Albumin and Globulin Fractions from Black Bean Seeds (Phaseolus vulgaris L.) used in the Management of Sickle Cell Disease (SCD) in the West Region of Cameroon have Antisickling and Antioxidant Properties
Kenmoe LR1, Kotue TC1*, Chandra K2, Djouhou FM1, Pieme AC3, Kansci G1, Fokou E1, Arumugam N2
1Laboratory for Food Science and Metabolism, Department of Biochemistry, University of Yaounde 1, Yaounde, Cameroon
2Department of Biotechnology, University of Pondicherry, India
3Laboratory of Biochemistry, Physiology and Pharmacology, Faculty of Medicine and Biomedical Science/UHC- University of Yaounde 1, Yaounde, Cameroon
*Corresponding Author: Kotue TC, Laboratory for Food Science and Metabolism, Department of Biochemistry, Faculty of Science, University of Yaounde 1, Yaounde, Cameroon
Received: 16 May 2020; Accepted: 18 June 2020; Published: 25 June 2020
Article Information
Citation:
Kenmoe LR, Kotue TC, Chandra K, Djouhou FM, Pieme AC, Kansci G, Fokou E, Arumugam N. Albumin and Globulin Fractions from Black Bean Seeds (Phaseolus vulgaris L.) used in the Management of Sickle Cell Disease (SCD) in the West Region of Cameroon have Antisickling and Antioxidant Properties. Journal of Biotechnology and Biomedicine 3 (2020): 78-92.
DOI: 10.26502/jbb.2642-91280029
View / Download Pdf Share at FacebookAbstract
Background: Peptides have shown anti-sickling effect and are able to reduce oxidative stress, thus contributing to the management of sickle cell disease.
Objectives: The present study was undertaken to characterize and to assess the antisickling and antioxidant properties of albumins and globulins fractions from black bean seed (Phaseolus vulgaris L.) used to manage sickle cell disease in West Region of Cameroon.
Methods: NaCl 5% (1:10 w/v) and dialysis were used for extracting albumins and globulins respectively. The concentration of proteins was determined by the Bradford method. Molecular size was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). In vitro antisickling properties, investigate was carried up using microscopic enumeration. Membrane stability effect and antioxidant potentials were determined using colorimetric method.
Results: In non-reducing buffer showed both albumin and globulin in native form, had the main proteins with dense band of about 130 and 37 kDa. Antisickling tests revealed the best inhibition (79.15 ± 3%) and reversibility (73.49 ± 6%) rates with globulin fraction at 3.82 mg/ml. Hemolysis decreased significantly with albumin and globulin fractions at different concentrations showing the stability effect on the membranes of erythrocytes. However the concentration of globulin at 3.82 mg/ml has shown the best activity (from 100 to 4.62%). The FRAP test has shown that the proteins extracted from black bean seeds (Phaseolus vulgaris L.) have a global antioxidant power of 48.38 ± 5 mg FeII/100g (albumin fraction) and 7.26 ± 4 mg Fell/100g (globulin fraction). The value obtained from the albumin fraction was significantly lower (p<0.05) than that obtained with gallic acid used as standard. However, the albumin fraction showed better anti-fre
Keywords
<p>Sickle cell disease, Black bean seeds, Proteins, Albumins, Globulins</p>
Article Details
1. Introduction
Many therapeutic proteins from plant have been used for a long time. This is the case for peptides extracted from Vigna unguiculata with anti-diabetic properties [1]. There are also cases like the use of albumin from potatoes, hemoglobin from tobacco leaves, aprotinin from corn seeds and several others used for different therapeutic treatments [2]. These traditional curative practices are beneficial in the treatment of so-called chronic, incurable and long life pathologies such as sickle cell disease [3]. In fact, sickle cells disease (SCD) also called weakens sickle-shaped or drepanocytosis, is a genetic disease which affects the red blood cell (RBC) [4]. At biochemical and molecular level, the genetically inherited sickle cell disease is characterized by a single base substitution in the gene encoding human β-globin subunit resulting in replacement of β6 glutamic acid (hydrophilic amino acid) in normal hemoglobin (HbA) by valine (hydrophobic amino acid) in abnormal hemoglobin, leading to the devastating clinical manifestations of SCD [5]. This substitution causes drastic reduction in the solubility of sickle cell hemoglobin (HbS) when deoxygenated. Under these conditions, the HbS molecules polymerize to form long crystalline intracellular mass of fibers that cause deformation of the biconcave erythrocyte into a sickle shape erythrocyte. The consequences of this defect are hemolytic anemia and tissue damage brought about by the blockage of blood vessels by the sickled cells. Other clinical manifestations of this condition are hyposthenuria, priapism, vascular necrosis, proliferative retinopathy, aplastic crises, cholelithiasis, delayed growth and sexual maturation, chronic pulmonary disease and chronic nephropathy. The complications can be severe and include retarded growth, periodic attacks of pain and progressive organ dysfunction leading in most cases to a much reduced life expectancy [6]. Likewise, reactive oxygen species (ROS) are considered to play a crucial role in the SCD pathogenesis. The chronically elevated oxidative stress in SCD might play a significant role in the development of SCD related organ complications [7]. Oxidative stress is a major cause of anemia and have a role in complicating anemia with other infectious diseases [8].
