Clinical Significance of Galectin-3 Expression in Squamous Cell Carcinoma of Lung
Saraswati Pokharel, MD, PhD1*, Umesh C Sharma, MD, PhD2, Kristopher Attwood, PhD3, Sharmeen Mansoor, MD1
1Department of Pathology and Laboratory Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
2Department of Medicine, Jacobs School of Medicine, University at Buffalo, Buffalo, NY, USA
3Department of Biostatistics and Bioinformatics, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
*Corresponding Author: Saraswati Pokharel, Department of Pathology and Laboratory Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA
Received: 27 June 2022; Accepted: 22 July 2022; Published: 22 August 2022
Article Information
Citation: Saraswati Pokharel, Umesh C Sharma, Kristopher Attwood, Sharmeen Mansoor. Clinical Significance of Galectin-3 Expression in Squamous Cell Carcinoma of Lung. Journal of Cancer Science and Clinical Therapeutics 6 (2022): 322-327.
View / Download Pdf Share at FacebookAbstract
Squamous cell carcinoma of lung is an aggressive disease with a poor a prognosis. While majority of these patients do not survive longer than five years, a minor proportion of patients go on to live longer without disease progression. Identification of biomarkers using easily available immunohistochemical assays could improve risk-stratification in lung cancer patients. Galectin-3, a lectin binding protein, expression has been linked to cancer progression and metastasis. We examined the prognostic impact of tumoral galectin-3 expression in 236 patients with completely resected squamous cell carcinoma of the lung and matching normal tissue using tissue microarray samples. In normal lung, galectin-3 staining is present in alveolar macrophages. Galectin-3 expression is detected in 87% of lung squamous cell carcinoma with a mean galectin-3 score of 2 (range 0-3). There was a significant association between galectin-3 expression and pathological stage (p=0.012) and nodal metastasis (p= 0.013). Galectin-3 expression level, however, was not associated with survival outcome. In conclusion, galectin-3 is expression is seen in alveolar macrophages and close to 90% of lung squamous cell carcinoma. Galectin-3 expression is not associated with survival outcome in North American cohort.
Keywords
<p>Lung; non-small cell lung cancer; Squamous cell carcinoma; Galectin-3; Prognosis</p>
Article Details
1. Introduction
Lung cancer is the third most common cancer accounting for 13% of all cancers and the number one cause of cancer deaths (22% of the total cancer deaths) [1]. The overall five-year survival rate is less than 20% [2], reflecting the advanced stage at diagnosis in majority of patients. However, even among early stage cancers, outcomes vary regardless of Tumor Node Metastasis (TNM) stage, with 5-year survival between 58%-83% at best [3]. A five-gene signature has been shown to be an independent predictor of relapse-free and overall survival among NSCLC patients [4]. However, these molecular assays are not readily avialable and are more labor intensive and expensive. Immunohistochemical analyses (IHC) is the most practical method for assessing change in protein expression and perhaps the most readily adaptable technique to clinical practice. It is critical to identify biomarkers using easily available and reproducible assays to improve risk-stratification in lung cancers that facilitate decision making for adjuvant therapy.
Galectin-3 (gal-3) is a beta-galactoside binding protein expressed by various cells including epithelial, neural, and inflammatory cells [5]. It plays an important role in cell-cell and cell-matrix interactions. Preclinical cancer models have shown gal-3 to be associated with tumor cell transformation, apoptosis, adhesion, invasive behavior, and metastasis [5-7]. Gal-3 is overexpressed in several malignancies, including gastric, breast, thyroid and colon cancers, however, higher gal-3 was associated with poorer outcome in colon cancer while better outcome was noted in gastric cancer [8-12]. Gal-3 was found to be overexpressed in non-small cell lung carcinomas, and its expression correlated with tumor progression in preclinical model [13], suggesting its important role in lung cancer development and metastasis. However, gal-3 expression profile varies among different types of lung cancer. For example, small cell lung cancer (SCLC) expresses gal-3 gene at very low levels or not at all, while non-small cell lung cancer (NSCLC) expresses high levels of gal-3 [14]. Previous study reported predictive relation between gal-3 expression and disease recurrence in relatively small number of patients with NSCLC [15]. NSCLC encompasses two major lung cancer types, including adenocarcinoma and squamous cell carcinoma. A recent study showed inhibition of tumor growth by gal-3 inhibitor in a preclinical model of lung adenocarcinoma [16]. Gal-3 expression profile and its clinical implication specifically in squamous cell carcinoma has not been examined.
