A Phase I dose escalation trial using Intensity-Modulated Radiotherapy with simultaneous integrated boost in Pelvic Chemoradiotherapy for Metastatic Rectal Cancer
Thibaut Lizée1, Valérie Seegers2, Julien Blanchecotte1, Emmanuel Rio3, Olivier Capitain4, Véronique Guérin-Meyer4, Florence Legouté1, Damien Autret5, Marc-André Mahé6, Amaury Paumier1
1Radiation oncology department, Institut de Cancérologie de l’Ouest, Angers, France
2Clinical research department, Institut de Cancérologie de l’Ouest - site Paul Papin, Angers, France
3 Radiation oncology department, Institut de Cancérologie de l’Ouest, Saint-Herblain, France
4Medical oncology Department, Institut de Cancérologie de l’Ouest, Angers, France
5Radiation physics department, Institut de Cancérologie de l’Ouest, Angers, France
6Centre François Baclesse, Caen, France
*Corresponding Author: Amaury Paumier, Institut de Cancérologie de l’Ouest, 15 Rue André Bocquel, 49055 Angers, France.
Received: 15 June 2021; Accepted: 12 July 2021; Published: 09 August 2021
Article Information
Citation:
Thibaut Lizée, Valérie Seegers, Julien Blanchecotte, Emmanuel Rio, Olivier Capitain, Véronique Guérin-Meyer, Florence Legouté, Damien Autret, Marc-André Mahé, Amaury Paumier. A Phase I dose escalation trial using Intensity-Modulated Radiotherapy with simultaneous integrated boost in Pelvic Chemoradiotherapy for Metastatic Rectal Cancer. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 334-346.
View / Download Pdf Share at FacebookAbstract
Background: In unresectable metastatic rectal cancers, surgery of primary tumor remains highly debated. Pelvic Chemoradiotherapy (CRT) could allow sufficient local control in order to avoid major and sometimes mutilating surgery. Dose escalated CRT could increase local control. The aim of this study was to evaluate the feasibility and tolerance of a CRT with radiation dose escalation using intensity modulated radiotherapy (IMRT) with simultaneous integrated boost (SIB), in metastatic low and middle rectal cancers.
Methods: This multicenter phase I study included six patients treated for unresectable synchronous metastatic low and middle rectal adenocarcinoma in two dose levels. Radiotherapy was delivered using IMRT with SIB. The dose escalation was 52.5 Gy (level 1) and 56.25 Gy (level 2) in the gross tumoral volume (GTV), in 25 fractions of 2.1 Gy and 2.25 Gy, respectively. High-risk clinical target volume (CTV) and low-risk CTV received respectively 50 Gy and 45 Gy in 25 fractions in the two levels. Concomitant chemotherapy was oral capecitabine and CRT was performed after four cycles of mFOLOX6 chemotherapy. The dose-limiting toxicity (DLT) was defined as toxicity requiring the interruption of radiotherapy for more than five consecutive fractions.
Results: All six patients received the full course of treatment at scheduled doses. No patients had acute toxicity requiring interruption of radiotherapy, therefore no DLT has been reported. No patients had acute toxicity ≥3. Concerning late toxicity, three patients experienced grade 3. No local progression occurred.
