Xenotransplantation of Human Adipose Tissue in SCID Mice: a Model to Study Proximal Interactions between Adipose Tissue and Tumors
Beaumel S1*, Geneste A1*, Duong MN2, Jordheim LP1, Delay E3, Valet P4, Perrial E1, Dumontet C1,5*
1Univ Lyon, Université Claude Bernard Lyon 1, INSERM 1052, CNRS 5286, Centre Léon Bérard, Centre de Recherche en Cancérologie de Lyon, Lyon, 69008, France
2Department of Oncology, Lausanne University Hospital Center (CHUV) and University of Lausanne, Epalinges, Switzerland
3Centre Léon Bérard, Chirurgie plastique reconstructrice et esthétique, 28 Promenade Léa et Napoléon Bullukian, 69008 Lyon, France
4Université de Toulouse, UPS, Institut de Médecine Moléculaire de Rangueil, Equipe n°3, UMR INSERM 1048, IFR31, Toulouse, France
5Hospices Civils de Lyon, Services d'Hématologie, 165 Chemin du Grand Revoyet, 69310 Pierre-Bénite, France
*both authors contributed equally to this work.
*Corresponding Author: Charles Dumontet, Univ Lyon, Université Claude Bernard Lyon 1, INSERM 1052, CNRS 5286, Centre Léon Bérard, Centre de Recherche en Cancérologie de Lyon, Lyon, 69008, France.
Received: 17 January 2023; Accepted: 24 January 2023; Published: 15 February 2023
Article Information
Citation: Beaumel S, Geneste A, Duong MN, Jordheim LP, Delay E, Valet P, Perrial E, Dumontet C. Xenotransplantation of Human Adipose Tissue in SCID Mice: a Model to Study Proximal Interactions between Adipose Tissue and Tumors. Journal of Cancer Science and Clinical Therapeutics. 7 (2023): 43-49.
View / Download Pdf Share at FacebookAbstract
Keywords
<p>Obesity; Immuno-histochemical; Trastuzumab</p>
Article Details
1. Introduction
While obesity rates are rising worldwide, population data link obesity to the increased incidence of several common cancers [1]. Obesity also portends worse cancer-specific outcomes after diagnosis in several tumor types including those of the breast, esophagus, colon, prostate and others [2, 3]. Furthermore, a higher risk of relapse after treatment has been described in overweight patients compared to lean patients. For breast cancer, several studies have shown a resistance phenotype in clinical, in vivo and in vitro studies. For example, treatment with the monoclonal antibody trastuzumab was associated with a reduced 10 year-survival in obese patients in comparison to lean patients [4]. In cell culture, adipocytes protected breast tumor cells from radiotherapy-induced death [5] and adipocyte-conditioned media reduced sensitivity of breast tumor cells to the cytotoxic activity of trastuzumab and gemcitabine [6, 7]. The exact mechanisms underlying the protumoral effects of obesity and tumor proximal adipose tissue are not yet well understood, making it imperative to develop relevant animal models. While there are several diet-induced and genetic murine models of obesity [8] there remains a lack of relevant models to study the interplay between proximal adipose tissue and tumor cells in vivo. For this reason, we chose to establish xenotransplants of normal human adipose tissue and proceeded to their immunohistochemical, immunophenotypical and functional characterization. We then determined their impact on the growth of human tumor xenografts implanted in direct contact with this adipose tissue.
2. Materials and Methods
2.1 In vivo Studies
All animal procedures were performed in accordance with European Union directive 86/609/EEC, under individual permit and in animal care facilities accredited by the French Ministry of Agriculture. The local animal ethics committee (Université Claude Bernard Lyon I, protocol number BH-2012-40) approved the study. Severe combined immunodeficiency (SCID) mice (Charles River) received a subcutaneous injection of 1 ml of abdominal adipose tissue obtained from patients. For tumor growth studies, tumor fragments obtained from tumor-bearing SCID mice were grafted subcutaneously in contact with the adipose xenotransplant, one week after the xenotranplantation of the adipose tissue. Tumor growth was directly measured using a caliper, based on the difference of consistencies between the lipoma and the tumor.
