Interaction between Physical Activity and Socioeconomic Determinants among Cancer Patients: A Systematic Mapping Review
Jean-Marie Nguyen1, Abdou Yacoubou Omorou1, 2, 3, Christine Rotonda1, 4, Cyril Tarquinio1, Sophie Gendarme4, Charles Martin-Krumm1, 6, 7, Aurélie Van Hoye1*
1Université de Lorraine, Nancy, France
2INSERM, CIC-1433 Clinical Epidemiology, Nancy University Hospital, Nancy, France
3National Clinical Research Platform for Quality of Life in Oncology, Besançon, France
4Université de Lorraine, Centre Pierre Janet, Metz, France
6Laboratoire de Psychologie de l’Ecole de Psychologues Praticiens de Paris, Paris, France
7Institut de Recherche Biomédicale des Armées (IRBA), Brétigny, France
*Corresponding Author: Aurélie Van Hoye, Université de Lorraine, APEMAC, 9 Avenue de la Foret de Haye, 54000 Nancy, France
Received: 22 July 2021; Accepted: 02 August 2021; Published: 17 November 2021
Article Information
Citation:
Jean-Marie Nguyen, Abdou Yacoubou Omorou, Christine Rotonda, Cyril Tarquinio, Sophie Gendarme, Charles Martin-Krumm, Aurélie Van Hoye. Interaction between Physical Activity and Socioeconomic Determinants among Cancer Patients: A Systematic Mapping Review. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 468-495.
View / Download Pdf Share at FacebookAbstract
Socioeconomic factors and physical activity (PA), have been recognized as key factors affecting survival and quality of life of cancer patients. Nevertheless, less is known about their interactions among cancer patients. A mapping systematic review was undertaken to identify gaps in the literature regarding the interactions of socioeconomic factors and PA and the identification of theoretical model to define this relationship. A search for peer-reviewed English articles published between January 2010 and March 2020 in Medline, PsycINFO, Web of Science and SportDiscus databases was realized using three keywords: physical activity, cancer, socioeconomic. Cancer location and time, socioeconomic factor measurement, PA measurement, intervention and theoretical model were analyzed. Of the 5163 articles found, 90 were included (86 observational studies and 4 interventions). While many studies evaluate socioeconomic factors and PA, authors often do not consider their interactions, but test them separately. Socioeconomic factors identified in the studies ranged among 12 categories (age, sex, ethnicity, education, income, occupation, residence, green space exposure, marital status, household, social support, Health insurance). A high diversity of measurements within each category led to a huge variation in the definition of socioeconomic factors and refrained comparison between studies. Similar conclusions could be drawn with regard to the diversity of PA measurements. Only few studies mobilized theoretical models, without considering the interactions between socioeconomic factors and PA. The definition of socioeconomic factors as well as theoretical modeling of how socioeconomic factors interact with PA among cancer patients needs to be clarified.
Keywords
<p>Physical activity; Cancer; Socioeconomic determinant; Mapping systematic review</p>
Article Details
Abbreviation:
PA- physical activity; SES- socioeconomic status
1. Introduction
Every year, 18.1 million new cases of cancer are diagnosed worldwide, and 9.6 million people die of cancer [1]. The most frequently diagnosed cancer types and leading causes of cancer deaths vary across countries and within each country, depending on the degree of economic development and associated social and lifestyle factors [1]. Physical activity (PA) has been recognized as one of the key lifestyle factors increasing cancer survival and quality of life during cancer [2]. PA has been defined as “any bodily movement that results in energy expenditure” [3]. Considered a non-pharmacological intervention, the benefits of PA before [4], during and after cancer treatment [5, 6] have been largely demonstrated. For example, PA improves the quality of life of the person affected by the cancer and reduces the risk of death or recurrence [7, 8], fatigue [9] and depression [10]. Several literature reviews have shown that PA interventions could be effective for well-being and physical, mental and social health [11].
Moreover, evidence-based recommend-dations have been produced and political agendas have considered PA a conditional part of care for all cancer survivors [12]. Despite this evidence regarding both benefits and interventions, people with cancer have a lower PA level than the general population [13, 14], and numerous studies have shown that their PA level decreases after the cancer diagnosis [15], which questions which individual, interpersonal and community factors could support PA practices among cancer patients. In this regard, the literature has shown that PA practice has been associated with the socioeconomic characteristics of the individual, especially different determinants of PA, such as age, sex, income, education, and socioprofessional category [16]. For example, low income has been negatively correlated with recreational PA [17]. Although socioeconomic factors are considered major determinants of PA [18], their definition, as well as their measurement seems a major issue, when looking at their relationships. Indeed, different concepts, such as socio-economic status [19], socioeconomic inequalities [20], socioeconomic background [21] as well as plenty of other indicators (e.g., education, income) have been identified as belonging to socioeconomic factors [18]. An umbrella review analysing correlations between socioeconomic status and PA based the selection of socioeconomic factors on previous studies [17, 22], without justifying the choice of these indicators. Moreover, in previous literature reviews, the definition of socioeconomic concepts used was missing or too broad [18]. Nevertheless, a previous review showed similar patterns of association with PA among the different indicators of socioeconomic position (e.g., high education level associated with high PA level) [22], but studies of cancer patients are rare [23].
In addition, previous studies of the general population have underlined the need to deeply understand how socioeconomic factors and PA could interact, as well as which mediators and moderators of the relationships could be identified [18]. Recent research has demonstrated that PA could compensate for low socioeconomic status in terms of poor self-related health and low quality of life [24].
To our knowledge, no recent systematic review has summarized results of studies analysing interactions between socioeconomic factors and PA among cancer patients. Expected results could help in developing a theoretical model of interactions between socioeconomic factors and PA among cancer patients. Indeed, theoretical models have been described as being helpful to enable the understanding of behaviour change, especially PA, and serve as strategies in interventions [25]. To achieve these aims, this literature review aimed to identify the association between socioeconomic factors and PA, from the diagnosis to remission. The review aimed to 1) describe indicators of socioeconomic background for cancer patients, 2) identify the relation between this socioeconomic background and PA level before the cancer diagnosis, during treatment and during remission and 3) identify a theoretical model framing the relation between socioeconomic background and PA.
2. Material and Methods
A systematic mapping review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26]. Indeed, the objective of a mapping review is to systematically search for and appraise research evidence and the main gaps in the literature [27].
2.1 Search strategy
The following electronic databases were searched from January 2000 to March 2020: Medline (PubMed), PsycINFO, Web of Science and SPORTDiscus. Electronic databases were searched for each possible combination of the following keywords as well as MeSH terms: physical activity, cancer, socioeconomic factor (see supplementary file 1).