Conclusions: Dose escalation at 56.25 Gy to the GTV was possible. This radiotherapy schedule needs to be evaluated in a larger study, in order to avoid mutilating surgery for metastatic patients. Tr
Keywords
<p>Rectal cancer; Dose escalated radiation therapy; Intensity-modulated radiotherapy; Simultaneous integrated boost; Pelvic chemoradiotherapy; Phase I trial</p>
Article Details
Abbreviations:
CRT- chemoradiotherapy; CT- computed tomography; CTV-HR- high-risk clinical target volume; CTV-LR- low risk clinical target volume; DLT- Dose-limiting toxicity; DPD- dihydropyrimidine dehydrogenase; IMRT- intensity-modulated radiation therapy; GTV- gross tumor volume; Gy- grays; MRI- magnetic resonance imaging; MTD- maximum tolerated dose; pCR - pathological complete response; PTV- planning target volume; SIB- simultaneous integrated boost; 18-FDG PET- 18 fluoro-deoxy-glucose positron emission tomography; 3D-CRT- three-dimensional conformal radiotherapy
1. Background
The management of metastatic rectal cancer depends on the resectability of the metastases. In cases of resectable metastases, a rectal conventional chemoradiotherapy (CRT) or an exclusive short radiotherapy (5 x 5 grays (Gy)) is proposed, then surgery of the primary tumor and metastases could be considered. In case of unresectable metastases, the treatment is based on chemotherapy with reassessment of the resectability [1]. The benefits of systematic surgical management of the primary tumor in terms of overall survival, progression-free survival, local complications, remain highly debated in unresectable synchronous metastatic patients [2-6]. If the primary tumor becomes or remains symptomatic, a CRT can be proposed. In case of good local response, surgical abstention could be considered, in order to preserve the quality of life, especially in case of mutilating surgery (abdominoperineal amputation). Indeed, several studies have shown the possibility of a surgical abstention after a complete response after CRT, including in non-metastatic patients [7,8]. The complete response usually occurs within 10 to 12 weeks after the end of CRT but may sometimes occur after several months [9-11]. This argument is in favor, particularly in metastatic patients, of a wait-and-see attitude and local monitoring after CRT. Salvage surgery may then be proposed in case of local evolution or symptoms. Increasing the dose delivered during CRT could increase the overall response rate [11,12]. The objective of this study was to evaluate the feasibility and tolerance of a CRT with radiation dose escalation using simultaneous integrated boost (SIB) intensity-modulated radiation therapy (IMRT), in patients with an unresectable synchronous metastatic low or middle rectal cancer.
2. Patients and Methods
2.1 Study design
The design of this prospective non-randomized, multicenter, phase 1 study is reported in Figure 1. It was based on three dose levels. According to the modified Fibonacci method (3+3 design), the number of patients required was three to six patients for each dose level (Figure 2).
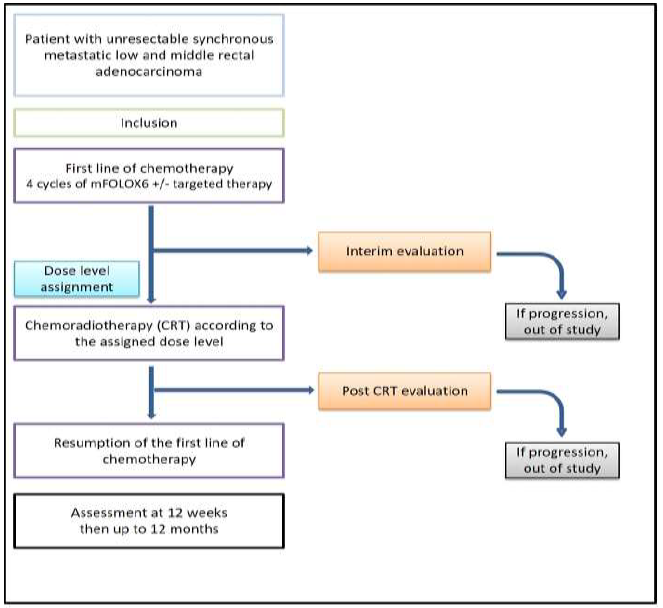
Figure 1: Study design
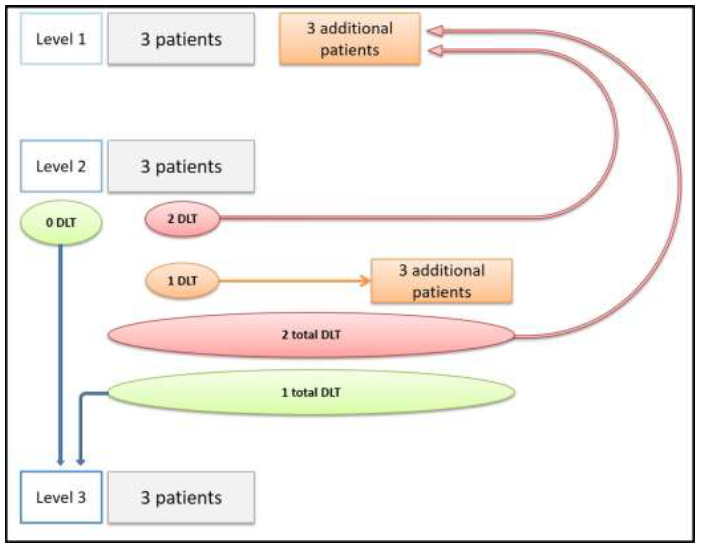
Figure 2: Dose escalation methodology according to the modified Fibonacci method called "3 + 3".