We hypothesized that gal-3 overespression correlates with advanced stage disease and decreased overall survival in lung squamous cell carcinoma. Gal-3 expression was evaluated by immunohistochemistry in tissue collected from cancer registry patients with lung squamous cell carcinoma who underwent surgical resection.
2. Materials and Methods
2.1 Patients and tissue samples
The Roswell Park Cancer Institute Institutional Review Board (IRB) approved this retrospective project in compliance with federal, state, and local requirements. Two hundred thirty-six patients diagnosed with squamous cell carcinoma of lung, from 1994 to 2011, who had sufficient tissue for tissue microarray, were included in the analysis. Tumor specimens and matched normal lung tissue were collected from patients who underwent surgical resection of lung cancer at Roswell Park Cancer Institute (RPCI), Buffalo, NY. Patients with adenocarcinoma, carcinoid, large cell, and large cell neuroendocrine carcinoma were excluded from this study. All clinical and outcome data were de-identified.
2.2 Tissue microarrays (TMA)
TMAs were constructed from formalin-fixed paraffin-embedded tissues with tumors. TMAs containing lung tumors and matched normal tissues from two hundred and thirty-six samples were prepared with each tumor and normal core sample in triplicate. Each patient had three lung tumor tissue cores spread over three TMA slides. Three 0.6-millimeter tissue cores from formalin-fixed paraffin embedded donor blocks were precisely arrayed into a new recipient paraffin block. Slides were analyzed by a board-certified pathologist blinded to clinical and pathologic data.
2.3 Immunohistochemical staining
The immunohistochemical staining was performed with gal-3 antibody (Abcam). For antigen retrieval, slides were heated in the microwave for 10 minutes in citrate buffer (pH 6.0), followed by a 15-minute cooling period. Endogenous peroxidase was quenched with aqueous 3% H2O2 for 10 minutes and washed with 1xPBS using 0.5% Tween 20 solution. Slides were loaded on a DAKO auto Stainer and blocked with serum-free protein block solution (Dako #X0909) for five minutes and then probed with Gal-3 primary antibody (Abcam) for one hour, followed by the biotinylated goat anti-mouse IgG (Jackson Immuno Research Labs, #115-065-062) for 30 minutes, then by the Elite ABC Kit (Vectastain, #PK-6200) for 30 minutes, and the DAB chromogen (Dako, #K4007) for five minutes. The slides were counterstained with hematoxylin.
2.4 Gal-3 scoring
The staining intensity and percentage of triplicate tumor cores were scored semi-quantitatively as: 0, negative, 1 weak, 2, moderate, and 3 strong. Maximum score on each core was recorded and average score among 3 cores from each patient was calculated. The maximum score was defined by the highest intensity of staining in at least 30% of tumor cells on each core. The average score of <1 was regarded as no expression, 1 to <3 was regarded as low expression, and 3 was regarded as high expression.
2.5 Statistical Methods
Patient characteristics were summarized by gal-3 expression using the mean, median, and standard deviation for continuous variables, and frequencies and relative frequencies for categorical variables. Comparisons were made using the Kruskal-Wallis and Fisher’s exact tests, as appropriate. Overall and recurrence-free survivals were summarized by galectin-3 expression using standard Kaplan-Meier methods, with comparisons made using the log-rank test.
Overall and recurrence-free survivals were summarized in the overall sample using standard Kaplan-Meier methods. The association between survival outcomes and patient demographic/clinical characteristics were evaluated using univariate and multivariate Cox regression models. Time-dependent ROC curves were used to evaluate the association between galectin-3 expression and survival outcomes; where, for each time-point, the AUC(t) and sensitivity/specificity correspond to the Youden’s index were obtained. These values were plotted over time and reflect the prognostic ability of galectin-3 expression. A similar analysis was conducted using the multivariate models. All analyses were conducted in SAs v9.4 (Cary, NC) at a significance level of 0.05.
3. Results
3.1 Clinicopathologic characteristics
There was a total of 248 patient samples included in the TMA, of which 236 met the criteria for analyses, including squamous cell histology and gal-3 levels available. Table 1 summarizes the clinical and pathologic characteristics of patients whose tumor specimen were utilized for this study. The median age of all patients at diagnosis was 69 years (range 39 to 86 years). There were 138 (63%) cases of stage I, 56 (26%) cases of stage II and 25 (11%) cases of stage III and IV disease in this cohort. Disease stage was not recorded for remaining 17 cases. Median follow-up duration was 76 months (range 1 to 175 months).
