Shared Inflammatory Pathways in Psoriasis and Atherosclerosis: Bacterial Contributions and Therapeutic Implications
Felicia Hung and Reena Lamichhane-Khadka*
Department of Biomedical Education, California Health Sciences University College of Osteopathic Medicine, Clovis, California, USA
*Corresponding author: Reena Lamichhane-Khadka, Department of Biomedical Education, California Health Sciences University College of Osteopathic Medicine, Clovis, California, USA.
Received: 22 January 2026; Accepted: 27 January 2026; Published: 07 February 2026
Article Information
Citation: Felicia Hung and Reena Lamichhane- Khadka. Shared Inflammatory Pathways in Psoriasis and Atherosclerosis: Bacterial Contributions and Therapeutic Implications. Archives of Microbiology and Immunology. 9 (2026): 01-08.
View / Download Pdf Share at FacebookAbstract
Psoriasis and atherosclerosis are chronic inflammatory conditions linked by overlapping immunological pathways. Psoriasis, an autoimmune skin disorder characterized by keratinocyte hyperproliferation and plaque formation, is associated with an increased risk of cardiovascular diseases, including atherosclerosis. Epidemiologic studies demonstrate that this increased risk persists even after adjustment for traditional cardiometabolic risk factors, highlighting psoriasis as an independent contributor to cardiovascular disease. Atherosclerosis, a vascular disease marked by lipid accumulation and immune cell infiltration, can lead to severe cardiovascular events such as myocardial infarction and stroke. The immune mechanisms driving both conditions overlap, with psoriasis contributing to endothelial dysfunction and atherogenesis through the activation of Th1 and Th17 cells, which produce proinflammatory cytokines that exacerbate vascular inflammation. Key shared mediators include tumor necrosis factor-α (TNF-α), interleukin-17A (IL-17A), interleukin-23 (IL-23), and interferon-γ (IFN-γ), which promote macrophage activation, foam cell formation, and plaque progression. In this review, we examine the contributions of the bacteria Chlamydia pneumoniae, Helicobacter pylori, and Porphyromonas gingivalis to the formation of atherosclerotic plaques by promoting chronic inflammation. These pathogens induce endothelial dysfunction, oxidative stress, and cytokine release through innate immune signaling pathways that overlap with those activated in psoriasis. We also explore the shared immunological and molecular pathways of psoriasis and atherosclerosis and the potential role of psoriasis treatments in mitigating the progression of atherosclerosis by targeting these specific bacteria. Specifically, biologic therapies targeting TNF-α and IL-17A, commonly used in the management of psoriasis, may also offer therapeutic benefits in reducing atherosclerotic risk by modulating bacteria-induced vascular inflammation, supporting further investigation into their cardiovascular effects.
Keywords
Atherosclerosis, psoriasis, immune, bacteria, inflammatory response
Article Details
Introduction
Psoriasis and atherosclerosis are two distinct yet interconnected chronic inflammatory conditions with multifactorial etiologies, characterized by systemic and localized manifestations. Psoriasis is a common, chronic autoimmune skin disorder affecting approximately 2-4% of the adult population in the United States (Mehta et al. 2010; Armstrong et al. 2021). Globally, psoriasis prevalence among adults is highest in Australasia (1.99%), Western Europe (1.92%), Central Europe (1.83%;), and Canada and the United States (1.5%) (Parisi et al, 2020; Zhang et al, 2022). Psoriasis is characterized by sustained inflammation leading to hyperproliferation of keratinocytes and subsequent plaque formation (Rendon et al, 2019). These plaques are commonly found on the elbows, knees, scalp, and lower back (Guo et al, 2023). Clinical types of psoriasis include psoriasis vulgaris, guttate psoriasis, inverse psoriasis, pustular psoriasis, and erythrodermic psoriasis, with psoriasis vulgaris accounting for approximately 90% of cases (Rendon et al, 2019).
Atherosclerosis is a chronic inflammatory vascular disease marked by the accumulation of lipids, immune cells, and fibrous matrix proliferation, culminating in arterial stenosis and occlusion (Zhu et al, 2018). Atherosclerosis itself is not fatal; however, thrombosis and rupture of atherosclerotic plaque can lead to acute coronary syndromes and stroke (Falk, 2006). The global prevalence of atherosclerotic cardiovascular diseases is estimated to be around 523 million people (Nedkoff et al, 2023).
