Development of Nomogram for Predicting Axillary Pathologic Complete Response after Neoadjuvant Therapy in Breast Cancer Patients without Distant Metastasis
Jian Zhang1†, Hong-Ming Cao1†, Gao-Yuan Wang2, Chang-Bo Nie3, Shou-Min Bai4,5, Shuang Ma5*
1Department of Radiation Oncology, Shenshan Medical Center, Memorial Hospital Of Sun Yat-sen University, Shanwei, 516600, People’s Republic of China
2Department of Radiation Oncology, Sun Yat-sen University Cancer Center, the State Key Laboratory of On-cology in South China, Collaborative Innovation Center for Cancer Medicine, Center
for Precision Medicine of Sun Yat-sen University, Guangzhou, 510120, People’s Republic of China
3School of Medical and Life Sciences, Chengdu University of TCM, Chengdu, 610000, People’s Republic of China
4Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 510120, People’s Republic of China
5Department of Radiation Oncology, Sun Yat- sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 510120, People’s Republic of China
†These authors contributed equally to this work
*Corresponding author:Shuang Ma, Department of Radiation Oncology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 510120, People’s Republic of China.
Received: December 14, 2023; Accepted: January 02, 2024; Published: January 10, 2024
Article Information
Citation:
Jian Zhang, Hong-Ming Cao, Gao-Yuan Wang, Chang-Bo Nie, Shou-Min Bai, Shuang Ma. Development of Nomogram for Predicting Axillary Pathologic Complete Response after Neoadjuvant Therapy in Breast Can-cer Patients without Distant Metastasis. Journal of Cancer Science and Clinical Therapeutics 8 (2024): 47-58.
View / Download Pdf Share at FacebookAbstract
Background: For N+ breast cancer patients treated with neoadjuvant therapy, the response to the treatment, especially the probability of axillary pathological complete response (apCR), can guide the choice of subsequent surgical strategy.
Method: 50 N+ breast cancer patients were treated with neoadjuvant therapy, with the response to neoadjuvant therapy guiding subsequent surgical modalities. Logistic regression was used to calculate the coefficients of the significant predictors for axillary pathologic complete response (apCR), and a nomogram was developed based on the logistic model and internally validated.
Results: 4 variables were found to be related to the probability of apCR: pathological grade and molecular subtype (HER2+), neu-trophil-to-lymphocyte ratio (NLR), and monocyte-to-lymphocyte ratio (MLR). The nomogram based predictive cooperating pathological features and hematological test results can be used to predict apCR in N+ breast cancer patients who had received neoadjuvant chemotherapy (NAC). The receiver operating characteristic (ROC) curve for the nomogram model is 0.929 [95% confi-dence interval (CI): 0.859–0.998], indicating a good discrimination.
Conclusion: A comprehen-sive predictive model using clinical data is a useful tool to predict the probability of apCR in N+ breast cancer patients receiving NAC.
Keywords
<p>Breast cancer, Neoadjuvant therapy, Axillary lymph node, Axillary pathologic Complete response, Nomogram.</p>
Article Details
Introduction
In recent years, the incidence of breast cancer has increased by 0.5% per year [1]. In addition, female breast cancer (11.7%) is the most commonly diagnosed cancer, over-taking lung cancer (11.4%), according to data from Global Cancer Statistics 2020 [2]. With the help of the integrated multidisciplinary comprehensive model of diagnosis and care, breast cancer treatment has obviously favourable clinical effects, and the 5-year overall survival (OS) rate can reach 80%. The current research direction is mainly to improve the quality of life of patients without affecting their survival. Neo- adjuvant chemotherapy can result in significant down staging among breast cancer patients [3]. The effect of the treatment can also help the clinicians to determine the postoperative adjuvant therapy plan. Consequently, it is increasingly applied to patients with axillary lymph node metastasis and some HER-2 positive or (triple negative breast cancer) TNBC patients,
especially those with high tumor burden who are HER-2 positive or TNBC patients. For patients who were confirmed to have N+ nodes at initial diagnosis, axillary lymph node dissection is still the standard operation scheme after neo- adjuvant treat-ment, especially in patients with cN2-3. However, complications (e.g. lymphedema, numbness of upper limbs, and limited joint activity) often occur after axillary lymph node dissection (ALND) ,which seriously affects the quality of life of patients. For pa-tients with cN1 and axillary pathologic complete response (apCR) after neoadjuvant therapy , there is no uniform standard for whether the subsequent axillary cavity can be exempted from ALND or axillary radiation therapy after neoadjuvant therapy.Due to the false negative rate (FNR) of SLNB, the Z1071 study [4] and SENTINA study [5] found that the false negative rate was significantly reduced when using combined tracer to detect at least 3 sentinel lymph nodes .Presently, a maximum FNR of 10% is considered acceptable in studies of early breast cancer [6]. NSABP B32 trial [7] found that patients with negative sentinel lymph nodes, even if they had a 9.8% axillary false-negative rate, did not affect survival. The FNR of sentinel biopsy further significantly decreased in patients who were more likely to achieve complete lymph node pathological remission after neo-adjuvant therapy. If clinicians can accurately predict the lymph node remission status after the neo-adjuvant therapy in advance, the axillary lymph node dissection can be avoided if the initial N+ patients turn negative after the neo-adjuvant therapy. Histopathological grade and molecular subtype (HER2 status) have been proved to be able to predict apCR after neoadjuvant therapy [8]. Nowadays, new immunotherapy techniques and drugs continue to be developed rapidly. At the same time, biomarkers of immune inflamma-tion, such as neutrophils, monocytes and lymphocytes, are also widely studied, as they were simple, convenient and cheap immune inflammatory indicators. For instance, there is some evidence indicating that neutrophil-to-lymphocyte ratio (NLR) and monocyte- to-lymphocyte ratio (MLR) have prognostic value in various solid tumors [9, 10]. However, few previous studies explored whether these indicators can be applied to predicting lymph node status after neoadjuvant therapy. This study is aimed at exploring the predictive value of some common clinical indicators and hematological indicators for lymph node status after neoadjuvant therapy.
