culture. J. Food Drug Anal 24 (2016): 157-163.
- Bozoglu FT, Erkmen O. Food Preservation by Reducing Water Activity. Food Microbiology: Principles into Practice. Food Microbiol. Princ. into Pract 11 (2016): 44-58.
- Pahalagedara ASNW, Gkogka E, Ravn LW. The growth potential and thermal resistance of bacterial spores under conditions relevant for ambient acid dairy-based products. Food Control 152 (2023): 630.
- Gassem MA. Microbiological and chemical quality of a traditional salted-fermented fish (Hout-Kasef) product of Jazan Region, Saudi Arabia. Saudi J. Biol. Sci 26 (2019): 137-140.
- Petrus H, Purnomo E, Suprayitno. Physicochemical characteristics, sensory acceptability and microbial quality of Wadi betok a traditional fermented fish from South Kalimantan, Indonesia. Int. Food Res. J 20 (2013): 933-939.
- Tamang JP. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf 19 (2020): 184-217.
- Um MN, Lee CH. Isolation and identification of Staphylococcus sp. from Korean fermented fish products. Journal of Microbiology and Biotechnology 6 (1996): 340-346.
- Fox CJ, Folkvord A, Geffen J. Otolith micro-increment formation in herring Clupea harengus larvae in relation to growth rate. Mar. Ecol. Prog. Ser 264 (2003): 83-94.
- Jaworska D, Neffe K, Kolozyn-Krajewska D, et al. Survival during storage and sensory effect of potential probiotic lactic acid bacteria Lactobacillus acidophilus Bauer and Lactobacillus casei Bif3′/IV in dry fermented pork loins. Int. J. Food Sci. Technol 46 (2011): 2491-2497.
- Muzaddadi A, Mahanta P. Extension of shelflife of the fermented fish product, shidal by packaging in glass bottle and low temperature storage. Indian J. Fish 60 (2013): 135-143.
- Villegas E, Gilliland SE. Hydrogen peroxide production by Lactobacillus delbrueckii subsp. lactis I at 5°C. J. Food Sci 63 (1998): 1070-1074.


 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 