2.2 Inclusion and exclusion criteria
Inclusion criteria were 1) English-written peer-reviewed publications, 2) involving all types of adult cancer patients (18 years and older), and 3) including at least one socioeconomic factor as defined by a previous literature review [18] and one PA domain (measured in terms of frequency, duration or intensity [28]. Only original articles were considered; protocols and reviews were excluded. We excluded studies associating socioeconomic factors and cancer, or PA and cancer only.
2.3 Screening and data extraction
All relevant publications were extracted from databases and imported into Covidence software for title and abstract screening by 2 authors (JMN, AVH). Duplicate records were removed before abstract screening. If there was ambiguity regarding eligibility, a third author (AYO) was consulted. Any disagreements were resolved by discussion among authors (JMN, AVH and AYO). Then, full texts were retrieved for the retained articles and analysed by one author (JMN), with a random analysis of half of the included studies by AVH.
2.4 Data analysis
The following information was extracted into data tables from each included study: authors, journal, year of publication, country, objectives, inclusion and exclusion criteria, stage and type of cancer, socioeconomic factors and their measurement, PA outcome and its measurement, sample size and population characteristics, type of study and method used, type of association between socioeconomic factors and PA, and theoretical model used. In the results section, “sex” was used for sex and gender, and “ethnicity” for race and ethnicity. To analyse the data, a specific section was dedicated to socioeconomic factor measurement, another to PA measurement, and a section on methods used by selected studies.
3. Results
3.1 Descriptive analysis
Overall, 90 of the 5,163 screened articles were included in this literature review (Figure 1).
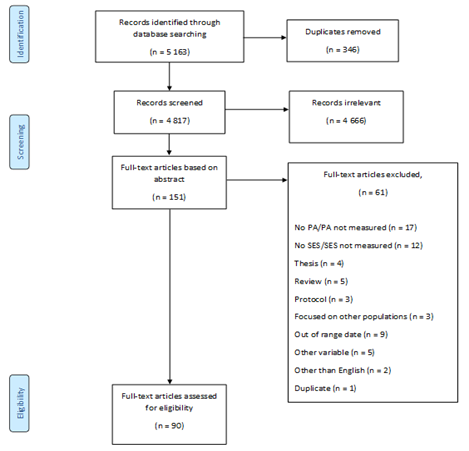
Plate 2: a) inoculated sheath blight Petri dish b) inoculated rice blast Petri dish.
A rather constant number of publications per year was found, with an increase in the last year. Indeed, one third of the studies (n=29) were published during 2018-2019 and 22 during 2013-2014. Almost half of the studies were performed in North America (37 in the United States and 7 in Canada). The other continents were less represented, with 18 studies conducted in Europe, 9 in Asia, 6 in Oceania, 5 in South America (all in Brazil) and none in Africa. Four multicountry studies were included [29-32].
The most commonly studied cancer locations were breast (n = 48), colorectum (n = 43) and prostate (n = 22). Among the 90 included studies, 45 focused on a single cancer; 16 studies did not mention any cancer location because they principally focused on primary cancer prevention. Forty studies involved before the cancer diagnosis (including primary and secondary prevention), 19 from diagnosis to the end of treatment and 27 cancer survivors. Only one study followed the patients during the cancer [33], 3 investigated both before cancer diagnosis and during remission [34-36] and 2 did not mention when the study took place [37, 38] (see Table 1 for details). We found high diversity with regard to sample size, ranging from 13 to 566 398 participants. Also, participants were 18 to 97 years old.
More than half (n = 56) of the studies included both sexes. Single-sex studies reported different cancer locations. For men, 4 studies included prostate cancer patients [39-42], and one targeted male breast cancer [43]. Studies of women investigated breast (n = 23), cervical (n = 3), colon and rectal (n = 1), epithelial ovarian (n = 1), and gynecologic (n = 1) cancers. Two studies focused on mortality rates due to cancer [37, 38]. Study designs were principally cross-sectional (n = 53), followed by cohort (n = 22), case–control (n = 8), qualitative (n = 2) and mixed methods (n = 1). Four interventional studies were included: 3 randomized controlled trials and one quasi-experimental study. The results of the observational studies will be presented, before dedicating a specific section to interventions.
3.2 Observational studies
3.2.1 PA measurement: Three studies used objective measures (2 studies combining objective and subjective measures) and 83 studies relied on only subjective measurement. The most frequently subjective PA assess-ment tools used were the International Physical Activity Questionnaire (n=14), the Godin Leisure-Time Exercise Questionnaire [41, 44-47], the Past Year Total Physical Activity Questionnaire [48], the Determinants of Physical Activity Questionnaire [49], the Patient-Centered Asses-sment and Counseling for Exercise questionnaire [50], and the Leisure-Time Physical Activity questionnaire [49, 51]. Other studies used single-item measures or non-validated scales. Objective measurements used were the GT1M [52, 53] and GT3X actigraph accelerometers [54]. In addition to the diversity of measurement tools and the frequent use of single-item measurement, the recall period ranged from 1 week to up to 3 years before cancer diagnosis. Also, the context of PA measured (global PA vs specific context such as transport, leisure or occupational) and the calculation ranged from “meeting PA guidelines or not” to time spend in minutes per week, which led to high heterogeneity of PA measurement.
3.3 Socioeconomic status variables
The analysis of socioeconomic status variables revealed a large diversity of variables measured, including a broad range of indicators. In total, 71 studies collected multiple socioeconomic variables analysed by PA level, but 16 studies focused on a single socioeconomic variable analysed by PA level (4 focusing on residence, 3 ethnicity, 3 income, 3 education, 2 green space exposure, and 1 occupation). To facilitate the analysis, variables were gathered into the following categories: education, income, occupation, residence, marital status and household, social support, exposure to green areas, lifestyle and Health insurance (see Table 2). The association between the factors in these categories and PA was analysed in terms of time of cancer, type of cancer and pattern tested.
3.3.1 Age: All studies collected age by asking for the date of birth or an age category. The authors of the publications classified age as a demographic, sociodemographic, medi-cal or gynaecologic variable. Only 17 studies analysed the relation between age and PA for a broad range of cancer types. Seven focused on before cancer diagnosis, 5 during treatment, and 5 after cancer treatment. Nine articles demonstrated that older adults (age 65 years and older) frequently had lower PA level, be inactive or be less active
than younger people [29, 43, 55-61].
3.3.2 Sex: Mentioned as “sex” or “gender”, all studies collected this variable. Although sex can be considered a biological variable and gender a cultural variable, many studies did not differentiate between the two. This variable was solely used as a control variable or analysed according to other socioeconomic variables, not with PA, which disallowed any conclusions on their interactions.