DLT: Dose-limiting toxicity
The escalation of dose at the next level was conditioned by the absence of limiting toxicity. Dose-limiting toxicity (DLT) was defined as the occurrence of toxicity requiring a radiotherapy discontinuation of more than five consecutive fractions. In the absence of DLT observed in the three patients of the current level, three new patients were then included at the next dose level. If one of the three patients in the current stage had a DLT, three additional patients were included at the same level. If no new DLT was observed among these three additional patients (ie one DLT on all six patients), then the dose escalation to the next level was allowed. If two or more DLT were observed among the six patients included in the same level, this dose level was then considered the maximum tolerated dose (MTD) and three new patients were included at the lower dose level. The maximum recommended dose (MRD) was defined as the level immediately below the level at which two toxic limiting doses (DLTs) occurred or the last level if two DLTs did not occur.
2.2 Patients
Patients included in the study had histologically confirmed lower or middle rectum adenocarcinoma, with synchronous metastases deemed unresectable. They must be over 18 years old, have an estimated life expectancy of more than three months, a performance status according to WHO from 0 to 2. They should not have received previous treatment with pelvic radiotherapy or chemotherapy, have a complete deficiency of dihydropyrimidine dehydrogenase (DPD), have a severe or unstable disease, or have diarrhea or neuropathy grade ≥ 2 at baseline intervention.
2.3 Chemotherapy
Before CRT, patients received four cycles of mFOLFOX6 chemotherapy administered every two weeks. This induction chemotherapy could be combined with targeted therapy (bevacizumab, cetuximab, panitumumab) based on KRAS / NRAS status. CRT started within two to four weeks after these four cycles of mFOLFOX6. Concomitant chemotherapy consisted of capecitabine at a dose of 800 mg / m² twice daily, five days a week. Targeted therapies were not allowed during radiation therapy. First-line metastatic chemotherapy was resumed after the end of CRT.
2.4 Radiation therapy
Irradiation was delivered with SIB-IMRT (Figure 3). The treatment was delivered in 25 fractions, five per week, and one per day, over five weeks. Macroscopic tumor, the Gross tumor volume (GTV), was defined using pre-chemotherapy rectoscopy, computed tomography (CT) and 18FDG positron emission tomography (PET). High-risk clinical target volume (CTV) was defined as the GTV with a margin of 10 mm excluding unaffected organs. Low risk CTV involved mesorectum and internal iliac node area. A margin of 5 to 7 mm was applied to generate planning target volume (PTV).
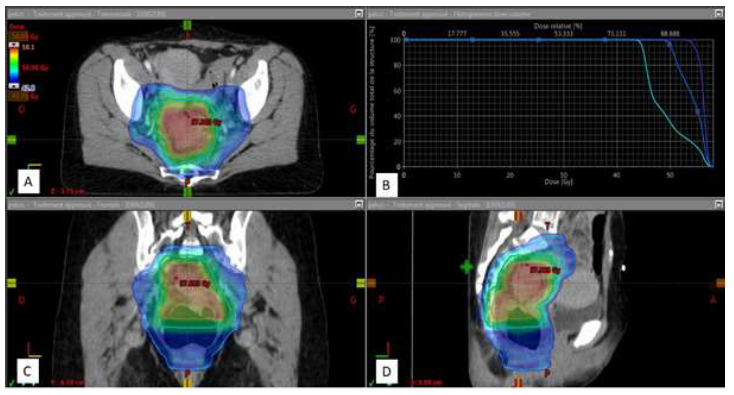
Figure 3: Dose distribution in IMRT with SIB.
Treatment plan with visualization of the dose distribution in color wash from 42.75 Gy (95% of the prescribed dose at PTV 1) to 58.09 Gy (maximum dose) with dark blue, sky blue and orange outline, respectively PTV 1, PTV2 and PTV 3.