Multivariate analyses showed a significant association between age at diagnosis and overall survival with lower survival among patients diagnosed at or after the age of 65 (hazard ratio, 1.67; p =0.01). Similarly, recurrence free survival was significantly lower in patients who were diagnosed at >= 65 years of age (hazard ratio 1.69; p = 0.009). Both overall survival and recurrence free survival correlated with pathological grade and stage with lower survival in grade III and stage III/IV patients, respectively (Table 1). There was no difference in survival outcome in relation to gender and race. The male to female ratio was 1.17 (129 male, 107 female). Majority (92%) identified themselves as white. Forty five percent of patients were current smokers, 51% were former smokers and 4% were never smokers.
3.2 Gal-3 expression
Gal-3 staining was diffuse and predominantly cytoplasmic in distribution. Gal-3 expression level was low in 17%, moderate in 67%, and high in 16% of lung tumors (Table 1). Median gal-3 score was 2. In normal lung, most of the pneumocytes did not express gal-3, but alveolar macrophages show moderate to strong staining for galectin-3. Figure 1 shows a representative image of the range of Gal-3 staining intensity.
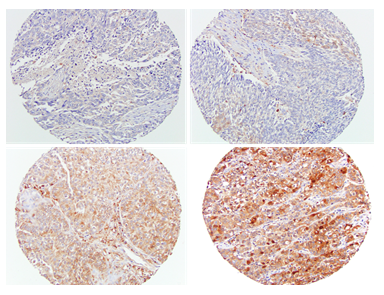
Figure 1: Representative images of gal-3 immunohistochemical staining. Gal-3 expression intensity scores; 0, negative (left upper), 1, weak (right upper), 2, moderate (left lower), 3, strong (right lower). Magnification 200 x.
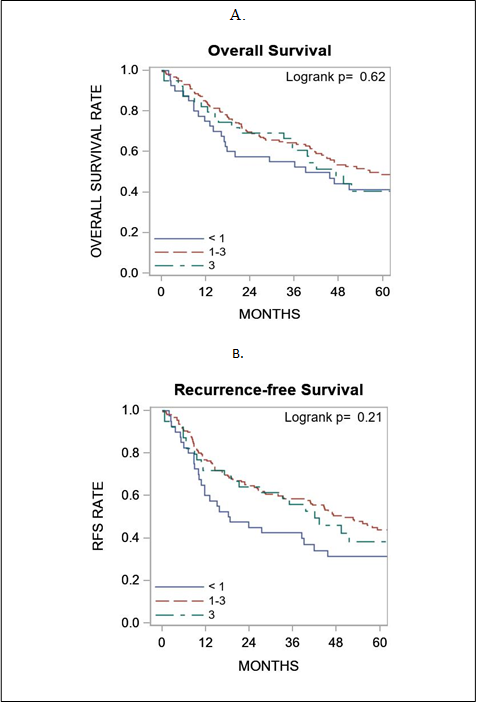
Figure 2: Association of galectin-3 expression with survival outcome. Panel A shows galectin-3 expression in relation to overall survival and panel B shows galectin-3 expression in relation to recurrence free survival. RFS, recurrence free survival.
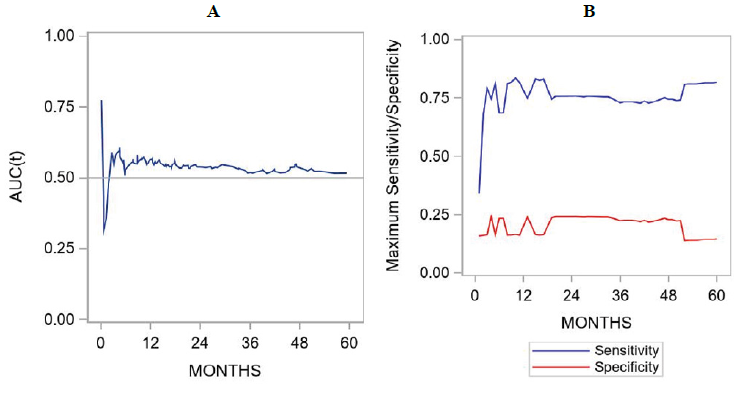
Figure 3: Time-dependent ROC curves showing the association between galectin expression and survival outcomes. For each time-point, the AUC(t) is depicted in panel A and sensitivity/specificity correspond to the Youden’s index are shown in panel B. These figures represent results derived from univariate analyses.