Materials and Methods
Inclusion and Exclusion Criteria
This retrospective cohort study was carried out in women who were diagnosed with breast invasive ductal carcinoma at Sun Yat-sen Memorial hospital between 2021 and 2022. The demographic and clinicopathological data of breast cancer patients, such as name, age, personal medical history, and information related to diagnosis and treatment of breast
cancer, were manually collected from the patient records of Sun Yat-sen Memorial Hospital. All patients had undergone thorough radiological examinations prior to neo-adjuvant treatment. If enlarged lymph nodes were clinically detected with the help of medical image, e.g. MRI, B ultrasound, CT images, and cytological examination of lymph node puncture would be recommended. According to the guidelines and specifications for diagnosis and treatment of breast cancer of Chinese Society of Clinical Oncology (CSCO) [11], women will be considered as menopausal if they meet any of the following criteria:
- Bilateral oophorectomy;
- Age ≥ 60 years, or age <60 years and
a) with amenorrhea occurring naturally after 12 or more months in the absence of chemotherapy, tamoxifen, and toremifene, or ovarian suppression and follicle stimulating hormone (FSH) and estradiol meeting the postmenopausal criteria, or
b) With taking tamoxifen or toremifene, and FSH and plasma estradiol levels of two consecutive measurements meeting post-menopausal criteria.
Before neoadjuvant treatment, the clinical T/N/M stage was obtained on the radiologic imaging according to the eighth edition AJCC cancer staging manual [12]. Histopathological grading was obtained from postoperative specimens after neoadjuvant therapy in accordance with the World Health Organisation (WHO) classification guide of breast tumors [13]. The pathologic information, including tumor histology, ER, PR, Ki67%, and HER2 was obtained on the extracted biopsies from the site of tumor under ultrasound guidance before the neoadjuvant treatment based on American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines updated version [14, 15]. More specifically, samples with 1% to 100% immunohistochemistry (IHC) staining of tumor nuclei positive for ER or progesterone receptor (PgR) are interpreted as positive. HER-2 positive samples should meet the following criteria:
- IHC result is 3+, or
- IHC result is 2+, and a) HER2/CEP17 ratio ≥ 2.0 and HER2 gene copy number ≥ 4.0, or, b) HER2/CEP17 ratio<2.0 and HER2 gene copy number ≥ 6.0.
The standard of apCR was that no lymph node metastasis was detected in the pathological results after neoadjuvant therapy. Hematological indicators include neutrophil count, lymphocyte count, and monocyte count were also collected before the first course of neoadjuvant therapy. Subsequently, NLR, and MLR were calculated.
Patients must have met all of the following inclusion criteria to be eligible for participation in this study.
- N Stage: N1/N2/N3 (axillary lymph node metastasis).
- Treatment: standard neoadjuvant therapy, and surgery after the completion of full course of neoadjuvant therapy. Neoadjuvant therapy comprised chemotherapy and targeted therapy. Common chemotherapy drugs included paclitaxel and Tumor-targeted therapy used one or both of the following drugs: trastuzumab and patuozumab.
All patients who 1) had no lymph node metastasis,
2) had pathologically negative lymph nodes, 3) had distant metastases; 4) had not completed a full course of chemotherapy, 5) had not received neoadjuvant treatment, 6) had distant metastasis (M1) prior to neoadjuvant treatment,
7) had inflammatory breast cancer, or 8) had preoperative axillary surgery or radiotherapy were excluded. In addition, the patients whose metastases were detected, operation time was delayed, or postoperative lymph node status, preoperative hematological indicators, or other clinical data were not clearly recorded were also eliminated. The end point used in this study was whether axillary lymph nodes were completely relieved after neoadjuvant therapy.
Statistical Analysis
Because the relationship between a variable and an outcome is not linear in general, this analysis transformed continuous variables (e.g. Age, Ki67, MLR and NLR) into categorical variables. The optimal cut-off was determined using Yoden’s index: maximum [sensitivity – (1-specificity)]. Then, differences in categorial variables between apCR or non-apCR groups were assessed using Pearson’s chi-square test and Fisher’s exact test when having expected number less than 1. Factors included in the model were selected based on the results of univariate descriptive statistical analysis. Multivariable logistic regression analysis with bi-directional stepwise method was done to detect the presence of possible predictors of apCR status. The odds ratios from multivariable logistic regression models were used to evaluate relative risks. The variables showing significance in the multivariable regression analysis were used to develop a nomogram-based model for predicting apCR status after neoadjuvant treatment in breast cancer patients without distant metastasis (M0). The area under the curve (AUC) of ROC is a discrimination measure which represents the ability of the model to assign higher probability of apCR patients than non-apCR patients [16]. The yield values were from 0.5 (no predictive power) to 1.0 (perfect prediction). A calibration plot represents the degree of agreement between the observed and predicted apCR. A calibration curve on the 45° diagonal line indicates perfect agreement. In addition, the false-negative means that achieving apCR patients are treated as non-apCR patients. The false-positive means that a non-apCR patient is considered to have achieved apCR. So, false-negative is less harmful than the false-positive. In decision curve analysis (DCA), the relative value of benefits of using the predictive model, the all model (all of the patients achieved apCR after neoadjuvant therapy), and the none model (none of the patients achieved apCR after neoadjuvant therapy) to predict outcomes against different threshold probability was plotted. A low-risk threshold probability might indicate that patients achieved non-apCR, so receiving follow-up treatment. In addition, calibration curve, receiver ROC curve, and decision curve analysis (DCA) based on 1,000 bootstrap samples were also plotted for the purpose of internally validating. All statistical analyzes were performed using R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). A p-value < 0.05 was considered statistically significant. 95% confidence interval (CI) was adopted for odds ratio (OR) and AUC. An AUC value > 0.7 is considered to have good discrimination.
Results
Univariate Descriptive Statistical Analysis
A total of 50 participants who met the Inclusion and Exclusion Criteria were included in this study. Demographic and clinical characteristics of the study population according to apCR status are shown in Table 1. The whole population’s mean age was 45.94 [SD 10.03] years old. apCR was achieved in 26 of 50 (52%). There are significant differences in pathological grade, HER2 status, NLR and LNR between apCR group and non-apCR group.