A: Axial section; B: Dose-volume histogram of PTV 1 (sky blue), PTV 2 (dark blue), PTV 3 (purple) used to report the dose received (abscissa) by a percentage of PTV volume (ordinate); C: Coronal section; D: Sagittal section
The dose delivered in low and high risk CTV were respectively 45 Gy and 50 Gy, 1.8 and 2 Gy per fraction, 5 fraction a week. Dose delivered to GTV increased from 52.5 Gy to 60 Gy in fractions of 2.1 to 2.4 Gy, respectively. The table 1 summarize the dose levels. Dosimetry followed the recommendations of ICRU 83. Image-guided radiation therapy was mandatory with daily three-dimensional image guidance. Patient was treated with helical tomotherapy or volumetric arc therapy.
|
Level |
Low risk CTV |
High risk CTV |
GTV |
|
1 |
45 Gy (1.8) |
50 Gy (2) |
52.5 Gy (2.1) |
|
2 |
45 Gy (1.8) |
50 Gy (2) |
56.25 Gy (2.25) |
|
3 |
45 Gy (1.8) |
50 Gy (2) |
60 Gy (2.4) |
Total radiation dose delivered (and dose per fraction) during chemoradiotherapy in the different volumes according to the dose level. CTV: Clinical Target Volume; GTV: Gross Tumor Volume
Table 1: Dose levels
2.5 Evaluation
The primary objective of the study was to determine the maximum tolerated dose of SIB-IMRT. The primary endpoint was dose-limiting toxicity (DLT), defined as the occurrence of toxicity requiring interruption of CRT for more than five consecutive fractions. The secondary objectives were: acute (up to 3 months after the end of CRT) and late toxicity according to NCI-CTCAE V4.0; the local response; local progression-free survival at 12 months; 2-year overall survival; local surgery; the quality of life assessed by the QLQ-C30 and QLQ-CR29 questionnaires at inclusion, at the end of treatment and at follow-up. Patients were followed clinically and biologically every two weeks during induction chemotherapy and weekly during CRT. An interim assessment was performed in the four weeks prior to CRT, including CT and 18FDG PET. An end-of-treatment assessment was made within six weeks of the end of CRT, including clinical and biological evaluation, rectal echo-endoscopy (or rectoscopy), CT, pelvic magnetic resonance imaging (MRI), 18FDG PET, quality of life questionnaires. Then follow-up was assed at 12 weeks and then every eight weeks for two years with at least a TAP CT scan, a biological assessment and quality of life questionnaires.
2.6 Ethics
This study received a favorable opinion from the committee for the protection of persons (CPP) (17 February 2014) and was authorized by the national agency for the safety of medicines (ANSM) (first April 2014). An independent committee has been appointed. All patients received from oral and written information, and signed a consent. The study was retrospectively registered on ClinicalTrials.gov (NCT03634202) (https://www.clinicaltrials.gov/ct2/show/NCT03634202) (16 August 2018).
3. Results
3.1 Primary objective
A total of seven patients were included in the trial between May 2015 and February 2017 in our institution. One patient was wrongly included and excluded from the study because he did not meet the inclusion criteria (grade 2 diarrheas at baseline) and was progressing before CRT. Finally, three patients were included in level 1 and three in level 2. No patients were included in level 3 because of low accrual. Patients and tumor characteristics are detailed in table 2. All six patients received four cycles of chemotherapy mFOLFOX6 and completed CRT. Five Patient were treated with helical tomotherapy and one with volumetric arc therapy. Acute side effects were mild (Table 3), no patient had acute toxicity requiring interruption of radiotherapy for more than five consecutive fractions: no DLT was therefore reported.
|
Patient |
Gender |
Age at diagnosis |
Performance Status |
TNM |
Primary tumor size (millimeter) |
Primary tumor location |
Metastatic sites |
|
1 |
Male |
75 |
1 |
T3N0M1 |
80 mm |
Middle and high rectum |
Liver, lungs |
|
2 |
Female |
62 |
1 |
T4N2M1 |
50 mm |
Middle rectum |
Liver, peritoneum |
|
3 |
Female |
66 |
1 |
T4N2M1 |
48 mm |
Low rectum |
Lungs, mediastinal lymph nodes |
|
4 |
Female |
59 |
0 |
T3N1M1 |
66 mm |
Middle and high rectum |
Lungs |
|
5 |
Male |
61 |
1 |
T4N2M1 |
120 mm |
Low, middle and high rectum |
Liver, lungs, inguinal lymph nodes |
|
6 |
Male |
69 |
1 |
T3N2M1 |
80 mm |
Middle and high rectum |
Liver |
Table 2: Patients and tumor characteristics.