Studies suggest that patients with psoriasis have a higher incidence and increased risk of cardiovascular disease compared to controls, including atherosclerosis (Wu et al, 2022). Psoriasis is linked to elevated risk of coronary artery disease and myocardial ischemia in both the European and East Asian populations, and a common genetic link for psoriasis and cardiovascular disease has been suggested (Zhang et al., 2022). In patients with severe psoriasis, the risk of myocardial ischemia is increased by threefold, the risk of stroke by 60%, and the risk of cardiovascular-related deaths by 40% (Piaserico et al, 2022). Genetic studies have demonstrated that IL-23 and TNF-α, both released in the pathogenesis of psoriasis, are associated with coronary artery disease (Piaserico et al, 2022). TNF-α has been recognized as one potent cytokine in causing atherosclerosis as it can cause reactive oxygen species formation and nitric oxide production in blood vessels, resulting in endothelial dysfunction and the first step in atherosclerosis (Kaptoge et al, 2014, Bai et al, 2017).
Recent research on how pathogenic bacterial species not normally found in microbiota may contribute or are associated with the formation of atherosclerotic plaques (Calandrini et al, 2014; Ziganshina et al, 2016; Karabulut, 2020). Analysis of the transcriptome of biopsies done on psoriatic skin lesions and atherosclerotic plaque shows that TNF-α and IFN- γ were upregulated, establishing a common pathogenic pathway (Reali et al, 2024). These bacterial species induce a particular inflammatory response that helps with the progression of atherosclerosis. This review aims to examine these pathways and determine whether the psoriasis medications target similar pathways and identify the potential benefits of psoriasis treatment in the prevention of atherosclerosis.
Methods
To find literature, a search was conducted in PubMed, Google Scholar, and Cochrane using a combination of the terms “atherosclerosis,” “psoriasis,” “immune,” and “bacteria” to identify relevant studies. Additional articles were identified through the bibliographies of selected papers. Peer-reviewed articles published from inception to October 1st, 2025, written in English, were included.
Discussion
Immunologic Pathophysiology of Psoriasis and Atherosclerosis
While the pathogenesis of psoriasis is not fully understood, it is suggested that excessive activation of the adaptive immune system plays a role (Nestle et al, 2009). In the initial steps of psoriasis pathogenesis, plasmacytoid dendritic cells, keratinocytes, natural killer T cells, and macrophages secrete the cytokines TNF-α, IFN-γ, IFN-α, and IL-1β which activate myeloid dendritic cells (Eder and Gladman, 2015; Armstrong and Read, 2023). These activated myeloid dendritic cells secrete two key cytokines: IL-12, which induces differentiation of naive T cells to TH1 cells, and IL-23, which is key in the survival and proliferation of TH17 and TH22 cells. (Eder and Gladman, 2015). In turn, the TH1 cells secrete TNF-α and IFN- γ, the TH17 cells secrete IL- 17A, IL-17F, IL-22, and the TH22 cells secrete IL-22 - all of which activate keratinocytes (Alwan and Nestle, 2015). Activated keratinocytes induce the production of antimicrobial peptides (LL-37 cathelicidin and β-defensins), proinflammatory cytokines (TNF-α, IL-1β, IL-6), chemokines (CXCL8, CXCL9, CXCL10, CXCL11, CCL20), and S100 proteins which feed back into the proinflammatory cycle, further activating the immune system (Nestle et al, 2009). The pathogenic process of atherosclerosis begins with accumulation of low-density lipoprotein (LDL) accumulates in the subendothelial space of vessels which undergo subsequent oxidation (Wolf and Ley, 2019). These LDLs are taken up by macrophages and trigger inflammation of the arterial wall by binding to TLRs (toll-like receptors) (Curtiss and Tobias, 2009). The macrophages, now foam cells and full of cholesterol, now activate the inflammasome, composed of nucleotide binding domain and leucine-rich repeat gene family that generates IL-1β (Duewell et al, 2010). IL-1β can enhance the expression of many pro- inflammatory cytokines, the macrophages form the lipid core of a plaque, and a fibrous cap covers the growing lipid core (Wolf and Ley, 2019).