SCD affects each year nearly five million people in the world. The prevalence of sickle cell disease (SCD) is 2% on an average in Africa with a life expectancy lower than 20 years [9]. In the other continents this prevalence is 0.02% [10]. In Africa, less than 50% of sickle cell patients attain the age of 5 years and less than18% reach at the adulthood [11]. Faced with this pathological epidemiology and troubling mortality, the WHO recognizes this disease as a major public health problem in many countries.
Even though people with sickle cell disease suffer from several pain crises every year, there is no cure for the majority of them. However, some clinicians tend to treat people with sickle cell disease with therapeutics. Different options that health care professionals apply are preventive measures, symptomatic treatment, bone-marrow transplantation (BMT) and some administer natural products that target different pathway. These treatments are at reducing the pain crises in a year while waiting for bone marrow transplant with the aim to cure from sickle cell disease [12]. The bone marrow transplant is a procedure used to replace damaged or destroyed bone marrow with healthy bone marrow stem cells from a donor. BMT is the only curative therapy available to date. The goal is to eliminate the sickle erythrocyte and its cellular progenitors and replace them with donor hematopoietic pluripotent stem cells that give rise to erythrocytes expressing no sickle hemoglobin (HbS), thereby reducing HbS levels in those associated with the trait [13]. Bone marrow transplantation has recorded good curative results in some Central Africa SCD children living in Belgium [14-17]. Its application in Africa is difficult because of its high cost (approximately one hundred thousand Euros) and lack of technical equipment [18].
The economics of treating sickle cell disease are of great importance in developing countries. The treatment is out of reach to the populations of Sub-saharian Africa. The most popular approach currently explored to prevent or reverse sickling in vitro and in vivo is to employ compounds which directly target the hemoglobin (Hb) molecule. Often, food plants and medicinal herbs are made use in the management of sickle cell associated morbidities by the less privileged classes of the society. Natural products like polypeptides from food plants and medicinal herbs are found to possess properties which prevent the erythrocytes from deformation and loss of integrity. Protein-based therapeutics have been reported to be highly successful in clinics and currently therefore enjoys unprecedented recognition for their potential. There seems to be a synergy between short and long polypeptide fragments that enable them to serve as anti-sickling agents [19]. Some oligopeptides act as potential anti-aggregation agents for deoxyhemoglobin S, antisickling and prevent the adherence of sickle cells to endothelial cells [20-22]. The preponderance of anti-sickling peptides in the seed extract of Vigna unguiculata probably must have been responsible for its use in the management of Sickle Cells Disease [23]. Recently, some investigations revealed that in west region of Cameroon, people use local black bean seeds (Phaseolus vulgaris L.) to manage sickle cell disease [24]. Infact, this common bean plays a vital role in the vegetarian diets and provides numerous health benefits connected with eating pattern [25]. Health benefits of beans are generally acquired from direct attributes, including their high content of dietary fibers, low saturated fat content, vitamins, minerals, phytochemical compounds and proteins [26]. The present study is an attempt to characterize, the antisickling and antioxidant properties of albumin and globulin fractions isolated from black bean seed (Phaseolus vulgaris L.) in order to explore the new sources of proteins with therapeutic value.
2. Methodology
2.1 Collection and preparation of plant materials
The seeds of black beans sample (figure 1) were obtained from sickle cell patients families and authenticated as PNN, a wild variety at the Agricultural Institute of Research for the Development of Foumbot station, Cameroon. At the Department of Biotechnology of the Pondicherry University, India, the seeds (1 kg) were weighted (Infra Digi balance), pulverized using an electrical grinding machine (Preethi). The flour material was stored in cold room at 4°C for further analysis.

figure 1: Photograph of black beans seeds (Phaseolus vulgaris L.) PNN (wild variety) collected from local sickle cell patient's families of West Region of Cameroon.
2.2 Protein fractions
About 400 g of bean flour was defatted overnight in a horizontal shaker using hexane (1:10 g/ml) at room temperature. The protein concentration in the defatted flour was determined. After this step, the mixture was filtered using Whatman paper No1. The defatted flour was left in a chapel of exhaust gases at room temperature for solvent evaporation.
2.3 Extraction of total albumin and globulin
The extraction of albumin and globulin fractions was performed according to the methodology described by [27]. About 5 g of the defatted flour were dispersed in NaCl 5% (1:10 w/v) and subjected to mechanical stirring for 30 minutes. Then, the suspension was centrifuged at 12 600 rpm for 15 minutes at 4ºC, obtaining the supernatant S1 and the residue R1. The residue (R1) was suspended again in NaCl 5% (1:10 w/v) and protein was extracted twice. This yielded supernatants S2 and S3 and the residue R2. The supernatants S1, S2 and S3 were mixed and subjected to dialysis in water for 24 hours at 4°C with several water exchanges during the period. This resulted in precipitation of globulin from the extracts. The dialyzed material was centrifuged at 12 600 rpm for 15 minutes at 4°C. Precipitate containing the globulin (salt-soluble protein) and the supernatant containing the albumins (water-soluble protein) were collected separately and stored at 4°C. The globulin fraction was then dissolved in water to get rid of the traces of albumin, which might have been dragged with the precipitate. The solution was centrifuged at 12 600 rpm at 4°C for 15 minutes and the residue was stored 4°C. Albumin and globulin have been lyophilized and the powders so obtained were used for antisickling in vitro and antioxidant essay.