For further exploration for these variables as possible independent predictors of apCR status in breast cancer patients without distant metastasis (M0) who have received neoadjuvant treatment, multiple binary logistic regression with bi-directional stepwise method was done. In the final model, pathological grade, HER2 status, NLR, and LNR remained significantly associated with apCR status.
Multivariable Regression Analysis
Fit a multiple logistic regression model using the variables which show difference significance between non- apCR and apCR groups. The results are shown in Table 2. Higher pathological grade (0.061 [95% CI: 0.005–0.444])
and positive HER2 status (0.065 [95% CI: 0.006–0.412]) was associated with higher probability of achieving apCR. Higher NLR (22.451 [95% CI: 2.237–454.068]) or MLR (8.569
[95% CI: 1.215–88.915]) is more un-likely to achieve apCR.
Nomogram-Based Prediction Model
Multivariate logistic regression coefficients were then used to generate a nomo-gram-based predictive model. A nomogram is a tool that provides graphical illustration of all predictors in the model and enables the user to easily compute the probability of an individual outcome [17].
Table 1: Baseline characteristics of patients
|
Characteristics |
Overall |
apCR |
Non-apCR |
p value |
|
|
(n = 50) |
(n = 26) |
(n =24) |
|||
|
Age |
Mean ± SD |
45.94 ± 10.03 |
46.81 ± 11.05 |
45.00 ± 8.94 |
|
|
< 31.5 |
4 (8%) |
3 (11.54%) |
1 (4.17%) |
0.661 |
|
|
≥ 31.5 |
46 (92%) |
23 (88.46%) |
23 (95.83%) |
||
|
Menopausal |
No |
22 (44%) |
12 (46.15%) |
10 (41.67%) |
0.749 |
|
Yes |
28 (56%) |
14 (53.85%) |
14 (58.33%) |
||
|
Pathological classification |
01-Feb |
20 (40%) |
6 (23.08%) |
14 (58.33%) |
0.011 |
|
3 |
30 (60%) |
20 (76.92%) |
10 (41.67%) |
||
|
T stage |
cT1 |
7 (14%) |
5 (19.23%) |
2 (8.33%) |
0.243 |
|
Ct2 |
32 (64%) |
18 (69.23%) |
14 (58.33%) |
||
|
cT3 |
6 (12%) |
2 (7.69%) |
4 (16.67%) |
||
|
cT4 |
5 (10%) |
1 (3.85%) |
4 (16.67%) |
||
|
N stage |
cN1 |
30 (60%) |
15 (57.69%) |
15 (62.5%) |
0.387 |
|
cN2 |
15 (30%) |
7 (26.92%) |
8 (33.33%) |
||
|
cN3 |
5 (10%) |
4 (15.38%) |
1 (4.17%) |
||
|
ER status |
Negative |
20 (40%) |
10 (38.46%) |
10 (41.67%) |
0.817 |
|
Positive |
30 (60%) |
16 (61.54%) |
14 (58.33%) |
||
|
HER2 status (molecular subtype) |
Negative |
29 (58%) |
9 (34.62%) |
20 (83.33%) |
< 0.001 |
|
Positive |
21 (42%) |
17 (65.38%) |
4 (16.67%) |
||
|
Ki67 |
< 87.5 |
44 (88%) |
24 (92.31%) |
20 (83.33%) |
0.589 |
|
≥ 87.5 |
6 (12%) |
2 (7.69%) |
4 (16.67%) |
||
|
NLR |
< 2.535 |
34 (68%) |
23 (88.46%) |
11 (45.83%) |
0.001 |
|
≥ 2.535 |
16 (32%) |
3 (11.54%) |
13 (54.17%) |
||
|
MLR |
< 0.254 |
30 (60%) |
22 (84.62%) |
8 (33.33%) |
< 0.001 |
|
≥ 0.254 |
20 (40%) |
4 (15.38%) |
16 (66.67%) |
||
LNLR: neutrophil-to-lymphocyte ratio; MLR: monocyte-to-lymphocyte ratio.
Table 2: Results of multivariate logistic regression
|
Characteristics |
β |
OR [95%CI] |
p value |
|
|
Pathological grade |
01-Feb |
1.000 |
||
|
3 |
-2.790 |
0.061 [0.005–0.444] |
||
|
HER2 status (molecular subtype) |
Negative |
1.000 |
0.013 |
|
|
Positive |
-2.727 |
0.065 [0.006–0.412] |
||
|
NLR |
< 2.535 |
1.000 |
0.010 |
|
|
≥ 2.535 |
3.111 |
22.451 [2.237–454.068] |
||
|
MLR |
< 0.254 |
1.000 |
0.019 |
|
|
≥ 0.254 |
2.148 |
8.569 [1.215–88.915] |
The output probability of apCR for an individual patient using the nomogram (Figure 1) is calculated as follows: obtain scores for all variable values, add all scores, and draw a bottom line to the probability axis (the bottom axis) to obtain the probability of event using the total score axis.
Validation of Nomogram-Based Prediction Model
The receiver operating characteristic (ROC) curve for the nomogram model is shown in Figure 2a with AUC (area under the curve) of 0.929 [95% CI: 0.859–0.998]. Then, the AUC
of the ROC (Figure 2b) was also calculated by generating 1000 smoothed bootstrap replications. The AUC value is 0.933 [95% CI: 0.816–0.973]. The results confirms that the nomogram has good ability discriminate between non-apCR and apCR patients.
Calibration curves can show the goodness of a fitting model in an absolute sense. In Figure 3a, there is only small departure between the calibration curve and the diagonal line denoting perfect calibration over the interval. Although the
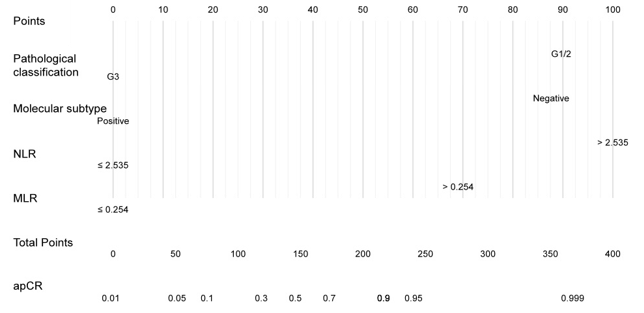
Figure 1: Nomogram for predicting axillary pathologic complete response (apCR) after neoadju-vant therapy in breast cancer patients without distant metastasis. Calculate the probability of apCR for an individual patient using the nomogram: obtain scores for all variable values, add all scores, and draw a bottom line to the probability axis (the bottom axis) to obtain the probability of event using the total score axis.
