Mycobacterium Bovis-Related Abdominal Aortic Aneurysm after Intravesical Bacillus Calmette-Guérin Therapy for Non-Muscle Invasive Bladder Cancer
Arkhjamil Angeles1, Jason Faulds2, Elaine Ni Mhurchu3, Gang Wang4, Monty Martin3, and Lucia Nappi1,5*
1Department of Medical Oncology, BC Cancer, Vancouver, BC, Canada
2Department of Surgery, Vancouver General Hospital, University of British Columbia, Vancouver, BC, Canada
3Department of Radiology, BC Cancer, Vancouver, BC, Canada
4Department of Pathology, BC Cancer, Vancouver, BC, Canada
5Department of Urologic Sciences, Vancouver Prostate Centre, University of British Columbia, Vancouver, BC, Canada
*Corresponding Author: Lucia Nappi, Department of Urologic Sciences, Vancouver Prostate Centre, University of British Columbia, Vancouver, BC, Canada.
Received: 28 December 2022; Accepted: 09 January 2023; Published: 24 January 2023
Article Information
Citation: Arkhjamil Angeles, Jason Faulds, Elaine Ni Mhurchu, Gang Wang, Monty Martin, and Lucia Nappi. Mycobacterium Bovis-Related Abdominal Aortic Aneurysm after Intravesical Bacillus Calmette-Guérin Therapy for Non-Muscle Invasive Bladder Cancer. Journal of Cancer Science and Clinical Therapeutics. 7 (2023): 21-24.
View / Download Pdf Share at FacebookAbstract
Background: Systemic infection after intravesical Bacillus Calmette- Guérin (BCG) therapy for non-muscle invasive bladder cancer (NMIBC) is a rare complication. Case
Presentation: Here, we describe an 81-year-old man with a history of multiple, recurrent NMIBC who was treated with intravesical BCG. Twelve months after his last BCG treatment, he developed back pain and constitutional symptoms. Investigations revealed a new 6.2 cm abdominal aortic aneurysm. He underwent open repair using a femoral vein conduit. Post-operatively, he was started on anti-tubercular therapy, and was discharged home with outpatient follow-up at the tuberculosis (TB) clinic.
Discussion: Mycotic aneurysms are a rare vascular complication of intravesical BCG. Maintaining a high degree of suspicion is key to early diagnosis and management.
Keywords
Intravesical Bacillus Calmette-Guérin; Mycobacterium Bovis; Mycotic Aortic Aneurysm; Non-Muscle Invasive Bladder Cancer
Article Details
1. Introduction
Bacillus Calmette-Guérin (BCG) is a live-attenuated strain of Mycobacterium bovis. It was initially developed as a vaccine against tuberculosis (TB) [1], but has become widely adopted as an adjuvant treatment for intermediate and high risk, non-muscle invasive bladder cancer (NMIBC). BCG is administered intravesically after transurethral resection of the bladder tumor (TURBT). Treatment regimens depend on the estimated risk of recurrence, but typically consist of induction therapy followed by a series of maintenance therapies over the course of one or three years [2]. The exact mechanism by which BCG exerts its antitumor effect has not been fully elucidated. It is thought that BCG is internalized and processed by both bladder cancer cells and benign urothelial cells, which leads to host immune activation and immune cell recruitment. A cellular inflammatory response to BCG ensues involving CD4 T-cells, natural killer cells, and antigen presenting cells. This results in the production of cytokines with antitumor activity and cell-mediated cytotoxicity of bladder cancer cells [3]. BCG can also directly exert cytotoxicity against bladder cancer cells [3]. Clinically, this functions to reduce the risk of disease recurrence, delay tumor progression, decrease the need for cystectomy, and improve patient overall survival [4]. Intravesical BCG therapy is generally well tolerated with its most common adverse effect being a hypersensitivity reaction secondary to treatment-induced cytokine production, occuring in up to 85% of patients [5]. This toxicity is often self-limited, and manifests as fever, malaise, and mild irritative bladder symptoms such as urinary frequency, dysuria, and hematuria [5]. Mycobacterium bovis-related infections, either local or systemic, can also be expected. Local complications often occur 3 months after intravesical BCG instillation. They can involve any site along the genitourinary tract, including the bladder (cystitis), prostate (prostate abscess, granulomatous prostatis), penis (balanitis), scrotum (granulomatous orchitis, testicular abscess), and kidneys (pyelonephritis, renal abscess) [6]. Systemic complications can occur within days to weeks after treatment, but can also present years after treatment completion. They are less common, but have been reported in a variety of sites including the liver (granulomatous hepatitis), lungs (pneumonitis), connective tissues (intamuscular abscess, hardware infection, osteomyelitis), and vasculature (mycotic pseudoaneurysms) [6]. Here, we report a case of Mycobacterium bovis-related abdominal aortic aneurysm as an infectious complication of intravesical BCG treatment in a patient with multiple, recurrent NMIBC.