2.4 Determination of protein content
NaCl 5% (1:10 w/v) and dialysis were used for extracting albumins and globulins respectively Protein content of each of the fraction was determined following the Bradford method [28]. Spectrophotometer calibration was performed using analytical reference solutions of BSA.
2.5 Separation of proteins by polyacrylamide gel electrophoresis
The protein fractions obtained were further analysed by SDS-PAGE for determination of the presence of any protein subunits and their estimated molecular weights. The path was performed according to a procedure of [29]. Both native (non-reducing) and SDS (reducing) PAGE were performed. The SDS-PAGE was carried out on a vertical slab gel (4% stacking gel, 15% separating gel) in SDS-Tris-Glycine discontinuous buffer system. Protein solutions (2 μg protein/μL) were prepared in reducing (62.5 mM Tris-HCl pH 6.8; 2% SDS; 10% glycerol; and 0.05% bromophenol blue) and nonreducing buffer solutions (62.5 mM Tris-HCl pH 6.8; 2% SDS; 10% glycerol; 0.05% bromophenol blue, and 0.05% 2-β-mercaptoethanol). Ten microliters of the solution was loaded onto the gel. Electrophoresis was performed at a constant current of 45 mA for approximately 45 min. The gel was stained by 0.1% Coomassie brilliant blue in acetic acid/ethanol/water solution (10/40/50, v/v/v) and destained in acetic acid/ethanol/water solution (10/20/70, v/v/v). Approximate molecular sizes of the proteins were determined using molecular size standards.
2.6 Blood samples collection
After receiving an ethical clearance with number 906/CRERSHC/2019, consent from all blood donors was read and signed by all the patients participating in the study after being informed of the research objectives. The blood samples used in the evaluation of the antisickling activity of the protein fractions in this study were taken from 16 confirmed sickle cell patients (8 males and 8 females) between 17 and 39 years old known to have sickle cell disease, attending monthly Hematology outpatient clinic at the Yaounde Central Hospital. 4.5 mL of blood samples were collected in the sodium EDTA tubes and stored for the experiment.
2.7 In vitro Antisickling activity
All anti-sickling experiments and hemolysis test were performed as reported earlier [30, 31].
2.8 Antioxidant activity of albumin and globulin fractions
The Total antioxidant activity by ferric reducing antioxidant power assay (FRAP) was used to determine the total antioxidant activity which measures the reduction of ferric ion to the ferrous form in the presence of antioxidant compounds according the method perform by [32]. The DPPH free radical scavenging assay was carried out for the evaluation of the antioxidant activity using the method described by [33]. However, hydroxyl radical scavenging activity radical was measured according to a previously method proposed by [34].
2.9 Statistical analysis
The results were expressed as mean ± standard deviation. Data was analyzed using Analysis of Variance (ANOVA) of Kruskall-Wallis with the software Sigma Start version 3.01A analysis software. Statistical data were considered significantly different at 95% confidence interval (p <0.05).
3. Results
3.1 Protein content of each group
The black bean seeds (Phaseolus vulgaris L.) used to manage sickle cell disease in the West Region of Cameroon contained 11.768 g/100g total protein. The fractionation of protein yielded 56.483 % albumin and 17.088% globulin (Table 1).
|
Fractions of protein |
Protein content |
Total protein |
|
|
(mg/g) |
(g/100g) |
% |
|
|
Black bean |
117.68 ± 1.3 |
11.768 ± 1.3 |
- |
|
Albumins |
66.47 ± 0.78 |
6.647 ± 0.78 |
56.483 ± 0.78 |
|
Globulins |
20.11 ± 0.45 |
2.011 ± 0.45 |
17.088 ± 0.45 |
Table 1: Protein content of different fractions of proteins extracted from black bean seeds.
figure 2 shows electrophoretograms of albumins and globulins in reducing and non-reducing buffers. Electrophoresis patterns showed simplicity in the structure of protein in albumin and globulin fractions. In reducing buffer solution that breaks down intermolecular disulfide bonds in the quaternary structure of albumins (1) showed a great variety of proteins. At least five bands with molecular weights in the range of 130 to 37 kDa could be observed. There were two which were less than 37 kDa. The globulin (2) fraction showed four bands, three in the range of 63-37kDa and one <37 kDa. (3) The electrophoretograms of the fractions in non-reducing buffer revealed albumins (4) and globulins (5) to be the main protein with dense bands. Albumins were represented by two bands with the molecular sizes of about 130 and 37 kDa, while globulins had only one dense protein with the molecular size of 130 kDa. A comparative account of results indicates that albumins and globulins protein fractions contain disulfide bonds in their protein structure. Sodium metabisulfite (SMB) 2%, added to sicklier red blood cells at equal volumes provoked sickling of red blood cells (figure 3). At time 0 hour, sickling percentage was 14.6%, and after 3 hours of incubation sickling increased on average from 14.6 ± 1.8% to 72.22 ± 2.8% and remained constant with time. The maximum number of sickled cells was obtained after 3 hours, suggesting that this is the time necessary to obtain maximum sickling.