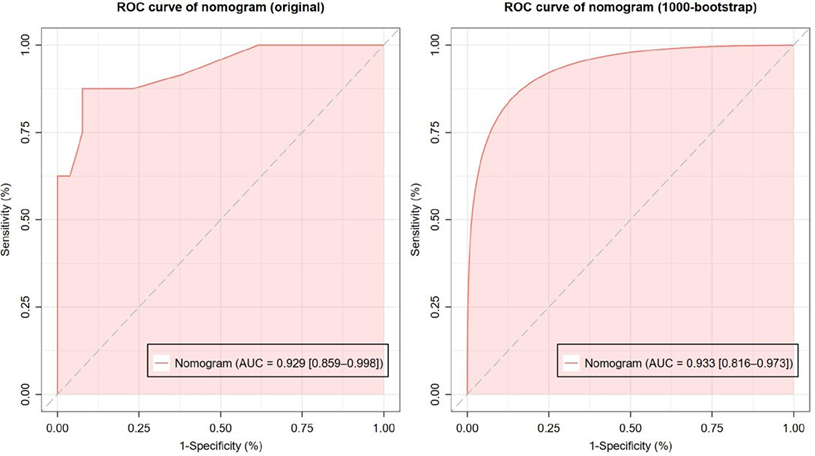
Figure 2: Receiver operating characteristic (ROC) curve of the nomogram. (a) ROC curve without bootstrapping (AUC [area under the ROC curve] = 0.929 [95% CI: 0.859–0.998]); (b) Smoothed ROC with 1000-time bootstrapping (AUC = 0.933 [95% CI: 0.816–0.973]).
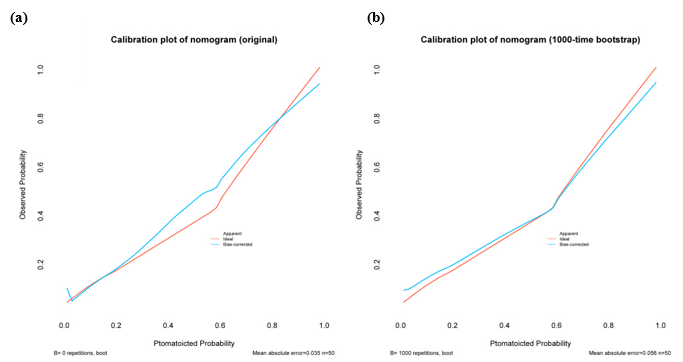
Figure 3: Calibration curve of the nomogram. (a) Calibration curve without bootstrapping; (b) Calibration curve with 1000-time bootstrapping.
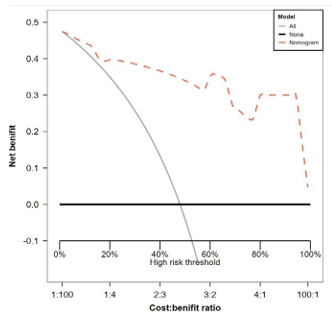
Figure 4: Decision curve analysis (DCA) plot using 1000-time bootstrap sampling. ALL: all of the patients achieved apCR after neoadjuvant therapy. NONE: none of the patients achieved apCR after neoadjuvant therapy.
calibration curve of the original model has been plotted, the bias-corrected curve with resampling validation is the more valid representation. The mean of the model’s calibration metric was computed across the 1000 simulation replicates using the bootstrap method, and the plot also shows the good agreement of the nomogram (Figure 3b).
Furthermore, the decision curve analysis (DCA) plot shown in Figure 4 using 1000 bootstrap samples, depending on comparing the net benefit of the nomogram model with that of a strategy of "ALL" (the grey line) and "NONE" (the black line parallel to the x axis at net benefit of zero), the nomogram model can be considered as the best model.
Discussion
Originally a means to downstage patients with inoperable locally advanced breast cancer, neoadjuvant therapy is now integral to comprehensive cancer treatment [18]. In 2021, the American Society of Clinical Oncology ASCO Consensus on Neo-adjuvant Therapy recommended that for patients with high-risk HER2-positive or triple-negative breast cancer (TNBC) should be administered neoadjuvant therapy prior to surgery, as an effective response to neoadjuvant therapy can result in downstaging of a tumor, hence enhancing tumor respectability, increasing the possibility of breast conversing, and reducing postoperative complications [19]. Neoadjuvant therapy for breast cancer includes neoadjuvant chemotherapy, neoadjuvant targeted therapy and neoadjuvant endocrine therapy. “Guidelines for Diagnosis and Treatment of Breast Cancer in China (2021 Edition)” released by Chinese Anti- Cancer Association, Committee of Breast Cancer Society (CACA-CBCS) divide patients with breast cancer into the group that treatment is a "must" (patients in need of downstaging) and the group that treatment is an "elective option" (patients who show signs of in-vivo drug sensitivity) [20]. Recently, in the realm of precision medicine, prospective clinical trials have been performed to evaluate the best- matched neoadjuvant treatment in accordance with the breast cancer patients’ molecular subtypes (HER2 status) [21]. For the patients who are suitable for neoadjuvant treatment, new chemotherapy drugs, targeted and immuno-therapy drugs are added to upgrade the standard of care without decrease in pathological complete response (pCR) rate. In patients who are not suitable for chemotherapy, degraded treatment strategy can be employed to protect patients from the toxic side effects of chemotherapy drugs. Since chemotherapy after surgery is not the best strategy to maximize the survival of patients, drug sensitivity results will be obtained according to residual lesions after neoadjuvant treatment, and subsequent intensive treatment will be adopted. On the other hand, some scholars claimed that whether surgery should be the first step in treating patients with early-stage breast cancer is an issue worth discussing [22]. Of course, there are still a small number (< 5%) of patients with disease progression or even loss of surgery opportunities in the process of neo-adjuvant treatment. Therefore, it is recommended that patients should carry out close efficacy evaluation, including clinical physical examination and imaging evaluation, so as to monitor the treatment response. Presently, there is less evidence showing that hematological indicators can be used to monitor the effect of neoadjuvant therapy. Studies have shown that patients who achieve pCR after neoadjuvant therapy have a better long- term outcome [23]. The 5-year survival rate of patients who reached apCR after neo-adjuvant treatment was significantly improved. For patients who did not achieve apCR after neo-adjuvant therapy, the number of residual tumor cells in different patients' lesions was also different, and the prognosis was not completely the same.