3.3.3 Ethnicity: Ethnicity was mentioned as “ethnicity” or “race” (with only one study distinguishing between “race” and “ethnicity”) [51]. The definition of ethnicity in the different publications was rare and depended on authors’ decisions in terms of classification, which led to studies defining groups such as “white and non-white” [43, 47, 61, 62], “multiracial reason” [46] or “others” [63, 64]. In most studies focusing on ethnicity, a large sample in the native population was compared with ethnic minority groups. For example, African-American ethnicity members (31.8%) were compared with Caucasian [65], and Danish (n = 152 356) with non-Danish samples (n = 9 927) [66]. Twenty-one studies collected data on PA level and ethnicity, but only 8 considered interactions between ethnicity and PA.
Four studies considered that white participants had a more active lifestyle than black or African-American participants [56, 67-69], but one study demonstrated no significant difference between ethnicities before cervical cancer diagnosis [70]. White cancer survivors were more active than non-white survivors in a Canadian study [47]. Non-Hispanic cancer patients were more engaged in routine PA than were Hispanic patients, specifically during and after treatment [50]. A comparison between Lebanese and American-Lebanese participants for predictors of breast cancer risk showed that the Lebanese-American group exercised more than the Lebanese group [31].
3.3.4 Education: Education was collected in 63 studies, but only 23 examined the relation between education and PA, for a broad range of cancer types. Nine focused on before cancer diagnosis, 3 during treatment, 10 after treatment, and 1 during and after cancer treatment. This variable was the single observed socioeconomic variable in 3 articles [32, 53, 71], 2 demonstrating that people with higher education frequently have a higher chance of meeting PA guidelines [71, 72]. Among the 23 studies analysing PA and socioeconomic status, 15 demonstrated that individuals with high education tended to have a high level of PA or meet PA guidelines, whatever the time of cancer. One study examining factors associated with breast cancer among women before diagnosis showed the reverse association [30], with high education linked to low PA level. Two studies showed no association between education and PA level among cancer survivors [73, 74].
3.3.5 Income: Income was collected in 44 studies, 14 examining the relation between income and PA, for a broad range of cancer types. Seven studies focused on before cancer diagnosis, 1 during treatment, 5 after cancer, and 1 before diagnosis and after cancer treatment. Three studies specifically focused on the cancer time [75-77]. A study examining change in health promotion behaviour among low-income cancer patients with diverse cancer types after diagnosis showed that they engaged in walking and were interested in learning more behaviours [77]. The second study demonstrated that cancer death rate was predicted by the mean income from the US county where patients resided and that this relation was strongly mediated by physical inactivity, accounting for 12% of the percentage mediated in a multivariable model [76]. The third article demonstrated that income did not affect PA level among Brazilian breast cancer patients during treatment [75]. Of the studies analysing income and PA, including other socioeconomic indicators, 8 demonstrated that individuals with high income tended to have a high level of PA or met PA guidelines, whatever the time of cancer (4 before, 1 during, 2 after and 1 before and after treatment) [36, 46, 48, 53, 70, 78-80].
3.3.6 Occupation: Occupation was collected in 41 studies, 14 examining the relation between occupation and PA, for a broad range of cancer types (4 focusing on before cancer diagnosis, 2 during treatment, 7 after cancer treatment, and 1 before cancer diagnosis and after treatment). Seven articles demonstrated that employed people tended to have a high level of PA or met PA guidelines, whatever the time of cancer [41, 44, 48, 53, 73, 81]. Regarding comple-mentary results, an international study of women with breast cancer showed that women who were unemployed had a high global physical inactivity level [30].
Moreover, mother’s employment played a role in women’s physical inactivity, whereas father’s employment seemed not related to physical inactivity [30]. A second study focusing on variations in PA level between before and after cancer diagnosis showed a significant decrease after diagnosis. This change was detected especially among professionally inactive patients for vigorous PA, with no changes in moderate PA or walking [35]. A third study showed that the odds of being unemployed due to health were appro-ximately 2.5-fold greater for inactive skin cancer survivors (i.e., who did not practice any leisure time aerobic activity lasting at least 10 min per week) [82].
3.3.7 Residence: Residence variables were collected in 32 studies, but only 8 (4 before cancer diagnosis and 4 after cancer treatment) examined the relation between residence and PA, for a broad range of cancer types [29, 30, 37, 38, 42, 52, 55, 83]. Four studies solely analysed this variable [29, 37, 38, 83]. A study comparing adherence to cancer prevention guidelines in 18 African countries showed that adherence to PA guidelines ranged from < 3% in Mauritius to 81% in Zambia for women and from < 5% in Mauritius to 84% in Zambia for men [29]. A second study compared the variation in cancer screening participation by geograp-hic area in Australia, showing insufficient exercise more likely for people living in inner regional areas and outer regional areas than in major cities [83]. Another study showed an increase in colorectal cancer mortality due to PA in Brazil (+0.66%), with a decrease observed in the rest of the world (-0.84%) between 1990 and 2015 [37]. Similar results were found for women with breast cancer (+0.77% in Brazil and -2.84% worldwide; [38].
3.3.8 Green space exposure: Green space exposure was collected in 2 studies, as a single studied variable, among populations before cancer diagnosis [60, 84]. A study of the association between green space and skin cancer showed that time spent outdoors and time spent in moderate to vigorous PA was higher among people living than not living in greener areas. As compared with people with 0% to 20% green space, for those with >80% green space, the adjusted odds of skin cancer were 9% higher, with only 1.6% of the association mediated by moderate to vigorous PA [84]. A second study showed an association between the presence of urban green areas and reduced risk of breast cancer but did not observe any mediation by PA level [60].
3.3.9 Marital status: Marital status was collected in 41 studies, only 8 studies examining the relation between marital status and PA, for a broad range of cancer types. All studies demonstrated that marital status did not affect PA level, whatever the time of cancer [40, 44, 63, 64, 70, 80,
85, 86].
3.3.10 Household: Only 6 studies collected indicators related to household [74, 81, 87-91], but no study analysed the association of these variables and PA because they were principally considered control variables.
3.3.11 Social support: Six studies collected indicators related to social support, but only 3 [42, 79, 86] examined the relation between social support and PA, for a broad range of cancer types. These articles demonstrated that having good social support is related to high level of PA or meeting PA guidelines, specifically during treatment [42, 79] and after treatment [86].
3.3.12 Health insurance: In total, 13 studies collected indicators related to insurance, but only one study analysed the association of insurance and PA level and showed a positive relation between access to health care and PA [67].