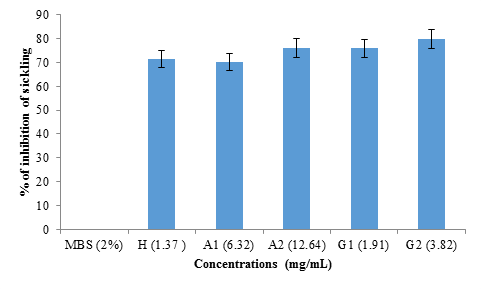
figure 4 shows the rate of inhibition of sickling of albumin and globulin fractions from black bean seeds at different concentrations (1.37 mg/mL; 6.32 mg/mL; 12.64 mg/mL; 1.91 mg/mL and 3.82 mg/mL) representing hemodya as references, albumin (A1), albumin (A2), globulin (G1) and globulin (G2) respectively. No significant difference (p>0.05) was observed between the percentage of inhibition obtained with hemodya and that of A1 (6.32 mg/mL) (70.09 ± 4%). However, G2 (3.82 mg/mL) showed significantly (P <0.05) highest sickling inhibition rate (79.15 ± 3%), followed respectively by A2 (12.64 mg/ml) (76.02 ± 1%) and G1 (1.91 mg/ml) (75.90 ± 5%).
The morphological states of patients red blood cells observed under the optical microscope (40x/0.65) after incubation at 37°C for 3 hours were presented in figure 5 bellow. When incubated with 2% SMB alone, there were approximately 17 sickled cells seen figure 5 (a); Incubation in a combination of 2% SMB and 3.82 mg/mL Globulin (G2) approximately there were only 3 sickled cells figure 5 (b).
The study of the reversibility of sickling was done in the absence of 2% SMB (figure 6). No significant difference (p>0.05) was observed between the reversibility rate obtained with hemodya (62.58 ± 6%) and that of A1 (6.32 mg mL) (62.39 ± 9%). However, G2 (3.82 mg/mL) showed significantly (P<0.05) the highest sickle reversibility rate (79.31 ± 6%), followed respectively by A2 (12.64 mg/mL ) (70.75 ± 8%) and G1 (1.91 mg/mL) (70.62 ± 7%).
figure 7 shows the effects of albumin and globulin from black bean seeds on hemolysis. The study was conducted using NaCl at concentrations ranging from 0 to 0.85% as a negative control. The hemodya, a phytodrug (1.37 mg/mL) commonly consumed by sickle cell anemia was used as the standard. Compared to the negative control, hemolysis decreased significantly with the concentrations of the protein fractions tested (P<0.05). Hemodya at 1.37 mg/ml concentration showed better stabilizing activity on the red blood cell membrane followed by G2 (3.82 mg/ml) and A2 (12.64 mg/ml).
Table 2 shows the evaluation of the reduction and scavenging properties of albumin and globulin fractions using gallic acid as references. With respect to the total antioxidant capacity expressed in mg FeII/100g, the fractions of albumin and globulin extracted from black bean seeds (Phaseolus vulgaris L.), respectively have showed 48.38 ± 5 mg FeII/100g and 75.26 ± 4 mg FeII/100g. However, these value are significantly lower (P<0.05) than that obtained with gallic acid (78.49 ± 6 mg FeII/100g) used as standard. The albumin and globulin fractions were significantly less active on the DPPH radicals (IC50 = 7.37 ± 0.2 mg/ml and IC50 = 13.56 ± 0.8 mg/ml) than gallic acid (IC50 = 2.67 ± 0.1 mg/ml) used as standard. However, a significant difference (P<0.05) was noted between these three values. For the hydroxyl radical, the albumin (IC50 = 5.96 ± 0.1 mg/mL) and globulin (IC50 = 13.41 ± 2 mg/mL) fractions presented significantly different values (P<0.05). These values were also significantly lower than that obtained with the standard gallic acid (IC50 = 0.32 ± 0.0 mg/mL).
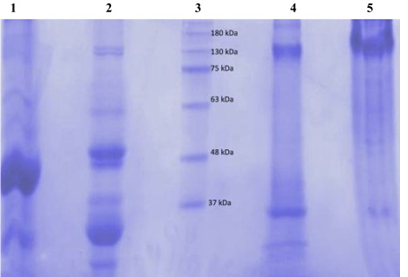
figure 2: Electrophoretic profile in 7.5% polyacrylamide gel of the black bean seeds protein fractions (globulin and albumin) using reducing (Lanes 1 and 2) and non-reducing buffers (Lanes 4 and 5). Lane 3 was the marker molecular weight protein standards.