Currently Known Connection between Psoriasis and Atherosclerosis
Helper T cell type I chronic inflammation in psoriasis plays a pivotal role in the pathophysiology of insulin resistance, atherosclerosis, and plaque rupture, leading to thrombotic events (Mehta et al, 2010). Psoriasis patients exhibit a higher prevalence of traditional cardiovascular risk factors, such as diabetes mellitus, hypertension, metabolic dyslipidemia, tobacco use, and obesity (Mehta et al, 2010). However, even after adjusting for these risk factors, psoriasis remains independently associated with an increased risk of myocardial infarction, coronary artery disease, stroke, diabetes, endothelial cell dysfunction, and atherosclerosis (Gelfand et al, 2006; Ludwig et al, 2007; Balci et al, 2009; Prodanovich et al, 2009). Psoriasis, characterized as a prototypical Th1 and Th17 inflammatory disease, involves Th1 cell-secreted factors that significantly contribute to the pathogenesis of atherosclerosis and myocardial infarction (O’Malley et al, 2001). Further supporting this connection, a study identified endothelial dysfunction as an early hallmark of atherosclerosis (Balci et al, 2009). Studies also indicate that psoriasis patients have increased intima-media thickness of the carotid arteries and impaired endothelial function compared to healthy controls, highlighting the heightened cardiovascular risk associated with psoriasis (Ramirez-Teran et al, 2022).
The relationship between psoriasis and cardiometabolic risk has also been demonstrated through systemic inflammatory biomarkers such as C-reactive protein and GlycA, a glycan biomarker of N-acetyl side chains of acute-phase proteins (GlycA) and C-reactive protein (Connelly et al, 2017). GlycA is correlated with body mass index, insulin resistance, markers of metabolic syndrome and has been associated with the presence of coronary artery disease, making it a reliable biomarker of cardiometabolic risk (McGarrah et al, 2016). Psoriasis patients have been shown to also have increased levels of GlycA, with initiating anti-TNF-α therapy reducing psoriasis severity, GlycA levels, and vascular inflammation (Joshi et al, 2016). In the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative and the Stockholm Psoriasis Cohort, patients with higher psoriasis severity scores were associated with elevated GlycA levels, and both measures correlated with coronary atherosclerosis and future cardiovascular events (Svedbom et al, 2025). Metabolic syndrome provides an important mechanistic bridge between psoriasis and heightened cardiovascular risk because it reflects the combined impact of central obesity, hypertension, dyslipidemia, and impaired glucose regulation, factors that are highly prevalent among individuals with psoriasis (Neimann et al, 2006; Love et al, 2011). Among the patients in the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative, one third met criteria for metabolic syndrome and demonstrated substantially greater systemic inflammation, insulin resistance, and an adverse lipoprotein profile compared with those without metabolic syndrome (Teklu et al, 2021). These patients also had markedly higher visceral adipose tissue volumes, a metabolically active fat depot that releases inflammatory mediators linked to endothelial dysfunction and atherosclerotic progression. Hypertension, another common component of metabolic syndrome in psoriasis, remained independently associated with noncalcified coronary burden even after adjustment for Framingham risk score, lipid-lowering therapy, and biologic use (Teklu et al, 2021). Together, these findings demonstrate that cardiometabolic abnormalities such as central obesity and elevated blood pressure compound the inflammatory burden of psoriasis and contribute to early coronary artery disease.
Bacteria in Atherosclerosis
Given the inflammatory nature of psoriasis and atherosclerosis, identifying specific aspects of the shared inflammatory pathways is key to understanding both. One way is to look at the bacteria in atherosclerotic plaques, which promote a pro-inflammatory state. The gut microbiota, consisting of Actinomycetota, Pseudomonadota, Bacillota, and Bacteroidota species, is increasingly recognized as an important modifier of cardiometabolic health, and dysbiosis contributes directly to the initiation and progression of atherosclerosis (Lima et al, 2025). Microbial metabolism of dietary fiber produces trimethylamine N-oxide (TMAO) which can modulate cholesterol and bile acid metabolisms, leading to lipid accumulation in macrophages, vascular inflammation, and subsequent development of atherosclerosis (Ghazalpour et al, 2016; Zhang et al, 2022; Shanmugham et al, 2023; Alexandrescu et al, 2024; Lima et al, 2025). In addition to TMAO, alterations in gut microbial composition reduce production of short chain fatty acids such as acetate, propionate, and butyrate which normally support epithelial barrier integrity and exert anti-inflammatory effects (Lima et al, 2025). Reduced short chain fatty acid production weakens tight junctions and allows bacterial components such as lipopolysaccharide to enter the circulation, where they activate Toll-like receptors and amplify systemic and vascular inflammation (El Hage et al, 2023; Lima et al, 2025). Dysbiosis also disrupts bile acid metabolism and impairs activation of receptors such as FXR and TGR5 that regulate lipid handling, glucose homeostasis, vascular tone, and cholesterol efflux (Porez et al, 2012; Xu et al, 2016; Salazar et al, 2023). Collectively, these pathways illustrate how gut microbiota influence endothelial function, lipid metabolism, immune activation, and plaque formation, and they highlight the gut microbiome as an important contributor to atherosclerotic disease. Aside from bacteria associated with microbiota, current research on bacterial species associated with atherosclerotic plaque formation identified Chlamydia pneumoniae, Helicobacter pylori, and Porphyromonas gingivalis as the three most common non-microbiota species (Pothineni et al, 2017; Morre et al, 2000; Karbulut et al, 2020; Munusamy and Shanmugham, 2022). The common non-commensal bacterial species with their associated immune mediators and inflammatory mechanisms are summarized in Table 1.