3.2 Secondary objectives
The median follow-up was 27.4 months. It is noteworthy that no local progression occurred. Regarding late side effects, three patients suffered from a grade 3 toxicity (Table 3). Patient 3, included in level 1, has developed pelvic pain and pre-occlusive syndrome 4 months after the end of CRT, requiring a colostomy. Rectoscopy did not show any carcinomatous proliferation but only fibrous and cicatricial changes. During the follow-up, he had no local complications and no need for further surgical management. Patient 4, included in level 2, has presented pelvic pain 4 months after the end of CRT, leading to a colostomy and then a posterior pelvectomy. Anatomopathology did not show any tumor infiltration but only cicatricial changes. Patient 5, enrolled in level 2, has presented a recto-vesical fistula 13 months after the end of CRT, requiring a suprapubic catheter. Interestingly, these three patients received targeted therapy during induction chemotherapy. The functional score for physical activity was improved or stable for five out of six patients, just one patient suffered from a transient decrease of physical activity after CRT (Figure 4). Pain decreased after CRT for four patients (Figure 4).
Table 3: Toxicity and follow-up
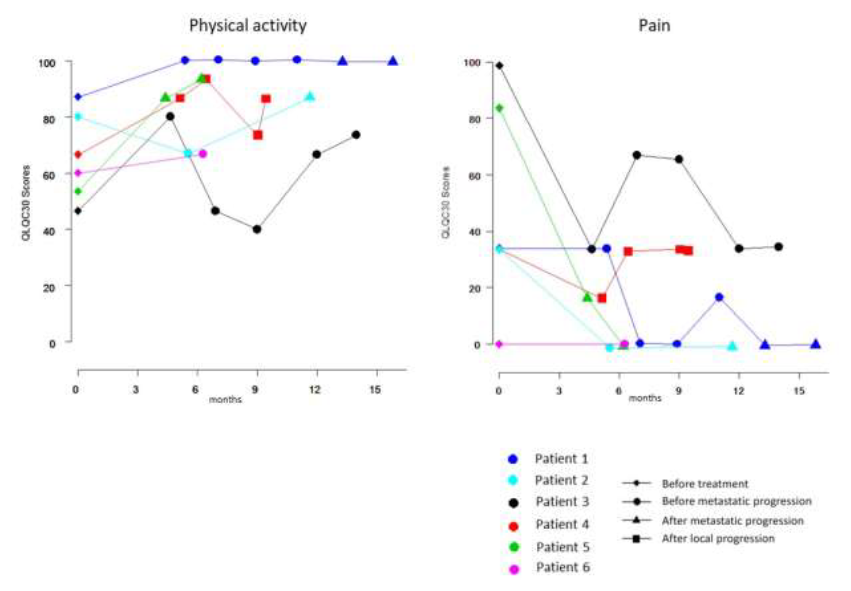
Figure 4: Symptoms according to QLQ-C30.
The first assessment was performed at inclusion, the second after RT-CT.
4. Discussion
This phase I trial showed that a dose escalation up to 56.25 Gy to the tumor is possible with SIB-IMRT. Indeed, no interruption of radiotherapy was needed. Three patients had late Grade 3 toxicity. Of these three patients, two had received anti-angiogenic treatment (bevacizumab) and one anti-EGFR treatment (panitumumab) with chemotherapy prior to CRT. The involvement of these targeted therapies, administered just prior to CRT, needs to be assessed in terms of toxicity. It should also be highlight that the included population is that of metastatic patients, with heavy treatments, and are potentially more fragile and prone to complications. The patient with recto-vesical fistula had undergone colostomy surgery before inclusion in the trial. Patients in the study also had advanced tumors (T3 or T4) with large volumes to irradiate. Patients with a less advanced disease could potentially benefit from higher dose escalation in a smaller volume, with good acute and late tolerance. Moreover, dose escalation assessed in our study provided good local control, no patient experienced local progression. This dose-escalation CRT strategy could provide sufficient local control in metastatic patients to avoid heavy and mutilating surgery throughout their management. SIB-IMRT is a slightly accelerated radiotherapy: the dose is escalated without increasing the overall treatment time, which allowed early resumption of first-line metastatic chemotherapy after CRT. It is noteworthy that chemotherapy following CRT can also participate to local control. Omitting pelvic surgery in this setting does not seem to jeopardize local control. Of course, the main limitation of this phase I feasibility study is the reduced size of the population (6 patients). Moreover, our trial was prematurely closed due to the difficulty of accrual, and the third step (60 Gy) was not evaluated. However, considering on the one hand manageable acute and late toxicities, and on the other hand the good local control, we think that our schedule of dose escalation up to 56.25 Gy is worth been assessed in a phase II study with a larger population.