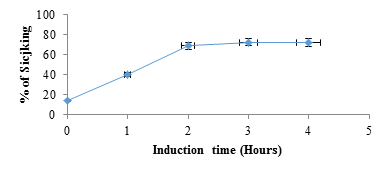
figure 3: Percentage of sickling as a function of time after induction of red blood cells with SMB (2%). At time 0 hour, SMB 2% was replaced by NaCl 0.9%.
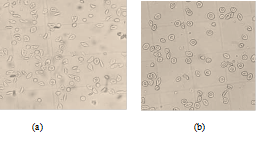
figure 5: Morphological states of patients red blood cells observed under the optical microscope (40x/0.65) after incubation at 37°C for 3 hours: (a) Incubation only with SMB 2% shows approximately 17 sickled cells; (b) Incubation with SMB 2% and the Globulin (G2: 3.82 mg/mL) shows approximately 3 sickled cells.
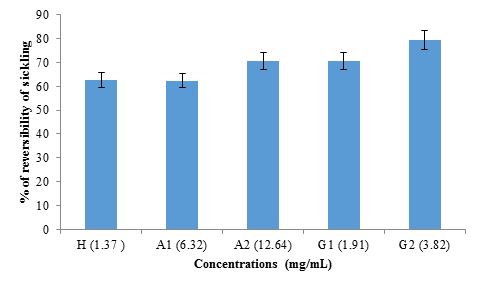
figure 6: Reversibility rate of albumin and globulin fractions from black bean seeds at different concentrations (1.37 mg/mL; 6.32 mg/mL; 12.64 mg/mL; 1.91 mg/mL and 3.82 mg/mL) for hemodya as references compared to albumin (A1), albumin (A2), globulin (G1) and globulin (G2) respectively.
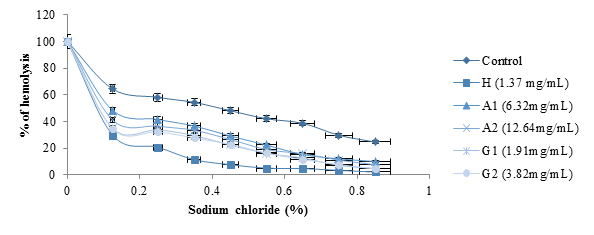
figure 7: Effects of albumin and globulin from black bean seeds on hemolysis.
|
Albumin |
Globulin |
Gallic acid |
|
|
FRAP (mgFeII/100g) |
48.38 ± 1.5a |
75.26 ± 4b |
78.49 ± 6b |
|
DPPH (IC50mg/mL) |
7.37 ± 0.2a |
13.56 ± 0.8b |
2.67 ± 0.1c |
|
OH (IC50mg/mL) |
5.96 ± 0.1a |
13.41 ± 2b |
0.32 ± 0.0c |
*Mean values from triplicate measurements ± standard deviation. values in the same row followed by different
superscripts are significantly different (p<0.05).
Table 2: Evaluation of the reduction and scavenging properties of albumin and globulin fractions using gallic acid as references.
4. Discussion
Albumins and globulins are the major proteins in blood. Serum albumins accounts for 55% and globulins make up 38% of blood proteins. These two proteins from black bean seeds and those found in the blood can play fundamental role in the management of SCD. In common beans, the total protein content ranges from 16% to 33%. The major protein fractions in Phaseolus beans are globulins and albumins [35].
Electrophoretic results showed that albumin fraction has five types of protein with molecular weights in the range of 130 to 37 kDa. There were two which were less than 37 kDa. The globulin fraction showed four bands, three in the range of 63-37 kDa and one < 37 kDa. These results are different from those obtained by [36], who had shown that black bean seeds were missing proteins in the molecular mass (MM) range of 60 to 97 kDa. In fact albumin belong to the class of alpha amylase inhibitor and trypsin family, these proteins are disturbed with defense mechanism of plant [37, 38]. The faseolamin (7s) is the major storage protein of black beans seeds. This main globulin fraction corresponds to 40% to 50% of the bean’s proteins. This oligomeric protein is composed of three polypeptide subunits –α, β and γ- with molecular weight between 43 kDa to 53 kDa [39]. While the other globulin’s fraction, the legumins (11S), correspond to only 10% of the bean’s proteins [40]. Electrophoretic bands correspondent to faseolin fraction (47 kDa and 44 kDa) were observed. Here, the results paradoxically show that the globulin fraction obtained was 17,088 ± 0.45%. Among several benefits of peptides reported, the most evident are anti-hypertension, antioxidant, antimicrobial and anti-carcinogenic activities and the control of lipid metabolism [41]. Some oligopeptides act as potential anti-aggregation agents for deoxyhemoglobin S, antisickling and prevent the adherence of sickle cells to endothelial cells [20-22].