Table 1: Non-commensal bacteria and associated immune mediators in atherosclerosis
|
Bacteria |
Immune Factors and Mechanisms Promoting Atherosclerotic Lesion Formation |
References |
|
Chlamydia pneumoniae |
IL-1α, IL-6, CCl2, IL-2: Early lesion progression, enhanced immune cell recruitment and Th1 polarization. |
Morre et al., 2000; Di Pietro et al., 2013; Pothineni et al., 2017; Karabulut, 2020 |
|
TNF-a, metalloproteases (MMPs): Plaque instability |
||
|
and rupture, extracellular matrix degradtion. |
||
|
IL17A: Enhancement of macrophage uptake of oxidized LDL and foam cell formation. |
||
|
Helicobacter pylori |
IL-1, IL-6, TNF-α: Induction of oxidative stress and endothelial dysfunction, increased endothelial adhesion molecule expression. |
Pothineni et al., 2017; Karabulut, 2020; Munusamy and Shanmugham, 2022; Aramouni et al., 2023 |
|
Porphyromonas gingivalis |
IL-1β, IL-18: Inflammasome activation, induction of endothelial oxidative stress. |
Pothineni et al., 2017; Karabulut, 2020; Zhang et al., 2021; Munusamy and Shanmugham, 2022 |
|
IL-1β, IL-6, TNF-α, and IFN-γ: Sustained plaque growth |
Chlamydia pneumoniae has been associated with multiple stages of atherogenesis through the induction of proinflammatory mediators (Table 1). In the early stages of lesion progression, the pathogen stimulates the production of IL-1α, IL-6, CCL2, and IL-12, which contribute to immune cell recruitment and the differentiation of naïve T cells into Th1 cells, thereby sustaining chronic vascular inflammation (Di Pietro et al, 2013). As plaques develop, C. pneumoniae promotes instability through upregulation of TNF-α and matrix metalloproteinases (MMPs). These factors contribute to vascular smooth muscle cell apoptosis and degradation of extracellular matrix components, weakening the fibrous cap and increasing the risk of plaque rupture (Di Pietro et al, 2013). Additionally, the infection enhances foam cell formation via induction of IL-17A, which promotes macrophage uptake of oxidized LDL and increases expression of scavenger receptors, contributing to lipid accumulation within the arterial wall (Di Pietro et al, 2013).
Helicobacter pylori has been shown to contribute to atherogenesis primarily through the induction of oxidative stress and vascular endothelial injury (Table 1). The infection triggers the release of proinflammatory cytokines, including IL-1, IL-6, and TNF-α, which disrupt endothelial function and promote a proatherogenic state (Aramouni et al, 2023). These mediators increase the expression of adhesion molecules and enhance leukocyte recruitment to the vascular wall, facilitating chronic inflammation. In addition, the oxidative stress generated by H. pylori infection contributes to lipid peroxidation and endothelial dysfunction, both of which are central to early atherosclerotic lesion development (Aramouni et al, 2023).