2.2 Adipokine Dosages
Adipokines were measured in the supernatants of explanted adipose xenografts obtained at different time points after implantation. These tissues were incubated for 4 hours in DMEM medium without serum. The supernatants were harvested, centrifuged and the Luminex technology was used to determine the concentrations of IL-6, IL-8, adiponectin, MCP-1, serpin E1 and PGE2.
2.3 Isolation of Human Adipose-Derived Stem Cells
Fresh adipose tissue was obtained by liposuction of abdominal fat from patients undergoing breast reconstruction in the Cancer Center Léon Bérard (Lyon, France) with oral informed consent for the use of residual material. Patient samples and adipose tissue xenografts were rapidly digested with collagenase at 5 mg/ml (Sigma-Aldrich, St Louis, MO, USA) at 37 °C with agitation for 30 minutes. Digestion was stopped by addition of complete DMEM/F-12 medium. After centrifugation at 300g for 7 minutes, cells corresponding to the stromal vascular fraction (SVF) were verified by flow cytometry to be CD14−, CD45−, CD73+, CD90+, CD105+ and HLA-ABC+ (human leukocyte antigen).
2.4 Flow Cytometry
SVF cells were labeled with antibodies for 15 minutes at room temperature and analyzed with a BD LSR II flow cytometer using BD FACSDiva software (BD Biosciences, San Diego, CA, USA) and FlowJo software (Tree Star, Ashland, OR, USA). The antibodies used were a human anti CD-73-APC (Beckton Dickinson, ref 56847) and a human anti-CD90-Vioblue (Miltenyi Biotec).
2.5 Reverse Transcription and Quantitative PCR
RNA was extracted using the QIAzol® Lysis Reagent from Qiagen by extraction successively with qiazol, chloroform, isopropanol and ethanol. cDNA was generated from the RNA using reagents from Invitrogen including random primers, M-MLV reverse transcriptase enzyme, DTT and dNTP. Quantitative real time RT-PCR (qPCR) was performed with primers (QIAGEN) using SYBR Green method and the LightCycler® (Roche) with a 40-cycle program. Primers used for qPCR are listed in Table 1.
2.6 Immunohistological Analysis
Samples were processed by CIQLE platform, University of Lyon. Immunohistochemistry was performed on an automated Discovery Xt (Roche) using a DAP Map Kit (ref 760-124). Primary antibodies (Anti Perilipin Ref #3470 Cell Signaling, diluted 1/100 and Anti PPARgamma Ref #2435 Cell Signaling, diluted 1/200) were incubated for 60 minutes at 37 °C then the secondary antibody (Anti rabbit Ref BA1000 Vector diluted 1/300) was incubated for 30 minutes at 37 °C. Routine staining was performed using hematoxylin and phloxin (HP).
2.7 Statistical Analysis
All experiments were performed at least three times. Mean ± SD values of representative experiments are shown. Statistical significance was evaluated using paired Student’s t-tests on the means of at least three independent in vitro experiments. Unpaired Student’s t-tests were used for in vivo experiments. p-values < 0.05 were deemed significant.
3. Results
3.1 Adipose Tissue Xenotransplants
Samples from five female patients were obtained and xenotransplanted into three separate mice with two implantations in each mouse. The patient BMI values ranged between 20 and 25. Tolerance to the subcutaneous implantation procedure was excellent with no local inflammation or necrosis in any of the animals. There was no impact on animal well-being.