3.4 Theoretical model
Among the observational studies, only 5 reported using a theoretical model. Models cited were the social deter-minants of health theoretical framework [67], the social cognitive theory-based theory [59, 92, 93], the theory of planned behaviour [45, 59, 94], the population intervention model [95], and the cause of death ensemble model [38].
3.5 Interventions
Among the 90 included studies, 4 were interventional studi-
es, taking place before diagnosis [94] as well as during [93, 96] and after treatment [92] (See Table 3). Three interventions took place in North America, and one in Europe. Two targeted breast cancers (only women) [92, 94], one prostate cancer [93] and the last a broad range of cancer types [96]. Three were randomized controlled trials [92-94] and one was a quasi-experimental trial [96]. The duration of follow-up varied, the shortest intervention lasting 12 weeks [92] and the longest over 1 year [94]. A single theoretical model was used: the social cognitive theory-based model [92].
Intervention strategies were diverse, including a specific training with an average of 200 min of supervised and unsupervised PA per week [94]; encouragement by use of a pedometer provided to count steps and encouragement to walk for at least 30 min per day, completed by a dietary journal [93]; an email intervention using social cognitive theory targeting PA [92]; and a free community-based exercise program including taking part in 30 weeks of individualized aerobic and resistance training with other participants. Among the 4 interventions, 2 considered PA practice as main outcomes [92, 94], and the 2 others focused on cancer-related fatigue or quality of life [96], BMI and body composition [93].
Results of the exercise intervention showed that 8% of the variance for supervised exercise was explained by cancer location and older age. For unsupervised exercise, 21% of the variance was explained by cancer location, a family history of breast cancer and increased vitality. Residence and age played an important role in PA practice among breast cancer patients [94]. Results of an email intervention showed a post-intervention difference in PA between the experimental and control group for self-reported moderate and vigorous PA among breast cancer patients. This study did not analyse the association of a socioeconomic variable with PA to explore the effect of variables, and the authors mentioned in the limitations section the focus on a single ethnicity and type of cancer [92]. For the dietary and PA intervention, 64% of patients provided a log sheet of daily step counts or time spent walking, but precise results on PA increase or decrease were not presented nor analysed by any socioeconomic variable [93]. In a community exercise intervention, PA was considered only as a predictor of cancer-related fatigue or quality of life, with no effect on either outcome [96]. The relation between socioeconomic status and PA was not tested in this intervention.
PA, physical activity; SES, socioeconomic status
Table 1: Characteristics of the included observational studies.
|
Age |
Age, date of birth |
|
Sex |
Sex, sex |
|
Ethnicity |
Ethnic group, ethnic background, race, ethnicity, place of birth, nationality |
|
Education |
Education, education level, university degree, level of education, low educational attainment, educational attainment, formal education at 15 years of age, first language other than English |
|
Income |
Before-tax household income, financial strain, family income, income ranges, low-income, societal factors low family income, annual household income, monthly income, annual household income, economic stability, perceived economic status, household income, monthly income per household unit, poverty income ratio, capita income, household income level, personal income, county-level income, ratio of family income, financial security, income categories, and income adequacy, house net-wealth, perceived economic difficulties |
|
Occupation |
Occupation, occupational status, type of occupation, employment, employment status, type of employment, work status, vocational status, job, professional work intensity, civil service employment grade |
|
Residence |
Area of residence, metropolitan or regional, urban residence, rural residence from postal codes, origin or residence, location, place of residence, place of residency, living area, residential location, living situation, area-level SES, residence, living conditions, home ownership, geographical area lived, postcode, residence (rural-urban), residential area based socio-economic, length at residence in years, country, neighbourhood-level SES |
|
Green space exposure |
Green space exposure, presence of urban green areas, presence of agricultural areas, and surrounding greenness |
|
Marital status |
Marital status, paired relationship, married or cohabitant, partner status |
|
Household |
Household, household size, family size, family structure, number of children, cohabitation status, family burden, household appliances |
|
Social support |
Family support, individual, social factors, perceived discrimination, social support, social environment, social inequality, support group participation, close friends |
|
Health insurance |
Access to health care, insurance status, treatment center, screening center, type of insurance, distance of residence from medical center, insurance coverage, health insurance, social security, care for children |
SES, socioeconomic status
Table 2: Category of socioeconomic variables and indicators used for each variable in included studies.
|
Brunet et al. 2020 [96] |
Courneya et al. 2012 [94] |
Hatchett et al. 2013 [92] |
O’Neill et al. 2015 [93] |
|
|
Date, country, cancer, time of treatment |
2020, Canada, several cancer type, after |
2012, Canada, breast, prevention |
2013, USA, breast, after |
2015, UK, Prostate, during |
|
Study design |
Prospective, quasi-experimental single-group repeated measures design |
Randomized controlled trial |
Randomized controlled trial |
Randomized controlled trial |
|
Population sample |
N: 224 |
N: 160 |
N: 74 |
N: 94 |
|
Age: ≥ 18y |
Age: 50-74 years |
Age: ≥ 18 years |
Age: range not precise |
|
|
Sex: male and female |
Sex: female |
Sex: female |
Sex: men |
|
|
Comparison: no |
Comparison: yes |
Comparison: yes |
Comparison: yes |
|
|
Volunteers adults who enrolled in Wellspring Cancer Exercise Program few years ago |
Postmenopausal women |
Volunteers survivors |
Planned to receive a cancer therapy for at least 6 months |
|
|
By mails, posters and brochures, media campaigns |
By mass email and written letter solicitation |
|||
|
Intervention strategy |
Community exercise program |
Exercise program |
Email program |
Dietary Intake and walking program |
|
PA assessment |
Godin Leisure Time Exercise Questionnaire |
Weekly minutes of total supervised and unsupervised exercise |
7-day physical activity recall questionnaire |
7 Day Physical Act-ivity Recall Questi-onnaire; Phone call to monitor compliance |
|
Results |
Physical activity practice did not affect cancer-related fatigue or quality of life |
Completion of 95% of supervised exerc-ise and 79% of un-supervised exercise |
Time spend in moderate to vigorous PA |
64% of patients provided a log sheet of daily step counts or time spend walking |
|
Theoretical model |
No |
No |
Social cognitive theory-based email evaluated |
No |
Table 3: Interventions for physical activity (PA) among cancer patients.
4. Discussion
The present systematic mapping review analysed the interactions between socio-economic factors and PA among cancer patients. The analysis of the 86 observational and 4 intervention studies showed several gaps in the literature. First, despite the data collection of both socioeconomic factors and PA, only a few studies considered their interactions, and often these variables were not crossed in studies, but their effect on a third variable (quality of life, survival, etc.) was tested separately. In other words, the interactions between PA and socioeconomic variables to predict cancer evolution or related variables were not tested. However, most articles described an exploratory model testing a multivariate association between socio-economic variables, PA and other predictors with cancer-related variables, which prevents an understanding of the complexity of PA practice among cancer patients.