2. Case Presentation
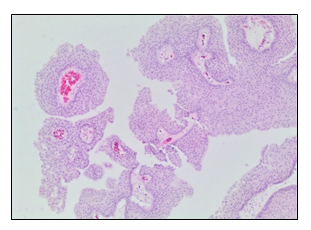
An 81-year-old man presented with a 3-month history of intermittent low back pain, fatigue, night sweats, and unintentional weight loss of 30 lbs. He had a history of low-grade, papillary, NMIBC initially diagnosed 5 years before the presentation of these symtoms (Figure 1). He underwent TURBT for the NMIBC, but he experienced local disease recurrences that were managed with several local fulgurations and resections followed by adjuvant gemcitabine and three separate courses of intravesical therapy with BCG. His last intravesical BCG treatment was one year prior to the current presentation, and was complicated by prolonged gross hematuria following TURBT and clinical evidence of chronic cystitis on cystoscopy. The remainder of his medical history included hypertension, dyslipidemia, bilateral cataract surgery, glaucoma, and mild carotid artery stenosis. He had no history of immunosuppression. His medications included amlodipine, ramipril, aspirin, and latanoprost eye drops.
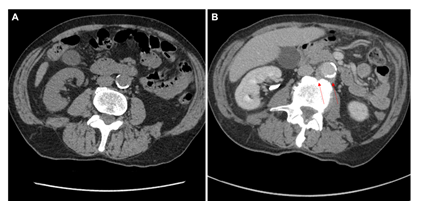
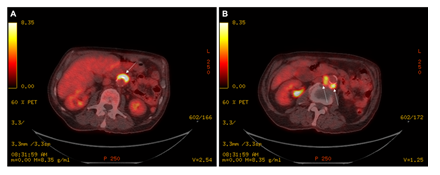
He completed several investigations for his presenting symptoms, including: blood cultures which were negative, upper and lower gastrointestinal endoscopies which were unremarkable, and a cystoscopy which did not demonstrate any evidence of bladder tumor recurrence but did show urothelial changes suggestive of marked chronic inflammation. A computed tomography (CT) of the chest, abdomen, and pelvis showed bilateral subcentimeter lung nodules, hepatic cysts, a thickened urinary bladder wall, a mildly enlarged prostate, and no abdominopelvic lymphadenopathy (Figure 2A and 2B). The lung nodule findings were further investigated with a bronchoscopy. Bronchial alveolar lavage was negative for acid-fast bacteria (AFB) and mycobacterial growth. Bronchial brushings were negative for malignant cells. Biopsy of the right lower lobe lung nodule revealed benign lung alveolar tissue with focal non-necrotizing granulomatous inflammation and hemosiderin deposition. A positron emission tomography (PET) scan demonstrated a lobulated fluorodeoxyglucose (FDG)-avid tissue measuring 1.0 x 2.6 cm closely contacting the abdominal aorta anteriorly between the superior and inferior mesenteric artery origins (standardized uptake values (SUV) max 12.0), and posteriorly near the origin of the inferior mesenteric artery measuring 1.9 x 3.7 cm (SUV max 8.4) (Figure 3A and 3B). These findings were favored to represent activity in the para-aortic lymph nodes. The bilateral small pulmonary nodules did not exhibit FDG activity. On retrospective review of his initial CT scan (Figure 2B), this did show low attenuation soft tissue posterior to the aorta.
Figure 2: (A) Non-contrast CT KUB evaluating for renal colic four years prior to presentation showed no peri-aortic abnormalities for comparison. (B) Split bolus urographic and portal venous phase post contrast CT abdominal image showing low attentuation soft tissue posterior to the aorta (solid red arrow).