Porphyromonas gingivalis, a key periodontal pathogen, has been associated with atherosclerosis through its ability to trigger endothelial oxidative stress and amplify inflammatory signaling (Table 1). It activates the TLR-NFκB pathway, leading to the upregulation of IL-1β and IL-18, which contribute to endothelial dysfunction and promote a local proinflammatory environment (Zhang et al, 2021). Infected endothelial and immune cells also produce elevated levels of IL-6, TNF-α, and IFN-γ, which further sustain vascular inflammation and encourage plaque development. These cytokines enhance leukocyte adhesion, vascular permeability, and smooth muscle cell proliferation—factors that collectively facilitate the progression and expansion of atherosclerotic lesions (Zhang et al, 2021).
Aside from these specific bacteria, it is noted that lipopolysaccharide, a major component of gram-negative bacterial cell walls, may lead to release of TNF-α, IL-1β, and IL-6 which work to promote endothelial activation, oxidative stress, and vascular dysfunction, all of which accelerate atherosclerotic plaque formation (Arya et al, 2025). Lipopolysaccharide also stimulates monocytes and macrophages to upregulate enzymes such as neuraminidase 1 and adipose differentiation related protein, which increase lipid deposition and enhance chemokine release within the vascular wall (Triantafilou et al, 2007; Arya et al, 2025; Jiang et al, 2025). In addition, lipopolysaccharide promotes generation of reactive oxygen species through NADPH oxidase, which contributes to smooth muscle cell proliferation, endothelial injury, and plaque instability (Griendling et al, 2000; Loffredo et al, 2020; Arya et al, 2025).
Current Psoriasis Treatments and Potential Effects on Bacteria associated with Atherosclerosis
The current biologic treatments for psoriasis target specific parts of the psoriasis immune pathophysiology (Guo et al, 2023). Table 2 summarizes the current immunomodulatory therapies used in the treatment of psoriasis, categorized by their molecular targets. These agents primarily target key cytokines involved in the pathogenesis of psoriasis, many of which also contribute to the inflammatory mechanisms of atherosclerosis. Both conditions are driven by a dominant Th1 and Th17 immune response characterized by increased production of IL-17A, IL-17F, IL-23, TNF-α, and IFN-γ, which promote sustained activation of keratinocytes in the skin and endothelial and smooth muscle cells within the arterial wall (Wang et al, 2022; Ponikowska et al, 2025). Importantly, therapeutic data reinforce the biologic overlap between these conditions. Targeting IL-17 and TNF-α pathways shows improvement in cutaneous inflammation seen with psoriasis and in vascular inflammation, carotid intima media thickness, pulse wave velocity, coronary inflammation, and noncalcified coronary plaque burden seem in atherosclerosis (Ji et al, 2024).
Table 2: Current immunomodulatory drugs used for psoriasis treatment and their targets.
|
Therapeutic Agent |
Molecular Target |
Primary Effect in Psoriasis |
Potential effect in Atherosclerosis |
References |
|
Etanercept, Infliximab, Adalimumab |
TNF-α |
Suppression of skin inflammation |
Enhancement of CIMT, PWV, CRP levels |
Joshi et al., 2016; Guo et al., 2023; Svedbom et al., 2025 |
|
Secukinumab, Ixekizumab, Brodalumab, Bimekizumab |
IL-17A, IL-17R |
Blocks IL-17-mediated keratinocyte activation |
Reduction of non-calcified plaque in CCTA; IL-17A crosstalk |
Merola et al., 2022; Guo et al., 2023; Ji et al., 2024 |
|
Ustekinumab (IL-12/23), Guselkumab, Tildrakizumab, Risankizumab (IL-23 p19) |
IL-12, IL-23 |
Reduction of Th1 and Th17 pathways |
Genetic linkage via IL-23R polymorphisms |
Piaserico et al., 2022; Guo et al., 2023 |
|
JAK inhibitors (Tofacitinib, Upadacitinib, Deucravacitinib) |
JAK/STAT pathway |
Broad suppression of cytokine signaling |
Not elucidated yet; potential benefit via IL signaling |
Guo et al., 2023 |
|
PDE4 inhibitors (Apremilast, Roflumilast) |
PDE4, cAMP |
Modulation of inflammation |
Direct link to atherosclerosis is not elucidated yet |
Guo et al., 2023 |
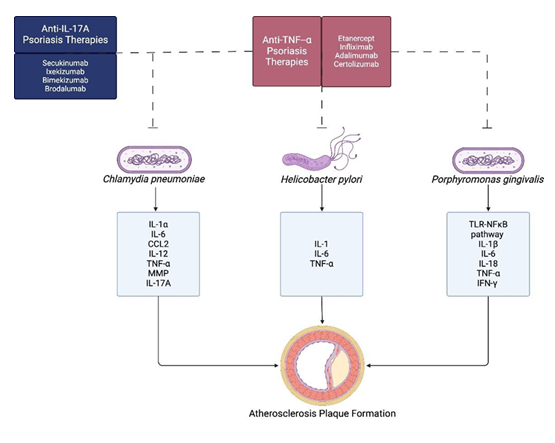
Considering that Chlamydia pneumoniae, Helicobacter pylori, and Porphyromonas gingivalis release cytokines implicated in the progression of atherosclerosis, biologic agents used in psoriasis may have the potential to influence this inflammatory pathway. A summary of these overlapping mechanisms is presented in Figure 1.