3.3 Gal-3 expression in relation to other patient characteristics
Using average gal-3 intensity score, correlation between gal-3 expression and several patient characteristics were analyzed. Gal-3 score was low in 40 patients (17%), moderate in 157 (66.5%) patients, and high in 39 (16.5%) patients. Univariate comparison of gal-3 scores as a continuous variable showed a significant difference in gal-3 expression and nodal metastasis as well as tumor stage with higher expression in advanced stage disease. There was no significant correlation with other clinicopathological variables, including age, gender, smoking status, and histologic grades (Table 1).
|
< 1 |
1-3 |
3 |
Overall |
P-value |
||
|
Overall |
N |
40 (16.9) |
157 (66.5) |
39 (16.5) |
236 (100%) |
|
|
Galectin |
Mean/Std/N |
0.3/0.3/40 |
1.8/0.6/157 |
3.0/0.0/39 |
1.7/0.9/236 |
<.001 |
|
Median/Min/Max |
0.3/0.0/0.7 |
1.7/1.0/2.7 |
3.0/3.0/3.0 |
1.7/0.0/3.0 |
||
|
Age at Dx |
Mean/Std/N |
67.5/9.1/40 |
69.0/9.9/157 |
66.6/11.2/39 |
68.3/10.0/236 |
0.375 |
|
Median/Min/Max |
67.0/50.0/83.0 |
71.0/40.0/86.0 |
69.0/39.0/84.0 |
69.5/39.0/86.0 |
||
|
Age at Dx |
< 65 |
14 (35.0%) |
49 (31.2%) |
14 (35.9%) |
77 (32.6%) |
0.801 |
|
>= 65 |
26 (65.0%) |
108 (68.8%) |
25 (64.1%) |
159 (67.4%) |
||
|
Race |
White |
37 (92.5%) |
144 (91.7%) |
36 (92.3%) |
217 (91.9%) |
1.000 |
|
non-White |
3 (7.5%) |
13 (8.3%) |
3 (7.7%) |
19 (8.1%) |
||
|
Sex |
Male |
17 (42.5%) |
92 (58.6%) |
20 (51.3%) |
129 (54.7%) |
0.175 |
|
Female |
23 (57.5%) |
65 (41.4%) |
19 (48.7%) |
107 (45.3%) |
||
|
Smoking Status |
Former |
18 (45.0%) |
81 (52.9%) |
18 (46.2%) |
117 (50.4%) |
0.631 |
|
Never |
1 (2.5%) |
6 (3.9%) |
3 (7.7%) |
10 (4.3%) |
||
|
Current |
21 (52.5%) |
66 (43.1%) |
18 (46.2%) |
105 (45.3%) |
||
|
Path Stage |
I |
18 (48.6%) |
99 (68.3%) |
21 (56.8%) |
138 (63.0%) |
0.012 |
|
II |
17 (45.9%) |
31 (21.4%) |
8 (21.6%) |
56 (25.6%) |
||
|
III/IV |
2 (5.4%) |
15 (10.3%) |
8 (21.6%) |
25 (11.4%) |
||
|
Grade |
I/II |
18 (47.4%) |
72 (46.8%) |
18 (47.4%) |
108 (47.0%) |
1.000 |
|
III |
20 (52.6%) |
82 (53.2%) |
20 (52.6%) |
122 (53.0%) |
||
|
Path Nodes |
Negative |
21 (53.8%) |
118 (76.6%) |
25 (64.1%) |
164 (70.7%) |
0.013 |
|
Positive |
18 (46.2%) |
36 (23.4%) |
14 (35.9%) |
68 (29.3%) |
||
Table 1: Demographic and clinical characteristic in relation to galectin-3 expression levels.
3.4 Galectin-3 score and survival outcome
Overall and recurrence-free survivals were summarized by galectin-3 expression using standard Kaplan-Meier methods, with comparisons made using the log-rank test. Comparison of gal-3 score as a continuous variable did not correlate with survival outcome (figure 2). ROC curve analysis did not show a significant performance of gal-3 expression levels to death. For overall survival and gal-3 expression, the AUC at 6-months, 1-year, 2-years, and 5-years was 0.53, 0.56, 0.54, and 0.52, respectively. For recurrence-free survival and Gal-3 expression, the AUC at 6-months, 1-year, 2-years, and 5-years was 0.53, 0.57, 0.55, and 0.53, respectively. The sensitivity and specificity figures indicated that this marker is more sensitive than specific (Figure 3).