Dose escalation probably increases local response rate and pathologic complete response (pCR) [11]. Firstly, dose escalation in rectal cancer was assessed with three-dimensional conformal radiotherapy (3D-CRT) [13]. Then, development of IMRT facilitated dose escalation through the SIB [14]. An additional dose delivered by SIB-IMRT, for an equivalent total dose, appears less toxic than a complement by 3D-CRT [15]. In a phase II trial, dose escalation up to 55 Gy with SIB-IMRT leads to 65% of pCR rate, while maintaining a good tolerance [16]. The retrospective study of Yamashita et al. showed a nonsignificant increase at 17% versus 11% (p=0.39) of pCR rates in non-metastatic patients who received 55 Gy with SIB-IMRT, compared to 50.4 Gy in 3D-CRT [17]. In a curative intent of nonmetastatic rectal cancer, some authors, such as Habr-Gama et al. and Maas et al, advocate for a non-operative approach (also known as a wait-and-see policy) for patient who experienced pCR [7,8]. They have reported their experience of sphincter preservation: patients with low rectal cancer presenting a pCR after CRT were not operated and were compared with patients who have undergone surgical treatment. No significant difference in progression-free survival and overall survival was found. Appelt et al. have also study the wait and see policy, they proposed a dose escalation with SIB-IMRT at 60 Gy, with an additional 5 Gy boost in brachytherapy in patients with T2-T3, N0-N1 of the lower rectum [18]. Of the 55 patients included, 40 patients had a pCR and were closely monitored. The local recurrence rate at 1 year was 15.5%. The treatment was well tolerated with the preservation of a good sphincter function. Delivering chemotherapy before the CRT has several interests. In our population of metastatic patients, it provides a systemic and rapid control of metastatic disease. It also has an effect on the local pelvic disease with a partial local response before CRT. PRODIGE 23 is a phase III randomised trial study which assessed neoadjuvant chemotherapy before CRT and surgery versus standard of care (CRT and surgery) for locally advanced rectal cancer [19]. Neoadjuvant chemotherapy both improved 3-year disease-free survival, 76% versus 69 % (p=0.034) and pCR, 28% vesus 12% (p<0.0001), for respectively experimental and standard arm. The study by Sloothaak et al. showed that out of 1,593 patients managed by preoperative CRT, pCR rate was maximal when surgery was performed at 14 weeks from the start of CRT, with a maximum plateau appearing to be reached at 17 weeks [20].
The observation that pCR could be reached up to 4 months after the end of CRT has also been noticed in retrospective study [10]. In order to further, improve pCR rate, chemotherapy can be start over after CRT. The study by Garcia-Aguilar et al. found an increasing rate of pCR rate in locally advanced tumors from 18%, 25%, 30% to 38% by adding zero, two, four, and six cycles of mFOLFOX6, respectively, after CRT [21]. The combination of different strategies could increase the pCR rate: neoadjuvant chemotherapy, radiation dose escalation by SIB-IMRT, chemotherapy after CRT and wait-and-see policy and could potentially increase the number of patients eligible for sphincter preservation and surgical abstention, both in metastatic and curative situation.
5. Conclusion
Radiation dose escalation up to 56.25 Gy using SIB-IMRT when treating rectal cancer was possible with acceptable side effects. No local progression occurred. Future studies with larger populations are needed to assess our dose escalation with the aim of avoiding mutilating surgery in metastatic patients. This dose escalation schedule could also be evaluated in order to increase the clinical complete response rate in non-metastatic patients.
Ethical Approval and Consent to Participate
This study received a favorable opinion from the committee for the protection of persons (CPP) (17 February 2014) and was authorized by the national agency for the safety of medicines (ANSM) (first April 2014). An independent committee has been appointed. All patients received from oral and written information, and signed a consent. The study was retrospectively registered on ClinicalTrials.gov (NCT03634202) (https://www.clinicaltrials.gov/ct2/show/NCT03634202) (16 August 2018).
Consent for Publication
All authors read and approved the final manuscript.