Figure 1: Overlap between biologic targets for psoriasis treatment and bacteria-induced cytokines in atherosclerosis. Created in BioRender. Hung, F. (2026) https://BioRender.com/sgqvaih
Drugs that inhibit TNF-α, such as etanercept, infliximab, and adalimumab disrupt a central proinflammatory pathway that is associated with both psoriasis and atherosclerotic plaque development (Guo et al, 2023). TNF-α contributes to atherosclerotic plaque formation by promoting endothelial dysfunction, leukocyte recruitment, and macrophage activation, thereby driving plaque development and instability. In psoriatic lesions, it stimulates keratinocyte proliferation and sustains inflammatory cell infiltration. Similarly, IL-17A inhibitors (secukinumab, ixekizumab, bimekizumab, and brodalumab) block a cytokine known to mediate both epidermal and vascular inflammation (Guo et al, 2023). IL-17A induces keratinocytes to secrete chemokines and antimicrobial peptides that amplify cutaneous inflammation, while in the arterial wall it enhances the uptake of oxidized LDL by the macrophages, promoting foam cell formation and atherogenesis. Consequently, inhibition of these cytokine pathways is likely to attenuate both psoriatic and vascular inflammatory processes. Targeting the IL-12/IL-23 axis, ustekinumab and the IL-23 p19 inhibitors guselkumab, tildrakizumab, and risankizumab downregulate Th1 and Th17 pathways, which are central to the pathogenesis of both psoriasis and atherosclerosis. Inhibition of IL-36 with spesolimab, as well as modulation of intracellular signaling via JAK/STAT inhibitors (tofacitinib, upadacitinib, deucravacitinib) and PDE4 inhibitors (apremilast, roflumilast), provides broader suppression of proinflammatory cytokine production (Guo et al, 2023). Therefore, although these agents are primarily used to treat psoriasis, they may also help attenuate atherosclerotic inflammation through their effects on common inflammatory pathways.
Conclusion
This study reviews the potential intersection between psoriasis treatment and atherosclerosis prevention through the targeting of atherosclerosis-associated bacteria. Current studies have shown that TNF-α inhibitors may improve subclinical atherosclerosis and cardiovascular events. However, this relationship is debated due to its association with weight gain (Mehta et al, 2010; Merola et al, 2022). Clinical trials on the IL-17A inhibitor secukinumab, IL-12/IL-23 inhibitor ustekinumab, demonstrate the possible reduction in aortic inflammation (Merola et al, 2022). In addition, there are limitations to using inflammatory markers in understanding the direct relationship between psoriasis and atherosclerosis (Svedbom et al, 2025). Further studies should focus on these specific psoriasis biologic therapies in the context of infections with Chlamydia pneumoniae, Helicobacter pylori, and Porphyromonas gingivalis. Additional research is required to elucidate the role of bacteria or their components within microbiota, their immunologic influence on atherosclerosis, and potential therapeutic approaches targeting these mechanisms.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
RLK and FH conceived and designed the study topic, wrote and reviewed the manuscript. FH created the figure using BioRender [Hung, F. (2026) https://BioRender.com/sgqvaih]. Both authors agree to be equally accountable for the content of the work.
Acknowledgements
The authors thank the Department of Biomedical Education, California Health Sciences University College of Osteopathic Medicine for their support. This work was presented and awarded second place for original research at the 12th Annual Osteopathic Physicians and Surgeons of California (OPSC) Poster Competition in Newport Beach, CA, on March 1, 2025.