To verify the antisickling activity of albumin and globulin fractions from black bean seeds, an in vitro bioassay was performed. The percentage of sickling obtained after 3 hours of induction in this study is lower than the values 73.25 ± 2% and 80.67 ± 3% obtained respectively by [42] and [43]. However, it is greater than 55.46% obtained by [44] after 2 hours of incubation. The increase in the percentage of sickle cells after induction is due to the fact that 2% sodium metabisulfite has the ability to deprive red blood cells of oxygen, thus causing a loss of their morphology. Indeed, in the absence of oxygen, hemoglobin S polymerizes inside the red blood cell giving it a form of fiber [45, 46].
As for the effect of the protein fractions extracted from black bean grains (Phaseolus vulgaris L.), they significantly inhibit (P <0.05) the sickling induced by SMB 2% in sickle cell red cells for all concentrations compared to the treated control without protein extract. For this purpose, the inhibition rates of induced sickling were 70.09 ± 4%; 76.02 ± 1% (respectively at the albumin fraction at concentrations A1: 6.32 mg/mL and A2: 12.64 mg/mL) and 75.90 ± 5%; 79.15 ± 3% (respectively at the globulin fraction at G1 concentrations: 1.91 mg/mL and G2: 3.82 mg/mL). This rate was 71.45 ± 8% for the hemodya positive control at the concentration of 1.37 mg/ml. These inhibition rates are less than 80.33 ± 1% obtained with the protein extract of the grains of Vigna unguiculata by [23] and above 21% obtained by [47] with a peptide fragment (His-Lys-Tyr-His). This effect could be explained by the ability of these protein fractions (albumin and globulin) to bind to certain sites of HbS in order to prevent their aggregation and therefore their polymerization leading to sickling. Indeed, [48] had shown that it was in this way that an oligopeptide segment (Val-His-Leu-Thr-Pro-Val-Glu-Lys-Ser-Ala…) inhibited sickling. The reversibility of sickling studied by incubation of red blood cells in the absence of 2% SMB showed reversibility rates of 62.39 ± 9%; 70.75 ± 8% (respectively at the albumin fraction at concentrations A1: 6.32 mg/mL and A2: 12.64 mg/mL) and 70.62 ± 7%; 79.31 ± 6% (respectively at the globulin fraction at concentrations of G1: 1.91 mg/mL and G2: 3.82 mg/mL). These reversibility rates are greater than 54.6 ± 5% obtained by [23] with the protein extract of the grains of Vigna unguiculata. Reversibility is the ability of sickle cells (sickle-shaped) to return to a normal form (biconcave form). This could be due to the aromatic amino acids contained in these protein fractions. In fact, aromatic amino acids such as Phe, Tyr and Trp have been shown to have the ability to interpose between polymerized deoxyHbS molecules, in order to competitively inhibit specific contacts between them [21]. As a result, the protein fractions could lift the binding of the HbS deoxy molecules initially joined together. Sickle cell red blood cells are fragile and susceptible to hemolysis. Compared with the control (p<0.05), the two protein fractions had a positive influence on the stability of the erythrocyte membranes. However, the hemodya standard at the concentration 1.37 mg/ml showed the best membrane stability, followed by the globulin fraction at the concentration 3.82 mg/ml. This effect could be explained by the fact that the protein fractions exert a counter-osmosis thus protecting the erythrocyte membrane from hemolysis.
The total antioxidant capacity of the protein fractions of black bean grains (Phaseolus vulgaris L.) is 48.38 ± 5 mg FeII/100g and 75.26 ± 4mg Fell/100g respectively for albumin and globulin. Thus, no significant difference (p<0.05) was observed between the value obtained with the globulin fraction and that of the gallic acid used as standard (78.49 ± 6mg FeII/100g). This effect could be explained by the presence in these protein fractions of the acidic (aspartic and glutamic acids) amino acids and basic (lysine, arginine, and histidine) amino acids which have strong global antioxidant properties [49]. The DPPH test made it possible to determine the ability of protein fractions to scavenge free radicals or act as a donor of hydrogen. The results obtained showed that the protein fractions extracted from black bean grains (Phaseolus vulgaris L.) trapped the free radicals of DPPH with an IC50 of 7.37 ± 0.2 mg/mL and 13.56 ± 0.8 mg/mL respectively for albumin and globulin. However, these values are significantly higher (p<0.05) than that of gallic acid (2.67 ± 0.1 mg/mL), which testifies to their weak anti-radical activity judged at the end. However, these values are greater than IC50 = 175 µg/mL, obtained by [50] with the crude extract of proteins from Leucas linifolia. The hydroxyl radical is known as the most aggressive radical because it commonly attacks several biomolecules such as DNA, proteins, lipids of the membranes, which alters the permeability of the latter [51]. Sickle cells have been shown to generate twice as many •OH radicals as normal cells [52]. The protein fractions extracted from black bean grains (Phaseolus vulgaris L.) have proven thier ability to trap these hydroxyl radicals with an IC50 of 5.96 ± 0.1 mg/mL and 13.56 ± 0.8 mg/mL respectively for albumin and globulin. However, these values are significantly higher than that obtained with gallic acid (IC50 = 0.32 ± 0.0 mg/mL), which testifies to their weak capacity to trap the OH radicals as compared to the latter. This trapping capacity could be linked to the presence of hydrophobic amino acids to which are attributed a high reducing power due to their capacity to emit electrons [53].