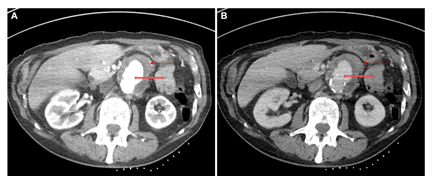
The para-aortic soft tissue lesion was concerning for malignancy, and a CT-guided biopsy was requested. However, during the procedure, there was evidence of several new aortic pseudoaneurysms at the intended biopsy site measuring 30 x 34 mm that were not previously seen on his CT or PET imaging (Figure 4). These findings were inconsistent with the expected evolution of para-aortic nodal metastases, and suspicious for an inflammatory or infectious process.
Figure 4: CT of the abdomen in (A) arterial and (B) portal venous phases showed a new 3 cm pseudoaneurysm of the aorta (open red arrow) with diffuse low attenuation circumferential peri-aortic and mural thickening (solid red arrow). Note that the patient was scanned in prone position with the biopsy markers on the back, and the images have been rotated.
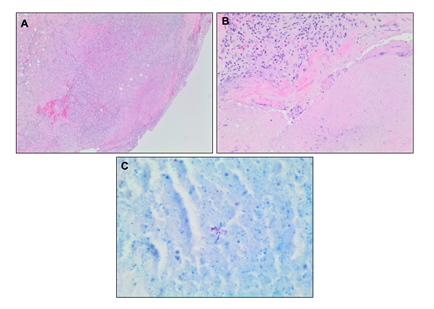
In light of these imaging findings, he was transferred emergently to an acute care hospital for admission under Vascular Surgery. On physical examination, he had a blood pressure of 136/90, heart rate of 89 beats per minute, and normal oxygen saturation. He was afebrile. A dedicated CT angiography of the aorta was performed, and this revealed a 6.2 cm abdominal aortic aneurysm with findings to suggest a contained rupture. He proceeded to the operating room the following day. The aorta was approached through a standard midline laparotomy for a transperitoneal aortic exposure. The infracolic aorta was exposed, and there were dense adhesions to the duodenum in keeping with an inflammatory or infectious process. There were no findings to suggest malignancy. There was no sign of aortic rupture. Both legs had been freely draped into the field, and the superficial femoral veins were procured from both thighs, preserving the profunda femoris vein and the popliteal veins bilaterally. The two femoral veins were then sewn together to form a large diameter tube to use as the conduit for the infrarenal aortic tube graft repair. After administration of intravenous heparin, the infrarenal aorta and both iliac arteries were cross-clamped. The aorta was opened, and pus and extensive necrotic debris were observed. This was completely debrided with specimens sent for microbiology and pathology. The femoral vein graft was then cut to the appropriate length and sewn in an end-to-end fashion to the infrarenal aorta and to the aorta just proximal to the iliac bifurcation. Hemostatsis was achieved. The femoral vein graft was covered with a tongue of omentum, which was brought through the mesentery of the transverse colon and loosely secured over the top of the femoral vein graft, completely excluding it from the visceral contents. The abdomen was closed in the standard fashion, and the patient recovered well without incident on the ward. An intraoperative sample of the aortic tissue was sent for microbiology and pathology. This came back positive for AFB, and TB polymerase chain reaction confirmed a mycobacterial strain related to BCG treatment. The excised aortic and retroperitoneal tissue showed evidence of mycobacterium-associated necrotizing granulomatous inflammation (Figure 5A and 5B) with a positive AFB stain (Figure 5C). The tissue culture grew M. bovis. There was no pathologic evidence of malignancy.
Infectious Disease was consulted for management. His echocardiogram did not show any cardiac valve vegetations. He was started on anti-tubercular therapy with rifampin, isoniazid, ethambutol, and pyridoxine, with a plan to follow up at the TB clinic on discharge.