Availability of Supporting Data
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Funding
This work was supported by the “Institut de Cancérologie de l’Ouest”
Author’s Contributions
Amaury Paumier and Valérie Seegers: protocol redaction and methodology
Thibaut Lizée and Amaury Paumier: results analyses and writing the article
Amaury Paumier, Thibaut Lizée, Julien Blanchecotte, Emmanuel Rio, Olivier Capitain, Véronique Guérin-Meyer, Florence Legouté, Marc-André Mahé: treatment and follow-up of patients
Damien Autret: dosimetry
Acknowledgments
The author thanks Pr Campone for promoting this study.
References
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27 (2016): 1386-1422.
- Verhoe C, Johannes H, Burger JW, et al. Surgery of the primary in stage IV colorectal cancer with unresectable metastases. Eur J Cancer 47 (2011): S61-S66.
- Venderbosch S, De Wilt JH, Teerenstra S, et al. Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Ann Surg Oncol 18 (2011): 3252-3260.
- Cirocchi R, Trastulli S, Abraha I, et al. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable Stage IV colorectal cancer. Cochrane Colorectal Cancer Group, ed. Cochrane Database Syst Rev 12 (2012): 19-23.
- Ferrand F, Malka D, Bourredjem A, et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: Results from the multicenter, randomised trial Fédération Francophone de Cancérologie Digestive 9601. Eur J Cancer 49 (2013): 90-97.
- Faron M, Pignon J-P, Malka D, et al. Is primary tumour resection associated with survival improvement in patients with colorectal cancer and unresectable synchronous metastases? A pooled analysis of individual data from four randomised trials. Eur J Cancer. 2015;51(2):166-176.
- Habr-Gama A, Perez RO, Nadalin W, et al. Operative Versus Nonoperative Treatment for Stage 0 Distal Rectal Cancer Following Chemoradiation Therapy: Long-term Results. Trans Meet Am Surg Assoc 72 (2004): 309-316.
- Maas M, Beets-Tan RGH, Lambregts DMJ, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 29 (2011): 4633-4640.
- Brierley JD, Cummings BJ, Wong CS, et al. Adenocarcinoma of the rectum treated by radical external radiation therapy. Int J Radiat Oncol Biol Phys 31(1995): 255-259.
- Wang Y, Cummings B, Catton P, et al. Primary radical external beam radiotherapy of rectal adenocarcinoma: Long term outcome of 271 patients. Radiother Oncol 77 (2005): 126-132.
- Hall MD, Schultheiss TE, Smith DD, et al. Effect of increasing radiation dose on pathologic complete response in rectal cancer patients treated with neoadjuvant chemoradiation therapy. Acta Oncol 55 (2016): 1392-1399.
- Wiltshire KL, Ward IG, Swallow C, et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: Effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int J Radiat Oncol 64 (2006): 709-716.
- Myerson RJ, Valentini V, Birnbaum EH, et al. A phase I/II trial of three-dimensionally planned concurrent boost radiotherapy and protracted venous infusion of 5-FU chemotherapy for locally advanced rectal carcinoma. Int J Radiat Oncol Biol Phys 50 (2001): 1299-1308.
- But-Hadzic J, Anderluh F, Brecelj E, et al. Acute toxicity and tumor response in locally advanced rectal cancer after preoperative chemoradiation therapy with shortening of the overall treatment time using Intensity-Modulated Radiation therapy with simultaneous integrated boost: A Phase 2 trial. Int J Radiat Oncol 96 (2016): 1003-1010.
- Bae BK, Kang MK, Kim J-C, et al. Simultaneous integrated boost intensity-modulated radiotherapy versus 3-dimensional conformal radiotherapy in preoperative concurrent chemoradiotherapy for locally advanced rectal cancer. Radiat Oncol J 35 (2017): 208-216.
- Tey J, Leong CN, Cheong WK, et al. A phase II trial of preoperative concurrent chemotherapy and dose escalated intensity modulated radiotherapy (IMRT) for locally advanced rectal cancer. J Cancer 8 (2017): 3114-3121.
- Yamashita H, Ishihara S, Nozawa H, et al. Comparison of volumetric-modulated arc therapy using simultaneous integrated boosts (SIB-VMAT) of 45 Gy/55 Gy in 25 fractions with conventional radiotherapy in preoperative chemoradiation for rectal cancers: a propensity score case-matched analysis. Radiat Oncol 12 (2017): 11-19.
- Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 16 (2015): 919-927.
- Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 22 (2021): 702-715.
- Sloothaak DAM, Geijsen DE, Van Leersum NJ, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer: Interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 100 (2013): 933-939.
- Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 16 (2015): 957-966.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 