5. Conclusion
This study recorded information on the quaternary structure of albumin and globulin fractions found in black bean seeds (Phaseolus vulgaris L.). They have five and fours sub-unit, respectively for albumin and globulin. These fractions have antisickling, anti-hemolytic and antioxidant properties. Globulin at 3.82mg/mL has shown the best properties. These results may justify the use of black bean seeds by sickle cell patients. The mode of action, nutraceutical capsules/functional food formulation with globulin at 3.82 mg/mL for SCD management will be the next focus of this work.
Conflict of Interest Statement
We declare that we have no conflict of interest.
Acknowledgements
We would like to thank CV Raman Fellowship/fiCCI, India for partial financial support.
References
- Barnes MJ, Uruakpa FO and Udenigwe CC. Influence of cowpea (Vigna unguiculata) peptides on insulin resistance. Journal of Nutritional Health and Food Science 3 (2015): 1-3.
- Faye L, Landry N, Lerouge P, et al. Production of proteins for biopharmaceutical applications in plants. médecine/sciences 17 (2001): 867-877.
- Mozouloua D. Traitement traditionnels de 150 maladies a base des plantes. experience de lokondo. Unité de Recherche en Sciences Appliquées au Développement (URSAD) 13 (2004): 111-116.
- La drépanocytose dans la région Africaine: situation actuelle et perspective. Comité régional de l’Afrique 52ème session (2006): 22.
- Wajcman H, Labie D. Aspects de la biologie de la drépanocytose. Annuaire Médecine Interne 132 (1981): 568-594.
- Svarch E, Hernández P, Ballester JM. Sickle Cell Disease in Cuba. Institute de Hematologiae Inmunologia (IHI) La Habana, Cuba (2001): 13-45.
- Xu W, Chi L, Row BW, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 126 (2004): 313-323.
- Wellems TE, Hayton K, Fairhurst RM. The impact of malaria parasitism: from corpuscles to communities. Journal of Clinical Investigation 119 (2009): 2496-2505.
- Gdadoe AD, Kampatibe N, Bakonde B, et al. Attitudes thérapeutiques chez le drépanocytaire en phase critique et inter critique au Togo. Méd. d’Afrique Noire 45 (1998): 155-162.
- Galactéros F. Drépanocytose, Encyclop. Orphanet (2000): 15.
- Arnal C and Girot R. Drépanocytose chez l’adulte. Encycl. Méd. Chir. (Editions scientifiques et Médicales Elsevier SAS, Paris). Hématologie J 16 (2002): 15-26.
- Kotue TC. Sickle cell disease: targeted pathways of antisickling product. Pharmacophore 7 (2016): 166-175.
- Parkman R. The application of bone marrow transplantation to the treatment of genetic diseases. Science 232 (1986): 1373-1378.
- Walters MC, Storb R and Patience M. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Blood 95 (2000): 1918-1924.
- Vermylen C, Cornu G, Philippe M, et al. Bone marrow transplantation in sickle cell anaemia. Archives of Disease in Childhood 66 (1991): 1195-1198.
- Vermylen C and Cornu G. Bone marrow transplantation in sickle cell anaemia. Blood Reviews 7 (1993): 1-3.
- Vermylen C, Cornu G, Ferster A, et al. Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transplant 22 (1998): 1-6.
- De Montalembert M et Tshilolo L. Les progrès thérapeutiques dans la prise en charge de la drépanocytose sont-ils applicables en Afrique subsaharienne? Médecine Tropicale 6 (2007): 612-616.
- Noguchi CT and Schechter AN. Effects of amino acids on gelatin kinetics and solubility of sickle hemoglobin. Biochem Biophys Res Commun 74 (1977): 637- 642.
- Shigeo K and Yang JT. Oligopeptides as potential antiaggregation agents for deoxyhemoglobin S. Proc. Natl. Acad. Sci. USA Biochemistry 74 (1977): 5431-5434.
- Górecki M., Votano RJ, and Alexander R. Peptide Inhibitors of Sickle Hemoglobin Aggregation: Effect of Hydrophobicity. Biochemistry 19 (1980): 1564-1568.
- Bernard JM, Thevenin, Ian C, et al. Band 3 Peptides Block the Adherence of Sickle Cells to Endothelial Cells In Vitro. Blood 90 (1997): 4172-4179.
- Egba SL, Ogugua N, Emmanuel TN, et al. Antisickling potential of the ethanol seeds extracts of Vigna unguiculata and Vigna subterranean. Full Length Research Paper 1 (2012): 2169-3048.
- Kotue TC, Pieme AC. et Fokou E. Ethnobotanicals usages in the management of sickle cell disease (SDC) in some localities of Cameroun. Pharmacophore 7 (2016): 192-200.
- Haddad EH, Tanzman JS. What do vegetarians in the United States eat? Am. J. Clin. Nutr 78 (2003): 626-632.
- Messina V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr 100 (2014): 437-442.