3.2 Tissue Specific Functions are maintained in Adipose Tissue Xenografts
Adipose tissue is a secretory organ and the cytokines it releases are involved in the functionality and metabolism of the tissue. For example, interleukins (IL) IL-6 and IL-8 are produced and secreted by adipocytes [9, 10]. The levels of these interleukins secreted and measured in the adipose xenograft-conditioned medium had a similar evolution, with a high level in the conditioned medium on the first day followed by a progressive decrease (Figure 1). This decrease was rapid for IL-8, IL-6, adiponectin and Serpin E1 while MCP-1 and PGE2 content decreased more slowly. Concentrations were found to be in the nanomolar range, which is consistent with previous data in the literature [11]. We observed the same trends with the concentrations of the serine protease inhibitor serpin E1 also called plasminogen activator inhibitor 1 (PAI-1), commonly secreted by adipose tissue [12]. Leptin and adiponectin are adipose tissue specific adipokines and the expression of their coding genes remained similar in explanted adipose xenografts [13]. The production and secretion of adiponectin by the adipose xenografts decreased with time but were still detectable at the end of the experiment (day 180). The levels of MCP-1 stayed high until 90 days then dropped and were poorly detectable at day 180. In parallel, we investigated the expression of several genes by rt-PCR in xenografts and compared these to those observed in the fresh adipose tissue, prior to implantation (Figure 2). Expression levels of ADRP and PPARG, which are both known to be expressed in differentiated adipocytes, were stable up to 90 days after transplant and similar to levels observed in primary tissue [14]. The transcription of the genes FABP4 and LPL were also maintained in the xenografts, suggesting a maintained ability to perform lipolysis. Expression levels of ADIPOQ (adiponectin) and LEP (leptin) were also stable. In the same manner, the inflammation marker HIF1a, which is expressed in adipocytes following the activation of lipolysis, remained stable in the explants up to 90 days. Overall these results suggest that the adipose tissue xenografts maintain tissue-specific ability for at least three months.
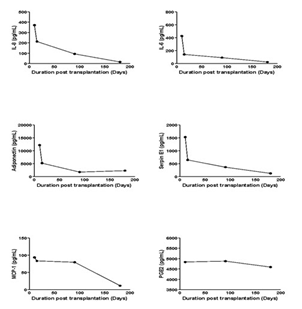
Figure 1: Evolution of secretome in xenotransplanted adipose tissue. Human adipose tissue was transplanted SC in scid mice and explanted at different time points up to 90 days then incubated ex vivo for 4 h. Measurements of IL-8, IL-6, adiponectin, serpin E1, MCP-1 and PGE2 were performed by Luminex.

Figure 2: Expression levels of selected genes in adipose tissue xenotransplants. Human adipose tissue was transplanted SC in scid mice and explanted at different time points up to 90 days. Total RNA was extracted and rt-qPCR analyses performed for target genes. Time 0 corresponds to the primary human adipose tissue used for implantation. Adiponectin and leptin are encoded by ADIPOQ and LEP genes, respectively.
3.3 Histological and Cytological Modifications in the Xenografts
Fresh adipose tissue is obtained by lipoaspiration and is likely to be structurally different from established xenografts. Interestingly, histological and immunohistochemical analyses of the samples showed a morphological aspect very comparable to that of a normal healthy adipose tissue. As shown in Figure 3, the xenografts maintained a classical adipose tissue structure for up to 90 days after transplantation, with maintained expression of perilipin and PPAR gamma. Cytometric analysis of the stromal vascular fraction (SVF) with antibodies anti-CD73 and anti-CD90 was performed both on fresh patient samples and the corresponding adipose tissue xenografts to assess the percentage of CD73+/CD90+ precursors. The percentages of this immature population were similar in fresh samples and in xenografts, in the order of 10%, and remained stable up to 90 days post-transplant. Overall, these results suggest that the adipose tissue xenografts maintain a classical adipose tissue morphology and contain CD73+/CD90+ precursor cells up to 90 days post-transplant.