With a 90% successful identification rate (SIR) and 8% FNR, sentinel lymph node mapping and biopsy after neoadjuvant therapy for early-stage breast cancer patients is a credible tool for planning treatment in this population as an alternative to completion ALND [24]. In the meanwhile, pre- treatment SLNB provides accurate axillary staging, identifying lymph node-negative patients who may avoid ALND [25]. Based on the re-sults of fine needle aspiration cytology (FNAC) combined with IHC staining, the rate of lymph node clearance can be lowered in the patients with negative sentinel lymph nodes. For initial axillary lymph node positive patients, lymph node negative patients after neoadjuvant therapy meet the following conditions: 1) cT1-3N1, 2) lymph node metastasis confirmed by dual tracer imaging; 3) positive sentinel lymph node (SLN)≥ 3; 4) place a marker clip on axillary lymph nodes with positive puncture biopsy before neoadjuvant chemotherapy and detect them during operation. After fully communicating with breast cancer patient, ALND can be avoided [11]. Adjuvant radiotherapy is recommended for axillary group I and II after operation. For patients with initial cN2-3, ALND is still recommended because the lymph node is negative after neo-adjuvant treatment. Clinicians can predict which patients will respond better to treatment according to the disease characteristics of patients, and may select the patients most likely to achieve apCR through sentinel lymph node biopsy after neo-adjuvant treatment. As shown in Tabel 2, the multivariate logistic regression- based prediction model demonstrates that patients with low pathological grade 3, HER-2+, NLR, and LMR are more likely to achieve apCR. The patients included in this study were N+, and N was not found to be an influ-encing factor , result similar to the Olga kantor model [26]. Although N is an important factor affecting the survival of patients, it is not related to whether or not the apCR can be achieved, which is different from what we think. This may be related to the low number of patients included in cN2-3. The later the N stage, the more likely it is that the disease will progress after neoadjuvant therapy, or because of disease factors, there is no regular reexamination, resulting in the exclusion of this part of patient data. Because the current controversy is more about patients with cN1, we hope to include more patients with cN1 for group analysis in the future.
T stage is also not an influencing factor. Clinically, there is no mass after minimally invasive atherectomy, and it is not possible to accurately assess the neoadjuvant T stage at this time. We compared the size of the surgical pathological mass with the size of the initial imaging mass after neoadjuvant and found that T was not the influencing factor. In fact, the most accurate is the imaging comparison before and after the neoadjuvary.Subgroup analysis of the Olga kantor model showed that HR+/HER2- patients had the lowest apCR rate. This study found that ER was not an influencing factor. Considering the small sample size of this study, only HR status was considered, and HER2-positive patients were not excluded. We should subsequently include more patients and analyze results with different HR status for the same HER2 status.This is where we need to improve. Finally, only 6 patients had a Ki67 of 87% or greater, and the result was negative. When Ki67 ≥ 14%, it is associated with apCR [27]. Previous studies have shown that HER-2 positive patients tend to achieve patho-logical complete remission after neo- adjuvant therapy. In 2014, Cortazar et al. [28] carried out the CTNeoBC pooled analysis including 11,955 neo-adjuvant therapy patients in 12 international multi-medical centers, and the results showed that HER-2 positive pCR was significantly related to event-free survival (EFS), and OS. For HER-2 positive type, various clinical research data emerge endlessly, hoping to improve pCR rate and survival. The NOAH trial [29] indicated that the addictive application of neoadjuvant and adjuvant trastuzumab to neoadjuvant chemotherapy should be considered for female patients with HER2-positive locally advanced or inflammatory breast cancer to improve survival, and clinical and pathological tumor responses. The NOAH study preliminarily explored the combination of neoadjuvant chemotherapy and targeted therapy, and the addition of trastuzumab can significantly improve the pCR rate. The phase II NeoSphere trial supports the approval of pertuzumab for use in combination with trastuzumab and docetaxel as neoadjuvant treatment for patients with HER2+ early breast cancer. This study reported lower pCR rates in hormone-receptor (HR)+ tumors as compared to HR- cases, but this did not reflected in OS analyzes [30]. The NeoALTTO20 and the CHER-LOB studies demonstrated significantly increased pCR rates using dual HER2-blockade in comparation with trastuzumab [31, 32]. For the triple negative breast cancer (TNBC) subtype, in the non-pCR post-neoadjuvant subgroup, standard therapy with adjuvant capecitabine (Create-X trial) was proved to improve OS [33]. In KATHERINE study, improved invasive DFS was shown for molecularly identified HER2+ breast cancer after a switch to PNACT with trastuzumab emtansine (T-DM1) [34]. As such, the personalised targeted post-neoadjuvant treatment based on pCR status will be administered in addi-tion to the standard of care according to the CREATEX trial and T-DM1 according to the KATHERINE trial [35].