Second, studies focused on a single time of cancer — before diagnosis, during treatment or after cancer treatment — which disallows examining the temporal dynamics in the interactions between socioeconomic factors and PA. Because previous studies have shown a decrease in PA practice from diagnosis to remission [15], studies providing evidence for these temporal patterns and their predictors are of primary importance for developing effective and tailored interventions. Third, the diversity of indicators to evaluate socioeconomic factors [18] and lack of definitions thereof are major weaknesses in comparing studies. The indicators varied among education, residence, health insurance, and marital status, and the measurements used among these categories also varied (e.g., country, town, and living area were considered variables in the residence category), which led to a high number of scales or classifications used. The authors’ classification of the variables as sociodemographic or socioeconomic or demographic did not help to identify them. Some authors considered this diversity by using a sociodemographic index (i.e., aggregation of scores on different socioeconomic variables), but no consistency was found across studies to calculate such an index, which led to even more variability in the measurement.
Fourth, the paucity of interventions for collecting and analysing socioeconomic factors reveal the difficulty in taking these variables into account when offering programs. Moreover, the use of PA to reduce social inequalities, as shown in a previous intervention for obese adolescents, has not been investigated [97]. Fifth, the lack of a theoretical model used in the observational study disallowed the ability to model and understand the interactions between the studied variables [25]. Sixth, the results, to be interpreted with caution with regard to the low number of studies in each socioeconomic category, demonstrated more similar patterns of association than in the general population, which questions the recurrence of the association patterns between socioeconomic factors and PA among vulnerable populations [18]. Different limitations to this study must be mentioned. First, studies involving a specific sport (i.e., yoga) and not measuring PA practice were not included, which limits the identification of evidence based on specific activities offered to cancer patients. Second, the review does not assess the quality of the studies but rather focuses on gaps in the literature. Third, we were not able to conceptualise a model of interactions between socio-economic factors and PA among cancer patients because of lack of a theoretical model and a path model tested in the literature as well as the diversity of measurement.
5. Conclusion
The identification of interactions between socioeconomic status and PA among cancer patients is at its early stage. The clarification of the definition of socioeconomic factors as well as the variables included in their measurement is highly necessary. Consistency in PA measurement, with use of a validated measurement tool, is needed to move the field forward. Despite finding 90 studies measuring PA behaviour and socioeconomic factors, few studies tested the association between these variables. In addition, the analysis of temporal patterns of PA at all times of cancer by socioeconomic factors is key to the development of an intervention theory adapted to patient profiles because patient compliance with PA post-diagnosis has been identified as weak.
Funding
This project was funded by the French Cancer League: “Projet de Recherche en Sciences Humaines et Sociales Ligue Contre le Cancer” and by the Grand-Est Region/Pole Biology-Medicine-Health of the University of Lorraine.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68 (2018): 394-424.
- Barbaric M, Brooks E, Moore L, et al. Effects of physical activity on cancer survival: A systematic review. Physiother Canada 62 (2010): 25-34.
- Caspersen CJ, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep (1985): 126-131.
- Des Guetz G, Uzzan B, Bouillet T, et al. Impact of physical activity on cancer-specific and overall survival of patients with colorectal cancer. Gastroenterol Res Pract 2013 (2013).
- Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med Oncol 28 (2011): 753-765.
- Lahart IM, Metsios GS, Nevill AM, et al. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol (Madr) 54 (2015): 635-654.
- Foucaut AM, Berthouze-Aranda SE, Touillaud M, et al. Reduction of health risk factors through an adapted physical activity program in patients with breast cancer. Support Care Cancer 22 (2014): 1097-1104.
- Touillaud M, Foucaut AM, Berthouze SE, et al. Design of a randomised controlled trial of adapted physical activity during adjuvant treatment for localised breast cancer: The PASAPAS feasibility study. BMJ Open 3 (2013).
- Brown JC, Winters-Stone K, Lee A, et al. Cancer, physical activity, and exercise. Compr Physiol 2 (2012): 2775-2809.
- Craft LL, VanIterson EH, Helenowski IB, et al. Exercise effects on depressive symptoms in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 21 (2012): 3-19.
- Speck RM, Courneya KS, Mâsse LC, et al. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J Cancer Surviv 4 (2010): 87-100.
- Buffart LM, Galvão DA, Brug J, et al. Evidence-based physical activity guidelines for cancer survivors: Current guidelines, knowledge gaps and future research directions. Cancer Treat Rev 40 (2014): 327-340.
- Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med 3 (1997): 215-226.
- Midtgaard J, Baadsgaard MT, Møller T, et al. Self-reported physical activity behaviour; exercise motivation and information among Danish adult cancer patients undergoing chemotherapy. Eur J Oncol Nurs 13 (2009): 116-121.
- Blanchard CM, Denniston MM, Baker F, et al. Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav 27 (2003): 246-256.
- Bauman AE, Reis RS, Sallis JF, et al. Correlates of physical activity: why are some people physically active and others not? Lancet 380 (2012): 258-271.
- Gidlow C, Johnston LH, Crone D, et al. A systematic review of the relationship between socio-economic position and physical activity. Health Educ J 65 (2006): 338-367.
- O’Donoghue G, Kennedy A, Puggina A, et al. Socio-economic determinants of physical activity across the life course: A “DEterminants of DIet and Physical ACtivity” (DEDIPAC) umbrella literature review. PLoS One 13 (2018).
- Duncan GJ, Daly MC, McDonough P, et al. Optimal indicators of socioeconomic status for health research. Am J Public Health 92 (2002): 1151-1157.
- Lee PR, Moss N, Krieger N. Measuring Inequalities in Health: Introduction. Public Health Rep 110 (1995): 302-305.
- Underhill ML, Habin KR, Shannon KM. Perceptions of Cancer Risk, Cause, and Needs in Participants from Low Socioeconomic Background at Risk for Hereditary Cancer. Behav Med 43 (2017): 259-267.
- Beenackers MA, Kamphuis CBM, Giskes K, et al. Socioeconomic inequalities in occupational, leisure-time, and transport related physical activity among European adults: A systematic review. Int J Behav Nutr Phys Act 9 (2012).
- Lund Nilsen T, Johnsen R, Vatten LJ. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br J Cancer 82 (2000): 1358-1363.
- Johansson LM, Lingfors H, Golsäter M, et al. Can physical activity compensate for low socioeconomic status with regard to poor self-rated health and low quality-of-life? Health Qual Life Outcomes 17 (2019): 33.