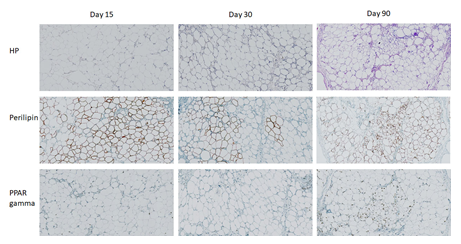
Figure 3: Immunohistochemical analyses of adipose tissue xenotransplants. Human adipose tissue was transplanted SC in scid mice and explanted at different time points. Standard staining with haematoxylin/phloxin (HP) and immunohistochemistry using anti-perilipin and anti-PPAR gamma antibodies was performed. These results show samples from a single adipose tissue donor and are representative of three different donors.
3.4 Impact of Proximal Adipose Tissue on Tumor Growth and Sensitivity to Doxorubicin
We evaluated the impact of proximal adipose tissue on the tumorigenesis of three human breast cancer lines MDA-MB-231, MDA-MB-436 and BT-474, corresponding to triple negative A, triple negative B, and luminal basal lines, respectively. We implanted subcutaneously small tumor fragments both near the adipose tissue xenograft two weeks after the xenotransplantation of the adipose tissue and in mice with no adipose tissue xenografts. In the case of the MDA-MB-436 and the MDA-MB-231 models, the growth curves were similar in the presence or absence of proximal adipose tissue (Figure 4). In the case of the BT-474 we observed a slightly, yet significantly, delayed tumor growth as we had previously reported [6]. Explantation of the adipose tissue and the tumors allowed us to confirm the proximity of both tissues (Figure 4). We evaluated the sensitivity to doxorubicin of the MDA-MB-436 model, in the presence or absence of proximal adipose tissue. As shown in Figure 5, the growth of tumors was significantly delayed by the administration of doxorubicin 1 mg/kg once weekly whereas tumors implanted in contact with the adipose xenografts were unaffected by this treatment.
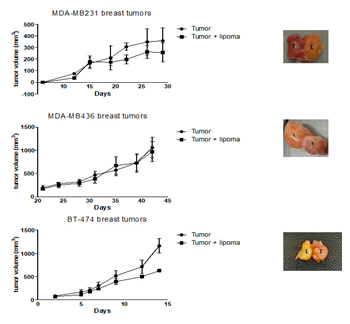
Figure 4: Impact of proximal adipose tissue on xenograft tumor growth. Human adipose tissue was transplanted SC in scid mice. Two weeks later tumor fragments of MDA-MB-231, MDA-MB-436 and BT-474 xenografts were implanted either in contact with the adipose xenotransplant or in mice devoid of adipose xenotransplants. T : tumor ; L : adipose tissue xenotransplant (or “lipoma”)
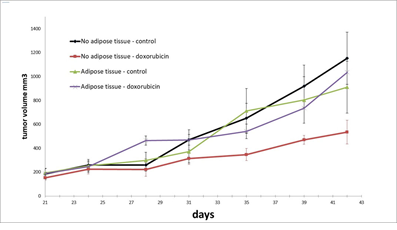
Figure 5: Impact of proximal adipose tissue on sensitivity of xenograft tumor to doxorubicin. Human adipose tissue was transplanted SC in scid mice. Two weeks later tumor fragments of MDA-MB-436 xenografts were implanted either in contact with the adipose xenotransplant or in mice devoid of adipose xenotransplants. Once tumor had reached a volume of 200 mm3 mice were treated systemically with doxorubicin 1 mg/kg once weekly.