The emergence of targeted drugs in breast cancer has improved the survival prog-nosis of HER-2 patients and the effect of neoadjuvant therapy. However, the HER-2 positive patients have different responses to the neo-adjuvant therapy. Therefore, some studies further analyzed whether the high HER2/CEP17 ratio indicates that the neo-adjuvant targeted therapy is better. In a clinical trial conducted in South Korea, pCR status was found to be highly associated with HER2/ CEP17 ratio in neoadjuvant an-ti-HER2 dual blockade [36]. A retrospective review in the National Cancer Database (NCDB) breast cancer patient cohort indicated higher HER2/ CEP17 ratio is an effective of predictor of pCR to neoadjuvant chemotherapy [37]. HER2, a member of the ErbB family of transmembrane receptor tyrosine kinases [38], is encoded by the gene ERBB2 [39]. HER2+ subtype is characterized by the absence of estrogen and progesterone receptors and overexpression of HER2 receptor, and ac-counts for 15– 20% of all breast cancers. HER2+ subtype patients tend to have higher likelihood of achieving pCR after neoadjuvant treatment. HER2+breast cancer patients who achieved pCR after new adjuvant treatment have more obvious benefits. Further-more, achieving pCR was associated with better survival [40]. It is estimated that patients with lower HER2 expression occupy 45%–55% of all the breast cancers [41]. In many previous studies [34], HER2-targeted therapy has shown very few benefits in patients with HER2-low breast cancer [35]. Trastuzumab deruxtecan (T-DXd), also known as DS-8201 or DESTINY-Breast 03 (DB 03), is an antibody-drug conjugate (ADC) consisted of an anti-HER2 antibody and cytotoxic topoisomerase I inhibitor [42]. T-DXd is a combination of a high drug antibody ratio (DAR) of eight molecules per antibody, plasma-stable linker that is selectively cleaved by tumor lysosomal proteases, and membrane-permeable payload. It is able to diffuse out into neighboring cells, thereby addressing tumor heterogeneity with a ‘‘bystander’’ effect, hence leading to impressive response rates in various HER2-expressing cancers including breast, lung, and gastric tumors [43]. In phase 3 trial of the drug involving patients with HER2-low metastatic breast cancer, T-DXd resulted in significantly longer progression- free and overall sur-vival than the clinicians’ choice of chemotherapy [44]. In the past, compared with current therapies such as endocrine therapy and chemotherapy, non– HER2-amplified breast cancer has extremely low chance of obtaining a pCR. It is hoped that applying this HER2-targeted ADC, which has shown many favourable benefits for breast cancer pa-tients with HER2-low, will also show beneficial results in the neoadjuvant setting [45].
It is widely known that many types of cancers are engendered from infection, chronic irritation, and inflammation. Furthermore, the tumor microenvironment, which is full of inflammatory cells, plays an indispensable role in the neoplastic process, pro-moting proliferation, survival and migration of tumor cells [46]. In recent years, more and more efforts have been made to fight against tumors in accordance the bodily internal immune system. As a simple, objective and cheap laboratory indicator, inflammatory immune related indicators have also been gradually applied to predict pCR. In general, lower lymphopenia indicators means that anti- tumor immune system is less activated and the treatment efficancy is better [47, 48]. Because the comparison before and after treatment of primary tumors is simple in clinical practice, most of the inflammatory immune related indicators predict primary tumors. There are few studies demonstrating correlation between clinical information and pCR. This study highlights the predictive efficacy of some immunity-related indicators as biomarkers for apCR status after neo-adjuvant therapy in breast cancer patients without distant metastasis. CD8+ T cells are considered as the key immune cells for killing cancer [49]. In addi-tion, neutrophils are important cells involved in inflammation, and chronic inflammation is one of the characteristics of tumor microenvironment. Neutrophils infiltrating tumors can produce a wide range of chemokines, which play an important role in tumor progression, metastasis, angiogenesis and immunosuppression [50-52]. Chemokines, such as CSF1, secreted by tumor cells will summon monocytes from peripheral blood circulation to the tumor microenvironment, and then differentiate into tumor-associated macrophages (TAM). TAM can be divided into two main phenotypes: the classical M1 macrophages and alternatively activated macrophages M2. In the breast malignant tumor, the main type is M2. TAM can promote tumor progression and immunosuppression through many ways. TAM can promote the proliferation of tumor cells by secreting EGF, PDGF, etc. Through the release of MMP2,
MMP9 and other tumor cell transfer factors to participate in tumor invasion and metastasis; By generating IL10, PGE2, TGF β. They are involved in the immune escape of tumor cells; It participates in the growth of tumor micro-vessels and lymphatic vessels by expressing VEGF. The study of Vitale et al. (2019) [53] depicted the impact of TAM metabolism on their ability to influence tumor growth, often as an outcome of altered immunomodulation. The increase of tumor-related inflammatory mediators produced by tumor cells, such as tumor necrosis factor (TNF)- α, interleukin (IL)-3, and IL-6, may lead to the elevation of neutrophils and tumor-related macrophages, and the decrease of lymphocytes. The levels of NLR and MLR will also increase accordingly. Therefore, low NLR and MLR indicates better efficacy and longer survival. In two rather recent meta-analyzes, a high NLR was shown to predict poor OS and disease free survival (DFS) among BC patients [54,55]. In the study of Tiainen et al. (2021) [56], the authors found that Trastuzumab was especially beneficial for those HER2+ breast cancer patients with a high baseline NLR or MLR, while the prognosis of the HER2+ patients with a low baseline NLR or MLR was better if they received adjuvant trastuzumab treatment. Multivariate logistic regression analysis performed by us showed that decreased level of NLR and MLR is associated with higher apCR rate after neoadjuvant therapy. This means that NLR and MLR can be used as independent predictors of apCR in breast cancer patients without distant metastasis after neoadjuvant therapy.