4. Discussion
In this study, we analyzed gal-3 expression in squamous cell carcinoma of lung and matched normal lung tissue using TMA. Gal-3 is expressed in majority of squamous cell carcinoma of the lung as well as in alveolar macrophages. This finding is important and provides rationale for investigating gal-3 expression in premalignant lesions as well as biomarker for disease progression into invasive lung cancer. High gal-3 expression is reported in various cancer including breast, thyroid, colon, and NSCLC of lung in general, but this is the first study to examine the prognostic implications of gal-3 expression specifically in squamous cell carcinoma of lung.
Gal-3 is a beta galactoside lectin protein of approximately 30 kDa size, composed of three domains: NH2 terminal domain (decides specific cellular targets), repetitive amino acid residues (serve as a substrate for matrix metalloproteinases) and a carboxyl terminal domain that contains carbohydrate-recognition domain (CRD) [17]. Galectins can be expressed in nucleus or cytoplasm and are secreted from cells into the extracellular matrix. Gal-3 has been implicated in various biological functions including cell-cell interactions. Gal-3 has been detected in various normal organs and tissues including colon, brain, ovary, stomach, liver, skin, capillary endothelium, and inflammatory cells [6, 17].
Several studies have examined gal-3 expression in various cancers and its value in determining the prognosis [1,12, 18]. Gal-3 overexpression is associated with an increased invasiveness of various types of tumor cells such as pheochromocytoma, ovarian, melanoma, thyroid and colorectal cancer cells [19]. It has been shown that overexpression of gal-3 enhances tumor cell adhesion to extracellular matrix (ECM) by its interaction with ECM glycoproteins such as fibronectins, collagen IV, elastin, and laminin and promotes the escape of tumor cells from the primary tumor sites [5, 7]. Nuclear galectin-3 can regulate β-catenin signaling pathway and enhance cyclin D1 and c-MYC expression and promote cell cycle progression in cancer cells [20]. Cytoplasmic gal-3, on the other hand, can interact with the activated GTP-bound K-Ras and cause constitutive activation of Ras-dependent PI3K and Raf-1 activation [21]. In our study, gal-3 expression was found predominantly in cytoplasmic distribution but a small subset of tumor showed both nuclear and cytoplasmic positivity. This supports the role of gal-3 in oncogenic potential. Earlier studies revealed that circulating gal-3 may play an important role in tumor metastases [22]. The level of circulating gal-3 is significantly higher in the bloodstream of cancer patients in particular those with metastasis compared to healthy people [23]. Higher level of circulating galectin-3 is associated with increased metastasis and poor prognosis in several types of cancers including colorectal, thyroid and ovarian cancer [7]. We studied relationship between nodal metastases and gal-3 levels. Gal-3 expression was associated with increased incidence of nodal disease in this cohort.
Castronovo et al. reported down regulation of gal-3 in cancers including colon, breast, ovary and endometrium compared to normal corresponding tissue [18]. However, other studies have shown overexpression of galectin-3 in colon and thyroid carcinomas [12]. Studies have also shown cytoplasmic expression in tongue and prostate carcinoma is associated with worse prognosis [24, 25]. Increased cytoplasmic expression has been reported in advanced stage of colon carcinoma and a correlation between high gal-3 expression in colorectal cancer and cancer progression, liver metastasis, and shorter disease-free survival rates has also been reported [12].
Higher level expression of gal-3 has been reported in non-small cell lung carcinomas using immunohistochemistry and RT-PCR technique [13, 14, 26]. Earlier studies published from Japan also reported predictive value of gal-3 expression in NSCLC recurrence [15]. This study used all subtypes of non-small cell lung carcinoma, including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma in their cohort. We selectively utilized lung carcinoma with squamous cell histology in our analyses. Our results showed that higher gal-3 expression is not directly associated with recurrence free and overall survival in this cohort of patients.
Limitations of our study include the use of TMA, which could account for results in discrepant results with whole tissue section due to the heterogeneity in gal-3 staining pattern within the same tumor. Additionally, we did not evaluate normal bronchial epithelium for gal-3 expression which could be impacted by cigarette smoking and potentially may have prognostic relation on risk of cancer recurrence. Additionally, there might be other confounding variables present in Asian patients that might explain the discrepant prognostic impact of gal-3 expression in this cohort of predominantly Caucasian population. The genomic changes found in NSCLC from Asian patients have a different profile from those found in patients from Western countries, even after controlling for histologic subtype [27]. These differences need to be studied in future investigations regarding the impact of gal-3 expression and lung cancer prognosis.