4. Discussion
Xenotransplantation of human adipose tissue in mice has already been performed to correct inborn errors of metabolism, but to our knowledge not to evaluate the influence on tumor growth and resistance to treatments [15]. We have already reported the use of this approach to study the efficacy of targeted therapies on breast tumor in a previous study and we wanted to determine the biological relevance, advantages and disadvantages of such a model [6]. Our main finding is that human adipose tissue transplanted subcutaneously in immunocompromised mice appeared to be well tolerated and stable for up to 90 days, with the presence of stem cells suggesting self-renewal, establishment of a murine vascularization, and maintenance of a secretory and an adipose-specific lipolytic activity. This model possesses several advantages to study the impact of adipose tissue on tumors. First, we can use immunodeficient mice and study tumors of human origin or from other species. Secondly, these mice do not need any preferential care or diets used in diet-induced obesity models [7, 16-18]. Thirdly, as the adipose tissue and the tumor are grafted subcutaneously, it is technically simple to evaluate tumor growth. Finally, these xenografts may easily be explanted without sacrificing the animals. Tumor growth was unaffected by the presence of proximal adipose tissue in two models and delayed in one model. This is unexpected since adipose tissue may be expected to favor tumor growth. Conversely, MDA-MB-436 tumors were found to be resistant to doxorubicin therapy when grown in the presence of adipose tissue. This suggests that proximal adipose tissue may have a complex impact, favoring a slow yet continued tumor growth unimpeded by therapeutic interventions. This is in keeping with prior observations from our group showing resistance to doxorubicin through the induction of the major vault protein, or to the targeted agent trastuzumab [6, 19]. The analysis of precursors of adipocytes was performed using CD90 and CD73. We had initially designed a panel to investigate other markers such as CD14, CD45, CD105, HLA-ABC and HLA-DR, as described in the literature [18]. Unfortunately, we did not obtain enough cells in the SVF fraction to perform complete immunophenotyping. The percentage of cells in this population appeared stable for three months and we saw an increased percentage at day 180.
Acknowledgments
We thank Aurore Cleret for excellent technical assistance, the CIQLE for immunohistochemistry studies and the Service Commun Animalerie Rockefeller for assistance with murine studies. This work was supported in part by the French National Cancer Institute INCa, grant INCA_6666).
References
- Obesity and overweight (2017).
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348 (2003): 1625-1638.
- Schmitz KH, Neuhouser ML, Agurs-Collins T, et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst 105 (2013): 1344-1354.
- Ewertz M, Jensen MB, Gunnarsdottir KA, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol 29 (2011): 25-31.
- Bochet L, Meulle A, Imbert S, et al. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem Biophys Res Commun 411 (2011): 102-106.
- Duong MN, Cleret A, Matera EL, et al. Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity. Breast Cancer Res 17 (2015): 57.
- De Angel RE, Blando JM, Hogan MG, et al. Stearoyl gemcitabine nanoparticles overcome obesity-induced cancer cell resistance to gemcitabine in a mouse postmenopausal breast cancer model. Cancer Biol Ther 14 (2013): 357-364.
- Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol (2012); Chapter 5:Unit5 61.
- Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 82 (1997): 4196-200.
- Bruun JM, Pedersen SB, Richelsen B. Regulation of interleukin 8 production and gene expression in human adipose tissue in vitro. J Clin Endocrinol Metab 86 (2001): 1267-1273.
- Skurk T, Alberti-Huber C, Herder C, et al. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92 (2007): 1023-1033.
- Scherer PE, Williams S, Fogliano M, et al. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270 (1995): 26746-26749.
- Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11 (2011): 85-97.
- Urs S, Smith C, Campbell B, et al. Gene expression profiling in human preadipocytes and adipocytes by microarray analysis. J Nutr 134 (2004): 762-770.
- Ablamunits V, Klebanov S, Giese SY, et al. Functional human to mouse adipose tissue xenotransplantation. J Endocrinol 212 (2012): 41-47.
- Behan JW, Yun JP, Proektor MP, et al. Adipocytes impair leukemia treatment in mice. Cancer Res 69 (2009): 7867-7874.
- Ehsanipour EA, Sheng X, Behan JW, et al. Adipocytes cause leukemia cell resistance to L-asparaginase via release of glutamine. Cancer Res 73 (2013): 2998-3006.
- Malvi P, Chaube B, Singh SV, et al. Weight control interventions improve therapeutic efficacy of dacarbazine in melanoma by reversing obesity-induced drug resistance. Cancer Metab 4 (2016): 21.
- Lehuede C, Li X, Dauvillier S, et al. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP). Breast Cancer Res 21 (2019): 7.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 