NLR and MLR are measures reflecting the balance between inflammation and im-munoreaction in cancer [57]. Furthermore, chronic inflammation and an inappropriate humoral immune response can contribute to tumorigenesis and malignant progression [58]. It is worth noting that molecular biology and signaling pathways in breast cancer are very important in tumor microenvironment. For example, IL-6 can make an extrinsic impact on tumor cells within the complex tumor microenvironment to sustain a pro-tumor condition by supporting cancer cell proliferation, angiogenesis and tumor evasion of immune surveillance [59]. On the other side, TGFβ signaling in the tumor microenvironment plays an important role in tumor initiation, progression, and metastasis by its capacity to regulate cross-talk between tumor cells and other components of the local environment [60]. Past studies revealed the causal relationship between inflammation, innate immunity and breast cancer and the therapeutic potential of targeting tumor microenvironment in breast cancer should be further analyzed. This study had several limitations. Firstly, this is a retrospective cohort study whereby the researchers designed the study, recruited subjects, and collected back- ground information of the subjects after the outcome of interest had been developed, and the results of the study may be affected by biases during data collection. Secondly, the strict exclusion criteria resulted in a relatively small sample size. Although the sample size was limited, the internal validation of the nomogram-based predictive model was performed to confirm that it is a reliable tool for predicting apCR status after neo-adjuvant therapy in breast cancer patients without distant metastasis. When only a single set is available, and all data are used to develop model, it is allowed to use resampling for development and validation [61]. Thirdly, breast cancer is a type of highly heterogeneous disease, and tumor cell biology is completely different in different pathological types. Only the most common pathological type, invasive ductal carcinoma (IDC), was investigated in this study. About 8 in 10 invasive breast cancers are IDC [62]. In addition, there exist some other factors that may be related to the effect of new adjuvant therapy, such as the site of the primary tumor, BMI, BRCA mutation status, race, etc., and these should be further analyzed. Lastly, the developed model lacked external validation. Future research should perform accuracy assessments using an independent data set.
Conclusions
In conclusion, clinical variables including pathological grade, HER2 status, and some routine hematological indicators can be utilized to build a nomogram-based model for predicting the complete remission probability of axillary lymph nodes after neoad-juvant therapy. A nomogram is widely considered as a reliable and concise tool to aid in predict patients’ outcome and clinical decision. Based on the predicted results of the model, clinicians can determine different surgery strategies for axillary lymph nodes after neoadjuvant treatment. This is the result of our initial exploration. Because complications (e.g. lymphedema, numbness of upper limbs, and limited joint activity) often occur after axillary lymph node dissection ,which seriously affects the quality of life of patients, for patients with early- stage breast cancer, ALND can be waived if it is judged that there is a high probability of achieving apCR. Also, it is necessary to inform the patients of the advantages and disadvantages in advance, and then make clinical decisions based on their requirements.
Declaration
Ethical Approval and consent to participate
This retrospective study was approved by the Ethics Committees of Sun Yat-sen Memorial Hospital, Sun Yatsen University, and the requirement to obtain informed consent was waived [SYSKY-2023-718-01]. All the data were analyzed anonymously. This study was conducted in accordance with the Declaration of Helsinki. Patient consent was waived due to the retrospective nature of reviewing medical records, which did not affect patients’ treatments nor any interventions that took place in patients’ treatments.
Consent to Publication
All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data available on request due to restrictions of medical record privacy. The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding
This work was supported by the Guangdong Province Natural Science Foundation (No: 2022A1515012497)
Author Contributions
Conceptualization, J.Z. and S.M.; data curation, J.Z. ; method-ology, J.Z., S.M.; formal analysis, H.-M.C. G.-Y.W. and S.M.; resources, H.-M.C., S.M.; writing—original draft preparation, J.Z. and S.M.; writing— review and editing, J.Z., H.-M.C. and S.-M.B.; validation, G.-Y.W., C.-B.N. and M.;
References
- American Cancer Society Key statistics for breast cancer (2022).
- Sung H, Ferlay J, Siegel RL, et Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin 71 (2021): 209-249.
- Deo SVS, Bhutani M, Shukla NK, et Randomized trial comparing neo-adjuvant versus adjuvant chemotherapy in operable locally advanced breast cancer (T4b N0-2 M0). Surg. Oncol 84 (2003): 192-197.
- Deo SVS, Bhutani M, Shukla K, et al. Randomized trial comparing neo-adjuvant versus adjuvant chemotherapy in operable locally advanced breast cancer (T4b N0-2 M0). Surg. Oncol 84 (2003): 192-197.
- Boughey J, Vera J Suman, Elizabeth A Mittendorf, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial 310 (2013): 1455-1461.
- Boileau J, Brigitte Poirier, Mark Basik, et al., Sentinel node biopsy after neoadjuvant chemotherapy in biopsy- proven node-positive breast cancer: the SN FNAC study 33 (2015): 258-264.
- Krag D, Thomas B Julian, Seth P Harlow, et NSABP-32: Phase III, randomized trial comparing axillary resection with sentinal lymph node dissection: a description of the trial 11 (2004): 208-210.
- Katayama A, Miligy IM, Shiino S, et al. Predictors of pathological complete response to neoadjuvant treatment and changes to post-neoadjuvant HER2 status in HER2- positive invasive breast cancer. Mod. Pathol 34 (2021), 1271-1281.
- Li Y.-X, Chang J.-Y, He M.-Y, et al. Neutrophil-to- lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR) predict clinical outcome in patients with stage IIB cervical cancer. J. Oncol (2021): 2939162.
- Song S, Li C, Li S, et Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther 10 (2017): 3145-3154.
- Jiang Z, Song E, Wang X, et al. Guidelines of Chinese Society of Clinical Oncology (CSCO) on diagnosis and treatment of breast cancer (2020 version). Breast Transl Cancer Res 1 (2020): 27.
- Edition S, Edge S, Byrd AJCC cancer staging manual. 8th ed.; Springer International Publishing: New York, NY, USA (2016).
- The WHO Classification of Tumours Editorial Board WHO classification of tumours, 5th edition, volume 2: breast tumours. IARC: Lyon 2 (2019).
- Allison KH, Hammond MEH, Dowsett M, et Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update 144 (2020):545-563.
- Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical practice guideline focused update. Arch Pathol Lab Med 142 (2018): 1364-1382.
- Rahmatinejad Z, Tohidinezhad F, Rahmatinejad F, et Internal validation and comparison of the prognostic performance of models based on six emergency scoring systems to predict in-hospital mortality in the emergency department. BMC Emerg Med 21 (2021): 68.
- Zlotnik A, Abraira V. A general-purpose nomogram generator for predictive logistic regression models. Stata J 15 (2015): 537-546.
- Cain H, Macpherson IR, Beresford M, et Neoadjuvant therapy in early breast cancer: treatment considerations and common debates in practice. Clin. Oncol 29 (2017): 642-652.
- Korde LA, Somerfield MR, Carey LA, et Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO Guideline. J Clin Oncol 39 (2021): 1485-1505.