Funding
This research was supported by Roswell Park Cancer Institute and National Cancer Institute Grant P30CA016056. SP received support from NIH/NHLBI R01 Grant (Award Number: HL150266).
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 61 (2011): 69-90.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 63 (2013): 11-30.
- Goldstraw P, Crowley J, Chansky K, et al. International Association for the Study of Lung Cancer International Staging C and Participating I. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2 (2007): 706-714.
- Chen HY, Yu SL, Chen CH, et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med. 356 (2007): 11-20.
- Matarrese P, Fusco O, Tinari N, et al. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int J Cancer. 85 (2000): 545-554.
- Matarrese P, Tinari N, Semeraro ML, et al. Galectin-3 overexpression protects from cell damage and death by influencing mitochondrial homeostasis. FEBS Lett. 473 (2000): 311-315.
- Newlaczyl AU, Yu LG. Galectin-3--a jack-of-all-trades in cancer. Cancer Lett. 313 (2011): 123-128.
- Baldus SE, Zirbes TK, Weingarten M, et al. Increased galectin-3 expression in gastric cancer: correlations with histopathological subtypes, galactosylated antigens and tumor cell proliferation. Tumour Biol. 21 (2000): 258-266.
- Irimura T, Matsushita Y, Sutton RC, et al. Increased content of an endogenous lactose-binding lectin in human colorectal carcinoma progressed to metastatic stages. Cancer Res. 51 (1991): 387-393.
- Le Marer N, Hughes RC. Effects of the carbohydrate-binding protein galectin-3 on the invasiveness of human breast carcinoma cells. J Cell Physiol. 168 (1996): 51-58.
- Niedobitek C, Niedobitek F, Lindenberg G, et al. Expression of galectin-3 in thyroid gland and follicular cell tumors of the thyroid. A critical study of its possible role in preoperative differential diagnosis. Pathologe. 22 (2001): 205-213.
- Nakamura M, Inufusa H, Adachi T, et al. Involvement of galectin-3 expression in colorectal cancer progression and metastasis. Int J Oncol. 15 (1999): 143-148.
- Chung LY, Tang SJ, Wu YC, et al. Galectin-3 augments tumor initiating property and tumorigenicity of lung cancer through interaction with beta-catenin. Oncotarget. 6 (2015): 4936-4952.
- Yoshimura A, Gemma A, Hosoya Y, et al. Increased expression of the LGALS3 (galectin 3) gene in human non-small-cell lung cancer. Genes Chromosomes Cancer. 37 (2003): 159-164.
- Kataoka Y, Igarashi T, Ohshio Y, et al. Predictive importance of galectin-3 for recurrence of non-small cell lung cancer. Gen Thorac Cardiovasc Surg. 67 (2019): 704-711.
- Vuong L, Kouverianou E, Rooney CM, et al. An Orally Active Galectin-3 Antagonist Inhibits Lung Adenocarcinoma Growth and Augments Response to PD-L1 Blockade. Cancer Res. 79 (2019): 1480-1492.
- Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 230 (2009): 160-71.
- Castronovo V, Liu FT, van den Brule FA. Decreased expression of galectin-3 in basal cell carcinoma of the skin. Int J Oncol. 15 (1999): 67-70.
- Ahmed H, AlSadek DM. Galectin-3 as a Potential Target to Prevent Cancer Metastasis. Clin Med Insights Oncol. 9 (2015): 113-121.
- Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 1760 (2006): 616-635.
- Elad-Sfadia G, Haklai R, Balan E, et al. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem. 279 (2004): 34922-34930.
- Yu LG. Circulating galectin-3 in the bloodstream: An emerging promoter of cancer metastasis. World J Gastrointest Oncol. 2 (2010): 177-180.
- Iurisci I, Tinari N, Natoli C, et al. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 6 (2000): 1389-1393.
- Honjo Y, Inohara H, Akahani S, et al. Expression of cytoplasmic galectin-3 as a prognostic marker in tongue carcinoma. Clin Cancer Res. 6 (2000): 4635-4640.
- van den Brule FA, Waltregny D, Liu FT, et al. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer. 89 (2000): 361-367.
- Buttery R, Monaghan H, Salter DM, et al. Galectin-3: differential expression between small-cell and non-small-cell lung cancer. Histopathology. 44 (2004): 339-344.
- Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 24 (2013): 2371-2376.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 