3. Discussion
In this patient with a history of NMIBC treated with intravesical BCG, the development of a para-aortic lesion should prompt a broad differential diagnosis. This would include malignant causes such as recurrence of urothelial carcinoma, lymphoma, sarcoma, or other metastatic lymphadenopathy; and non-malignant causes such as retroperitoneal fibrosis, aortitis, hemorrhage, abdominal aortic aneurysm, and mycotic aneurysm. Mycotic aneurysms caused by M. bovis after intravesical BCG administration are rare, but case reports have been reported in literature. In the last three decades, over 90 cases have been described. Most patients were of male gender with a median age of 73 (range 57-94). The aorta was the most common site of vascular involvement, accounting for about 88% of the reported cases. The median duration from the time of last BCG instillation to presentation was 18 months, but the range was highly variable from 1 to 84 months. Mycotic aneurysms most likely develop from hematogenous mycobacterial seeding of the arterial wall, or from local extension of an adjacent infective process [7]. Risk factors for systemic infection include: traumatic catheterization, active cystitis, persistent gross hematuria following transurethral surgery, immunosuppression, and advanced age ≥70 years [7, 8, 9]. Patients typically present with new onset back pain and constitutional symptoms such as fever, night sweats, weight loss, and fatigue. A pulsatile abdominal mass may be felt on examination. Radiographic evaluation using CT angiography is the preferred initial imaging modality [10]. Findings may include saccular or lobulated aneurysms, and perivascular pathology such as soft tissue masses, air and/or fluid collections, edema, and inflammation [10]. A definitive diagnosis is achieved by obtaining tissue for testing. Microbiological testing using AFB stain and mycobacterial culture have a sensitivity of about 25% and 40%, respectively [7]. Histopathologic examination shows necrotizing granulomatous inflammation in about 86% of cases [7]. Molecular testing using polymerase chain reaction can also be used to confirm mycobacterial infection, and can help identify the strain of mycobacterium involved. The management of systemic vascular M. bovis infections should involve a Vascular Surgeon and an Infectious Disease expert with experience in treating mycobacterial infections. Surgical excision of the aneurysm with removal of all infected and necrotic tissue is often warranted. Anti-mycobacterial therapy consists of an initial phase with isoniazid, rifampin, and ethambutol for two months, followed by a continuation phase with isoniazid and rifampin for seven months [7]. Pyrazinimide is omitted as M. bovis carries intrinsic resistance [11]. The treatment regimen should be individualized based on culture and susceptibility results when available. This case highlights the importance of considering mycotic aneurysm as a systemic infectious complication in patients treated with intravesical BCG therapy who present with new onset back pain and constitutional symptoms. Maintaining a high degree of suspicion is key to early diagnosis and management, and to prevent serious and fatal complications.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA 271 (1994): 698-702.
- Kassouf W, Traboulsi SL, Kulkarni GS, et al. CUA guidelines on the management of non-muscle invasive bladder cancer. Canadian Urological Association Journal 9 (2015): E690.
- Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nature Reviews Urology 11 (2014): 153-162.
- Herr HW, Schwalb DM, Zhang ZF, et al. Intravesical bacillus Calmette-Guérin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. Journal of Clinical Oncology 13 (1995): 1404-1408.
- Larsen ES, Joensen UN, Poulsen AM, et al. Bacillus Calmette–Guerin immunotherapy for bladder cancer: a review of immunological aspects, clinical effects and BCG infections. Apmis 128 (2020): 92-103.
- Green DB, Kawashima A, Menias CO, et al. Complications of intravesical BCG immunotherapy for bladder cancer. Radiographics 39 (2019): 80-94.
- Asín MA, Fernández-Ruiz M, López-Medrano F, et al. Bacillus Calmette-Guérin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: incidence, risk factors, and outcome in a single-institution series and review of the literature. Medicine 93 (2014).
- Heiner JG, Terris MK. Effect of advanced age on the development of complications from intravesical bacillus Calmette-Guérin therapy. In Urologic Oncology: Seminars and Original Investigations 26 (2008): 137-140.
- Catalona WJ, Ratliff TL. Bacillus Calmette-Guérin and superficial bladder cancer. Clinical experience and mechanism of action. Surgery annual 22 (1990): 363-378.
- Lee WK, Mossop PJ, Little AF, et al. Infected (mycotic) aneurysms: spectrum of imaging appearances and management. Radiographics 28 (2008): 1853-1868.
- Durek C, Rüsch-Gerdes S, Jocham D, et al. Sensitivity of BCG to modern antibiotics. European urology 37 (2000): 21-25.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 