- Rothman AJ. Is there nothing more practical than a good theory?: Why innovations and advances in health behavior change will arise if interventions are used to test and refine theory. Int J Behav Nutr Phys Act 1 (2004): 1-7.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6 (2009).
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Heal Inf Libr J 26 (2009): 91-108.
- Ancellin R, Gaillot-de Saintignon J. Bénéfices de l’activité physique pendant et après cancer: des connaissances scientifiques aux repères pratiques. Oncologie 19 (2017): 95-107.
- Akinyemiju TF, McDonald JA, Tsui J, et al. Adherence to cancer prevention guidelines in 18 African countries. PLoS One 9 (2014).
- Akinyemiju T, Ogunsina K, Okwali M, et al. Lifecourse socioeconomic status and cancer-related risk factors: Analysis of the WHO study on global ageing and adult health (SAGE). Int J Cancer 140 (2017): 777-787.
- Badr LK, Bourdeanu L, Alatrash M, et al. Breast cancer risk factors: A cross- cultural comparison between the west and the east. Asian Pacific J Cancer Prev 19 (2018): 2109-2116.
- Cirera L, Huerta JM, Chirlaque, et al. Socioeconomic effect of education on pancreatic cancer risk in western Europe: An update on the EPIC cohorts study. Cancer Epidemiol Biomarkers Prev 28 (2019): 1089-1092.
- Bock C, Schmidt ME, Vrieling A, et al. Walking, bicycling, and sports in postmenopausal breast cancer survivors - Results from a German patient cohort study. Psychooncology 22 (2013): 1291-1298.
- Fassier P, Zelek L, Bachmann P, et al. Sociodemographic and economic factors are associated with weight gain between before and after cancer diagnosis: Results from the prospective population-based NutriNet-Santé cohort. Onco-target 8 (2017): 54640-54653.
- Fassier P, Zelek L, Partula V, et al. Variations of physical activity and sedentary behavior between before and after cancer diagnosis: Results from the prospective population-based NutriNet-Santé cohort. Med (United States) 95 (2016).
- Khadanga S, Lakoski SG, Hart V, et al. Partnership status and socioeconomic factors in relation to health behavior changes after a diagnosis of ductal carcinoma In Situ. Cancer Epidemiol Biomarkers Prev 25 (2016): 76-82.
- Silva DAS, Tremblay MS, De Souza M de FM, et al. Mortality and years of life lost by colorectal cancer attributable to physical inactivity in Brazil (1990–2015): findings from the global burden of disease study. Beiki O, ed. PLoS One 13 (2018): e0190943.
- Silva DAS, Tremblay MS, Souza M de FM de, et al. Mortality and years of life lost due to breast cancer attributable to physical inactivity in the Brazilian female population (1990–2015). Sci Rep 8 (2018): 11141.
- Batty GD, Kivimäki M, Clarke R, et al. Modifiable risk factors for prostate cancer mortality in London: Forty years of follow-up in the Whitehall study. Cancer Causes Control 22 (2011): 311-318.
- Chipperfield K, Fletcher J, Millar J, et al. Factors associated with adherence to physical activity guidelines in patients with prostate cancer. Psychooncology 22 (2013): 2478-2486.
- Harrington JM, Schwenke DC, Epstein DR. Exercise preferences among men with prostate cancer receiving androgen-deprivation therapy. Oncol Nurs Forum 40 (2013).
- Hughes S, Egger S, Carle C, et al. Factors associated with the use of diet and the use of exercise for prostate cancer by long-term survivors. PLoS One 14 (2019).
- Andrykowski MA. Physical and mental health status and health behaviors in male breast cancer survivors: A national, population-based, case-control study. Psychooncology 21 (2012): 927-934.
- Stevinson C, Lydon A, Amir Z. Adherence to physical activity guidelines among cancer support group participants. Eur J Cancer Care (Engl) 23 (2014): 199-205.
- Tabaczynski A, Strom DA, Wong JN, et al. Demographic, medical, social-cognitive, and envi-ronmental correlates of meeting independent and combined physical activity guidelines in kidney cancer survivors. Support Care Cancer 28 (2020): 43-54.
- Philip EJ, Coups EJ, Feinstein MB, et al. Patient-provider discussion of physical activity among early stage lung cancer survivors. Psychooncology 24 (2015): 359-362.
- Naik H, Qiu X, Brown MC, et al. Socioeconomic status and lifestyle behaviours in cancer survivors: Smoking and physical activity. Curr Oncol 23 (2016): e546-e555.
- Aparicio-Ting FE, Friedenreich CM, Kopciuk KA, et al. Prevalence of meeting physical activity guidelines for cancer prevention in Alberta. Chronic Dis Inj Can 32 (2012): 216-226.
- Aparicio-Ting FE, Friedenreich CM, Kopciuk KA, et al. Intrapersonal and social environment correlates of leisure-time physical activity for cancer prevention: A cross-sectional study among canadian adults. J Phys Act Heal 11 (2014): 790-800.
- Diorio C, Lin M, Ginn E, et al. Psychosocial determinants of physical activity and dietary behaviors in adolescents and young adults with cancer and survivors. Pediatr Blood Cancer 65 (2018).
- Owusu C, Antognoli E, Nock N, et al. Perspective of older African-American and Non-Hispanic white breast cancer survivors from diverse socioeconomic backgrounds toward physical activity: A qualitative study. J Geriatr Oncol 9 (2018): 235-242.
- Ishii K, Shibata A, Oka K. Identifying environmental, social, and psychological correlates of meeting the recommended physical activity levels for colon cancer prevention among Japanese adults. J Sci Med Sport 16 (2013): 520-525.
- Ishii K, Shibata A, Oka K. Meeting physical activity recommendations for colon cancer prevention among Japanese adults: Prevalence and sociodemographic correlates. J Phys Act Heal 8 (2011): 907-915.
- Santos-Lozano A, Ramos J, Alvarez-Bustos A, et al. Cardiorespiratory fitness and adiposity in breast cancer survivors: is meeting current physical activity recommendations really enough?. Support Care Cancer 26 (2018): 2293-2301.
- Adams RJ, Piantadosi C, Ettridge K, et al. Functional health literacy mediates the relationship between socio-economic status, perceptions and lifestyle behaviors related to cancer risk in an Australian population. Patient Educ Couns 91 (2013): 206-212.
- Akinyemiju T, Moore JX, Pisu M. Mediating effects of cancer risk factors on the association between race and cancer incidence: analysis of the NIH-AARP Diet and Health Study. Ann Epidemiol 28 (2018): 33-40.e2.