References
- Alexandrescu L, Suceveanu AP, Stanigut AM, et al. Intestinal Insights: The gut microbiome's role in atherosclerotic disease: A narrative review. Microorg 12 (2024): 2341.
- Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol 33 (2015): S2-6.
- Aramouni K, Assaf RK, Azar M, et al. Infection with Helicobacter pylori may predispose to atherosclerosis: role of inflammation and thickening of intima-media of carotid arteries. Front Pharmacol 14 (2023): 1285754.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol 157 (2021): 940-946.
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 323 (2020): 1945-1960.
- Arya P, Sharma V, Singh P, et al. Bacterial endotoxin-lipopolysaccharide role in inflammatory diseases: An overview. Iran J Basic Med Sci 28 (2025): 553-564.
- Bai F, Zheng W, Dong Y, et al. Serum levels of adipokines and cytokines in psoriasis patients: a systematic review and meta-analysis. Oncotarget 9 (2017): 1266-1278.
- Balci DD, Balci A, Karazincir S, et al. Increased carotid artery intima-media thickness and impaired endothelial function in psoriasis. J Eur Acad Dermatol Venereol 23 (2009): 1-6.
- Calandrini CA, Ribeiro AC, Gonnelli AC, et al. Microbial composition of atherosclerotic plaques. Oral Dis 20 (2014): e128-e134.
- Connelly MA, Otvos JD, Shalaurova I, et al. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J Transl Med 15 (2017): 219.
- Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. J Lipid Res 50 (2009): S340-S345.
- Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464 (2010): 1357-1361.
- Eder L, Gladman DD. Atherosclerosis in psoriatic disease: latest evidence and clinical implications. Ther Adv Musculoskelet Dis 7 (2015): 187-195.
- El Hage R, Al-Arawe N, Hinterseher I. The Role of the Gut Microbiome and Trimethylamine Oxide in Atherosclerosis and Age-Related Disease. Int J Mol Sci 24 (2023): 2399.
- Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol 47 (2006): C7-C12.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA 296 (2006): 1735-1741.
- Ghazalpour A, Cespedes I, Bennett BJ, et al. Expanding role of gut microbiota in lipid metabolism. Curr Opin Lipidol 27 (2016): 141-147.
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86 (2000): 494-501.
- Guo J, Zhang H, Lin W, et al. Signaling pathways and targeted therapies for psoriasis. Signal Transduct Target Ther 8 (2023): 437.
- Ji L, Ravi S, Wright L, et al. Psoriasis treatments in the stabilization of atherosclerosis: a systematic review. Arch Dermatol Res 317 (2024): 159.
- Jiang D, Yang Y, Li D. Lipopolysaccharide induced vascular smooth muscle cells proliferation: A new potential therapeutic target for proliferative vascular diseases. Cell Prolif 50 (2017): e12332.
- Joshi AA, Lerman JB, Aberra TM, et al. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res 119 (2016): 1242-1253.
- Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 35 (2014): 578-589.
- Karabulut A. The role of microbiologic agents in the progression of the atherosclerosis: a comprehensive review. J Saudi Heart Assoc 32 (2020): 440-450.
- Lima T, Costa V, Nunes C, et al. From Dysbiosis to Cardiovascular Disease: The Impact of Gut Microbiota on Atherosclerosis and Emerging Therapies. Appl Sci 15 (2025): 7084.
- Loffredo L, Ivanov V, Ciobanu N, et al. Is there an association between atherosclerotic burden, oxidative stress, and gut-derived lipopolysaccharides? Antioxid Redox Signal 33 (2020): 8109.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: Results from the national health and nutrition examination survey, 2003-2006. Arch Dermatol 147 (2011): 419-424.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol 156 (2007): 271-276.
- McGarrah RW, Kelly JP, Craig DM, et al. A novel protein glycan-derived inflammation biomarker independently predicts cardiovascular disease and modifies the association of HDL subclasses with mortality. Clin Chem 63 (2017): 288-296.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J 31 (2010): 1000-1006.
- Merola JF, McInnes IB, Deodhar AA, et al. Effect of secukinumab on traditional cardiovascular risk factors and inflammatory biomarkers: post hoc analyses of pooled data across three indications. Rheumatol Ther 9 (2022): 935-955.
- Morré SA, Stooker W, Lagrand WK, et al. Microorganisms in the aetiology of atherosclerosis. J Clin Pathol 53 (2000): 647-654.a - A mini review. J Pure Appl Microbiol 16 (2022): 1-6.