- CACA-CBCS (Chinese Anti-Cancer Association. The Guideline and Specification on Diagnosis and Treatment of Breast Cancer (2021 Edition). China Oncol 31 (2021):954-1040.
- Denkert C, Seither F, Schneeweiss A, et al Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical Lancet Oncol 22 (2021): 1151-1161.
- Pusztai L, Foldi J, Dhawan A, et Changing frameworks in treatment sequencing of triple-negative and HER2- positive, early-stage breast cancers. Lancet Oncol 20 (2019): 390-396.
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26 (2008): 778-785.
- Kelly AM, Dwamena B, Cronin P, et al. Breast cancer: sentinel node identification and classification after neoadjuvant chemotherapy—systematic review and meta analysis. Acad. Radiol 16 (2009): 551-563.
- Pilewskie M, Morrow M. Axillary Nodal Management Following Neoadjuvant Chemotherapy: A Review. JAMA Oncol 3 (2017): 549-555.
- Kantor O, Sipsy L, Yao K, et A Predictive Model for Axillary Node Pathologic Complete Response after Neoadjuvant Chemotherapy for Breast Cancer 25 (2018): 1304-1311.
- Zheng W, Zhou P, Liu Y, et al. Prediction of axillary response after neoadjuvant chemotherapy in clinical node positive breast cancer 10 (2021): 2822-2830.
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled Lancet 384 (2014): 164-172.
- Gianni L, Eiermann W, Semiglazov V, et Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375 (2010): 377-384.
- Gianni L, Pienkowski T, Im -H, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2- positive breast cancer (NeoSphere): a multicentre, open- label, phase 2 randomised trial. Lancet Oncol 17 (2016):791-800.
- Baselga J, Bradbury I, Eidtmann H, et Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379 (2012): 633-640.
- Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J. Clin. Oncol 30 (2012): 1989-1995.
- Masuda N, Lee -J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med 376 (2017): 2147-2159.
- von Minckwitz G, Huang C.-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2- positive breast cancer. N. Engl. J. Med 380 (2018): 617-
- Pixberg C, Zapatka M, Hlevnjak M, et al. COGNITION: a prospective precision oncology trial for patients with early breast cancer at high risk following neoadjuvant chemotherapy. ESMO Open 7 (2022): 100637.
- Choi JH, Jeon CW, Kim YO, et Pathological complete response to neoadjuvant trastuzumab and pertuzumab therapy is related to human epidermal growth factor receptor 2 (HER2) amplification level in HER2-amplified breast cancer. Medicine (Baltimore) 99 (2020): 23053.
- Greenwell K, Hussain L, Lee D, et Complete pathologic response rate to neoadjuvant chemotherapy increases with increasing HER2/CEP17 ratio in HER2 overexpressing breast cancer: analysis of the National Cancer Database (NCDB). Breast Cancer Res Treat 181 (2020): 249-254.
- Hurvitz SA, Hu Y, O’Brien N, et al. Current approaches and future directions in the treatment of HER2-positive breast cancer. Cancer Treat. Rev 39 (2013): 219-229.
- Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554 (2018): 189-194.
- DeMichele A, Yee D, Berry D.A, et al. The neoadjuvant model is still the future for drug development in breast cancer. Clin. Cancer Res 21 (2015): 2911-2915.
- Tarantino P, Hamilton E, Tolaney SM, et al. HER2-low breast cancer: pathological and clinical J. Clin. Oncol 38 (2020): 1951-1962.
- Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382 (2019): 610-621.
- Makhlin I, DeMichele A. Trastuzumab deruxtecan: an antibody-drug conjugate embracing its destiny in breast cancer. Cell Rep Med 3 (2022): 100668.
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387 (2022): 9-20.
- Hurvitz SA, Wang LS, Chan D, et TRIO-US B-12 TALENT: phase II neoadjuvant trial evaluating trastuzumab deruxtecan with or without anastrozole for HER2-low, HR+ early-stage breast cancer. J. Clin. Oncol 40 (2022): TPS623-TPS623.
- Coussens LM, Werb, Inflammation and cancer. Nature 420 (2022): 860-867.
- Song X, Chen D, Yuan M, et Total lymphocyte count, neutrophil-lymphocyte ratio, and platelet-lymphocyte ratio as prognostic factors in advanced non-small cell lung cancer with chemoradiotherapy. Cancer Manag. Res 10 (2018): 6677-6683.
- Nishijima TF, Muss HB, Shachar SS, et al. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat. Rev 41 (2015): 971-978.
- Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A J. Cell. Physiol 234 (2019): 8509-8521.
- Tazawa H, Okada F, Kobayashi T, et al. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am. J. Pathol 163 (2003): 2221-2232.
- Galdiero MR, Garlanda C, Jaillon S, et al. Tumor associated macrophages and neutrophils in tumor progression. J. Cell. Physiol 228 (2013): 1404-1412.
- Coffelt SB, Wellenstein MD, de Visser Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16 (2016): 431-446.
- Vitale I, Manic G, Coussens LM, et Macrophages and metabolism in the tumor microenvironment. Cell Metab 30 (2019): 36-50.
- Guo W, Lu X, Liu Q, et Prognostic value of neutrophil- to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med 8 (2019): 4135-4148.
- Ethier J.-L, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 19 (2017): 2.
- Tiainen S, Rilla K, Hämäläinen K, et al. The prognostic and predictive role of the neutrophil-to-lymphocyte ratio and the monocyte-to-lymphocyte ratio in early breast cancer, especially in the HER2+ subtype. Breast Cancer Res. Treat 185 (2021): 63-72.
- Sahin AB, Cubukcu E, Ocak B, et al. Low pan-immune- inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep 11 (2021):
- Prestwich RJ, Errington, F, Hatfield P, et The immune system — is it relevant to cancer development, progression and treatment? Clin. Oncol 20 (2008): 101-112.
- Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol 26 (2014): 38-47.
- Caja F, Vannucci L. TGFβ: A player on multiple fronts in the tumor microenvironment. J. Immunotoxicol 12 (2015): 300-307.
- Collins G, Reitsma J, Altman D, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement 350 (2015): 7594.
- American Cancer Society Invasive breast cancer (IDC/ ILC) (2022).


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 