- Bersvendsen HS, Haugnes HS, Fagerli UM, et al. Lifestyle behavior among lymphoma survivors after high-dose therapy with autologous hematopoietic stem cell transplantation, assessed by patient-reported outcomes. Acta Oncol (Madr) 58 (2019): 690-699.
- Kaul S, Avila JC, Jupiter D, et al. Modifiable health-related factors (Smoking, physical activity and body mass index) and health care use and costs among adult cancer survivors. J Cancer Res Clin Oncol 143 (2017): 2469-2480.
- Lowe SS, Watanabe SM, Baracos VE, et al. Determinants of physical activity in palliative cancer patients: An application of the theory of planned behavior. J Support Oncol 10 (2012): 30-36.
- O’Callaghan-Gordo C, Kogevinas M, Cirach M, et al. Residential proximity to green spaces and breast cancer risk: The multicase-control study in Spain (MCC-Spain). Int J Hyg Environ Health 221 (2018): 1097-1106.
- Park SH, Strauss SM. Similarities and differences in the correlates of comorbidities in US male and female adult cancer survivors. Public Health Nurs 36 (2019): 478-487.
- Moskowitz MC, Feuerstein M, Todd BL. Job stress and physical activity related to elevated symptom clusters in breast cancer survivors at work. J Occup Environ Med 55 (2013): 93-98.
- Moss JL, Xiao Q, Matthews CE. Patterns of cancer-related health behaviors among middle-aged and older adults: Individual and area-level socioecono-mic disparities. Prev Med (Baltim) 115 (2008): 31-
- Petkeviciene J, Ivanauskiene R, Klumbiene J. Sociodemographic and lifestyle determinants of non-attendance for cervical cancer screening in Lithuania, 2006–2014. Public Health 156 (2018): 79-86.
- Lewis C, Xun P, He K. Physical activity in relation to quality of life in newly diagnosed colon cancer patients: a 24-month follow-up. Qual Life Res 23 (2014): 2235-2246.
- Friis K, Larsen FB, Nielsen C V., Momsen AMH, Stapelfeldt CM. Social inequality in cancer survivors’ health behaviours—A Danish popula-tion-based study. Eur J Cancer Care (Engl) 27 (2018): e12840.
- Asare M, McIntosh S, Culakova E, et al. Assessing Physical Activity Behavior of Cancer Survivors by Race and Social Determinants of Health. Int Q Community Health Educ 40 (2019): 7-16.
- Akinyemiju T, Wiener H, Pisu M. Cancer-related risk factors and incidence of major cancers by race, gender and region; analysis of the NIH-AARP diet and health study. BMC Cancer 17 (2017): 597.
- Hair BY, Hayes S, Tse CK, et al. Racial differences in physical activity among breast cancer survivors: Implications for breast cancer care. Cancer 120 (2014): 2174-2182.
- Rawl SM, Dickinson S, Lee JL, et al. Racial and socioeconomic disparities in cancer-related know-ledge, beliefs, and behaviors in Indiana. Cancer Epidemiol Biomarkers Prev 28 (2019): 462-470.
- Aarts MJ, Kamphuis CBM, Louwman MJ, et al. Educational inequalities in cancer survival: A role for comorbidities and health behaviours? J Epidemiol Community Health 67 (2013): 365-373.
- Hvidtfeldt UA, Lange T, Andersen I, et al. Educational Differences in Postmenopausal Breast Cancer - Quantifying Indirect Effects through Health Behaviors, Body Mass Index and Reproductive Patterns. PLoS One 8 (2013).
- Stalsberg R, Eikemo TA, Lundgren S, et al. Physical activity in long-term breast cancer survivors – A mixed-methods approach. Breast 46 (2019): 126-135.
- Berry NM, Miller, Woodman RJ, et al. Differences in chronic conditions and lifestyle behaviour between people with a history of cancer and matched controls. Med J Aust 201 (2014): 96-100.
- Pena GG, Maia YCP, Mendes MCS, et al. Physical activity is associated with malignant and benign breast diseases in low-income Brazilian women. Nutr Cancer 66 (2014): 707-715.
- O’Connor JM, Sedghi T, Dhodapkar M, et al. Factors Associated With Cancer Disparities Among Low-, Medium-, and High-Income US Counties. JAMA Netw open 1 (2018): e183146.
- Meraviglia MG, Stuifbergen A. Health-promoting behaviors of low-income cancer survivors. Clin Nurse Spec 25 (2011): 118-124.
- Amuta AO, Mkuu RS, Jacobs W, et al. Influence of Cancer Worry on Four Cancer Related Health Protective Behaviors among a Nationally Representative Sample: Implications for Health Promotion Efforts. J Cancer Educ 33 (2018): 1002-1010.
- Anderson RT, Peres LC, Camacho F, et al. Individual, social, and societal correlates of health-related quality of life among African American survivors of ovarian cancer: Results from the African American cancer epidemiology study. J Women’s Heal 28 (2019): 284-293.
- Smith SA, Ansa BE, Yoo W, et al. Determinants of adherence to physical activity guidelines among overweight and obese African American breast cancer survivors: Implications for an intervention approach. Ethn Heal 23 (2018): 194-206.
- Shah IA, Bhat GA, Rafiq R, et al. Strenuous occupational physical activity: Potential association with esophageal squamous cell carcinoma risk. Proc Singapore Healthc 28 (2019): 232-242.
- Weaver KE, Palmer N, Lu L, et al. Rural-urban differences in health behaviors and implications for health status among US cancer survivors. Cancer Causes Control 24 (2013): 1481-1490.
- Goodwin BC, Rowe AK, Crawford-williams F, et al. Geographical disparities in screening and cancer- related health behaviour. Int J Environ Res Public Health 17 (2020).
- Astell-Burt T, Feng X, Kolt GS. Neighbourhood green space and the odds of having skin cancer: Multilevel evidence of survey data from 267 072 Australians. J Epidemiol Community Health 68 (2014): 370-374.
- Noonan D, Dardas L, Bice-Wigington T, et al. Understanding Multiple Behavioral Risk Factors for Cancer in Rural Women. Public Health Nurs 33 (2016): 519-528.
- Ahmed AE, Almuzaini AS, Alsadhan MA, et al. Health-Related Predictors of Quality of Life in Cancer Patients in Saudi Arabia. J Cancer Educ 33 (2018): 1011-1019.
- Alazzeh AY, Azzeh FS. Active lifestyle patterns reduce the risk of colorectal cancer in the Mecca region, Saudi Arabia: A case-control study. Eur J Cancer Prev 27 (2018): 438-442.
- Dianatinasab M, Mohammadianpanah M, Daneshi N, et al. Socioeconomic Factors, Health Behavior, and Late-Stage Diagnosis of Breast Cancer: Considering the Impact of Delay in Diagnosis. Clin Breast Cancer 18 (2018): 239-245.