- Nedkoff L, Briffa T, Zemedikun D, et al. Global trends in atherosclerotic cardiovascular disease. Clin Ther 45 (2023): 1087-1091.
- Neimann AL, Shin DB, Wang X, et al. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 55 (2006): 829-835.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 361 (2009): 496-509.
- O’Malley T, Ludlam CA, Riemersma RA, et al. Early increase in levels of soluble inter-cellular adhesion molecule-1 (sICAM-1); potential risk factor for the acute coronary syndromes. Eur Heart J 22 (2001): 1226-1234.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ 369 (2020): m1590.
- Piaserico S, Orlando G, Messina F. Psoriasis and cardiometabolic diseases: shared genetic and molecular pathways. Int J Mol Sci 23 (2022): 9063.
- Ponikowska M, Hill L, Lee CS, et al. Cardiovascular disease and psoriasis. Dermatol Ther (Heidelb) 15 (2025): 1566.
- Porez G, Prawitt J, Gross B, et al. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res 53 (2012): 1723-1737.
- Pothineni NVK, Subramany S, Kuriakose K, et al. Infections, atherosclerosis, and coronary heart disease. Eur Heart J 38 (2017): 3195-3201.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol 145 (2009): 700-703.
- Ramírez-Terán AL, Vega-Memije ME, Torres-Tamayo M, et al. Carotid intima-media thickness in patients with psoriasis with and without metabolic syndrome. Arch Cardiol Mex 92 (2022): 305-311.
- Reali E, Caliceti C, Lorenzini A, et al. The use of microbial modifying therapies to prevent psoriasis exacerbation and associated cardiovascular comorbidity. Inflamm 47 (2024): 13-29.
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci 20 (2019): 1475.
- Salazar J, Morillo V, Suárez MK, et al. Role of gut microbiome in atherosclerosis: Molecular and therapeutic aspects. Curr Cardiol Rev 19 (2023): e020223213408.
- Shanmugham M, Bellanger S, Leo CH. Gut-derived metabolite, trimethylamine-N-oxide (TMAO) in cardio-metabolic diseases: Detection, mechanism, and potential therapeutics. Pharmaceut (Basel) 16 (2023): 504.
- Svedbom A, Mallbris L, González-Cantero Á, et al. Skin inflammation, systemic inflammation, and cardiovascular disease in psoriasis. JAMA Dermatol 161 (2025): 81-86.
- Teklu M, Zhou W, Kapoor P, et al. Metabolic syndrome and its factors are associated with noncalcified coronary burden in psoriasis: An observational cohort study. J Am Acad Dermatol 84 (2021): 1329-1338.
- Triantafilou M, Gamper FG, Lepper PM, et al. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4 and trigger inflammatory responses in human vascular endothelial cells. Cell Microbiol 9 (2007): 2030-2039.
- Wang Y, Zang J, Liu C, et al. Interleukin-17 links inflammatory cross-talks between comorbid psoriasis and atherosclerosis. Front Immunol 13 (2022): 835671.
- Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res 124 (2019): 315-327.
- Wu JJ, Kavanaugh A, Lebwohl MG, et al. Psoriasis and metabolic syndrome: implications for the management and treatment of psoriasis. J Eur Acad Dermatol Venereol 36 (2022): 797-806.
- Xu Y, Li F, Zalzala M, et al. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology 64 (2016): 1072-1085.
- Zhang L, Wang Y, Qiu L, et al. Psoriasis and cardiovascular disease risk in European and East Asian populations: evidence from meta-analysis and Mendelian randomization analysis. BMC Med 20 (2022): 421.
- Zhang J, Xie M, Huang X, et al. The effects of Porphyromonas gingivalis on atherosclerosis-related cells. Front Immunol 12 (2021): 766560.
- Zhang Q, Zhang L, Chen C, et al. The gut microbiota-artery axis: A bridge between dietary lipids and atherosclerosis? Prog Lipid Res 89 (2023): 101209.
- Zhu Y, Xian X, Wang Z, et al. Research progress on the relationship between atherosclerosis and inflammation. Biomol 8 (2018): 80.
- Ziganshina EE, Sharifullina DM, Lozhkin AP, et al. Bacterial communities associated with atherosclerotic plaques from Russian individuals with atherosclerosis. PLoS One 11 (2016): e0164836.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