- Doubeni CA, Major JM, Laiyemo AO, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst 104 (2012): 1353-1362.
- Howard R, Scheiner A, Kanetsky PA, et al. Sociodemographic and lifestyle factors associated with the neutrophil-to-lymphocyte ratio. Ann Epidemiol 38 (2019): 11-21.e6.
- Peiró-Pérez R, Salas D, Vallés G, et al. Walking, biking or sport: How Spanish women attending breast cancer screening meet physical activity recommendations? Eur J Public Health 25 (2015): 857-863.
- Hatchett A, Hallam JS, Ford MA. Evaluation of a social cognitive theory-based email intervention designed to influence the physical activity of survivors of breast cancer. Psychooncology 22 (2013): 829-836.
- O’Neill RF, Haseen F, Murray LJ, et al. A randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer. J Cancer Surviv 9 (2015): 431-440.
- Courneya KS, Karvinen KH, McNeely ML, et al. Predictors of adherence to supervised and unsupervised exercise in the alberta physical activity and breast cancer prevention trial. J Phys Act Heal 9 (2012): 857-866.
- Schootman M, Deshpande AD, Pruitt S, et al. Estimated Effects of Potential Interventions to Prevent Decreases in Self-Rated Health Among Breast Cancer Survivors. Ann Epidemiol 22 (2012): 79-86.
- Brunet J, Howell D, Au D, et al. Predictors of cancer survivors’ response to a community-based exercise program. Psychol Sport Exerc 47 (2020): 101529.
- Briançon S, Legrand K, Muller L, et al. Effectiveness of a socially adapted intervention in reducing social inequalities in adolescence weight. The PRALIMAP-INÈS school-based mixed trial. Int J Obes 44 (2020): 895-907.
- Advani PS, Reitzel LR, Nguyen NT, et al. Financial strain and cancer risk behaviors among African Americans. Cancer Epidemiol Biomarkers Prev 23 (2014): 967-975.
- AlSaeed EF, Tunio MA. Diet, Physical Activity, Marital Status and Risk of Cancer: A Case Control Study of Adults from Riyadh, Saudi Arabia. Gulf J Oncolog 1 (2017): 6-10.
- Andersen SW, Blot WJ, Shu XO, et al. Adherence to cancer prevention guidelines and cancer risk in low-income and african American populations. Cancer Epidemiol Biomarkers Prev 25 (2016): 846-853.
- Azevêdo IG, Carneiro ICLM, Tomiya MTO, et al. Gastric cancer and associated factors in hospitalized patients. Nutr Hosp 32 (2015): 283-290.
- Bifulco G, De Rosa N, Tornesello ML, et al. Quality of life, lifestyle behavior and employment experience: A comparison between young and midlife survivors of gynecology early stage cancers. Gynecol Oncol 124 (2012): 444-451.
- Chatterjee S, Chattopadhyay A, Levine PH. Between-ward disparities in colorectal cancer incidence and screening in Washington DC. J Epidemiol Glob Health 5 (2015): S1-S9.
- Chouhdari A, Yavari P, Pourhoseingholi MA, et al. The relationship between lifestyle and compliance with colonoscopy in first-degree relatives of patients with colorectal cancer. Int J Cancer Manag 12 (2019).
- Ekenga CC, Parks CG, Sandler DP. A prospective study of occupational physical activity and breast cancer risk. Cancer Causes Control 26 (2015): 1779-1789.
- Gunes-Bayir A, Kiziltan HS, Sentürk N, et al. A Pilot Study of Self-Reported Physical Activity and Eating Habits in Turkish Cancer Patients under Chemotherapy. Nutr Cancer 67 (2015): 906-911.
- Hang J, Cai B, Xue P, et al. The Joint effects of lifestyle factors and comorbidities on the risk of colorectal cancer: A large Chinese retrospective case-control study. PLoS One 10 (2015).
- Hastert TA, Ruterbusch JJ, Beresford SAA, et al. Contribution of health behaviors to the association between area-level socioeconomic status and cancer mortality. Soc Sci Med 148 (2016): 52-58.
- Inumaru LE, Irineu Gomes Duarte Quintanilha M, Aparecida Da Silveira É, et al. Risk and protective factors for breast cancer in Midwest of Brazil. J Environ Public Health 2012 (2012).
- Johannsen M, Christensen S, Zachariae R, et al. Socio-demographic, treatment-related, and health behavioral predictors of persistent pain 15 months and 7–9 years after surgery: a nationwide prospective study of women treated for primary breast cancer. Breast Cancer Res Treat 152 (2015): 645-658.
- Keegan THM, Shariff-Marco S, Sangaramoorthy M, et al. Neighborhood influences on recreational physical activity and survival after breast cancer. Cancer Causes Control 25 (2014): 1295-1308.
- Kim D, Masyn KE, Kawachi I, et al. Neighborhood socioeconomic status and behavioral pathways to risks of colon and rectal cancer in women. Cancer 116 (2010): 4187-4196.
- Kouloulias V, Platoni K, Kantzou I, et al. Physical activity, early first delivery and residence as parameters for breast cancer prevention: An observational study. J BUON 24 (2019): 1512-1515.
- Skrzypczak M, Czerniak U, ?ski P. Selected elements of socio-demographic status and lifestyle as factors determining subjective assessment of life in women after mastectomy. Wspolczesna Onkol 16 (2012): 569-575.
- Sözmen K, Unal B, Sakarya S, et al. Determinants of Breast and Cervical Cancer Screening Uptake among Women in Turkey. Asia-Pacific J Public Heal 28 (2016): 528-538.
- Venturelli F, Sampaolo L, Carrozzi G, et al. Associations between cervical, breast and colorectal cancer screening uptake, chronic diseases and health-related behaviours: Data from the Italian PASSI nationwide surveillance. Prev Med (Baltim) 120 (2019): 60-70.
- Vidrine JI, Stewart DW, Stuyck SC, et al. Lifestyle and cancer prevention in women: Knowledge, perceptions, and compliance with recommended guidelines. J Women’s Heal 22 (2013): 487-493.
- Wang L, Wang K, Liu X, et al. Association of education & lifestyle factors with the perception of genetic knowledge on the development of lung cancer. Indian J Med Res Suppl 143 (2016): 32-37.
- Wiedemann A, Wood AD, Luben RN, et al. Dimension of pain-related quality of life and self-reported mental health in men and women of the European Prospective Investigation into Cancer–Norfolk cohort: a population-based cross-sectional study. Br J Pain 12 (2018): 35-46.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 