- Sathe SK and Salunkhe DK. Isolation, Partial Characterization and Modification of the Great Northern Bean (Phaseolus vulgaris L.) Starch. Journal of Food Science 46 (1981): 617-621.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72 (1976): 248-254.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227 (1970): 680-686.
- Joppa KM, Vovora Eklu K, Agbonon A, et al. Effet de Morinda Lucida Benth (Rubiaceae) et de NewbouldiaLeavis p. Beau v. Bignoniaceae) sur la falciformation. Med. Trop 68 (2008): 251-256.
- Jaja SI, Kehinde MO, Gbenebitse S, et al. Effect of vitamin C on arterial blood pressure, irreversible sickled cells and osmotic fragility in sickle cell anemia subjects. Nig J Physio Sci 16 (2000): 14-18.
- Benzie I and Strain J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power: The FRAP Assay”. Analytical Biochemistry 239 (1996): 70-76.
- Jain A, Soni M, Deb L, et al. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leaves. J. Ethnopharmacol 115 (2008): 61-66.
- Yu LL, Beta T. Identification and antioxidant properties of phenolic compounds during production of bread from purple wheat grains. Molecules 20 (2015): 15525-15549.
- Vidigal FP, Gonçalves-Vidigal C, Hammerschmidt R, et al. Characterization and content of total soluble protein and amino acids of traditional common bean (Phaseolus vulgaris L.) cultivars collected in Paraná state, Brazil. Journal of Food Agriculture and Environment 3 (2011): 9143-9147.
- Rui X, Boye JI, Ribereau S, et al. Comparative study of the composition and thermal properties of protein isolates prepared from nine Phaseolus vulgaris legume varieties. Food Research International 44 (2011): 2497-2504.
- Tatham AS, and Shewry PR. Allergens in wheat and related cereals. Clinical and Experimental Allegy 38 (2008): 1712-1726.
- Shewry PR, Lucas JK. Plant proteins that resistance to pests and pathogens. Advances in Botanical Research 26 (1997): 135-192.
- Yin SW, Tang CH, Wen QB, et al. Functional properties an in vitro trypsin digestibility of red kidney bean (Phaseolus vulgaris L.) protein isolate: effect of high-pressure treatment. Food Chemistry 110 (2008): 938-945.
- Montoya CA, Lallès JP, Beebe S, et al. Phaseolin diversity as a possible strategy to improve the nutritional value of common beans (Phaseolus vulgaris L). Food Research International 43 (2010): 443-449.
- Roy F, Boye JI and Simpson B K. Bioactive proteins and peptides in pulse crops: pea, chickpea and lentil. Food Research International 43 (2010): 432-442.
- Nkenmeni DC, Kotue TC, Kumar P, et al. HPLC profiling, in vitro antisickling and antioxidant activities of phenolic compound extracts from black bean seeds (Phaseolus vulgaris L.) used in the management of sickle cell disease in the West Region of Cameroon. International Journal of Food and Nutrition Research, 30 (2019): 2572-8784.
- Kotue TC, Wirba LN, Jayamurthy P, et al. HPLC profiling, in vitro antisickling and antioxidant activities of amino acids from black bean seeds (Phaseolus vulgaris l.) used in de management of sickle cell disease (SCD) in the west region of Cameroon. International Journal of Current Research 11 (2019): 5872-5880.
- Nanfack P, Biapa NP, Pieme C, et al. The in vitro antisickling and antioxidant effects of aqueous extracts Zanthoxyllum heitzii on sickle cell disorder. BMC Complementary and alternative medicine 1 (2013): 1-7.
- Bunn HF and Forget BG. Hemoglobin: Molecular, Genetic and Clinical Aspects. The WB Saunders Co., Philadelphia (1986): 12.
- Edelstein SJ, Telford JN, Crepeau RH. Structure of fibers of sickle cell hemoglobin. Proc. Natl. Acad. Sci 70 (1973): 1104-1107.
- Franklin M, Cotter R, Cheetham R, et al. A potent new dipeptide inhibitor of cell sickling and haemoglobin S gelation. Eur. J. Biochem 136 (1983): 209-214.
- Shigeo K and Jen TY. Oligopeptide as potential antiaggregation agents for desoxyhemoglobin S. Proc. Nat. Acad. Sci 74 (1977): 5431-5434.
- Zhang Y, Zhang H, Wang L, et al. Influence of the degree of hydrolysis (DH) on antioxidant properties and radical-scavenging activities of peanut peptides prepared from fermented peanut meal. European Food Research and Technology 232 (2011): 941-950.
- Harsha R, Sushma S, Mamatharani DR, et al. Hydroxyl radical and DPPH scavenging activity of crude protein extract of Leucas linifolia: a folk medicinal plant. Asian Journal of Plant Science and Research 2 (2012): 30-35.
- Nur E, Biemond J, Otten H, et al. Oxidative stress in sickle cell disease; pathophysiology and potential implications for disease management. Am J Hematol 86 (2011): 484-489.
- Asakura TK, Minakata K, Adachi M, et al. Denatured hemoglobin in sickled erythrocytes. J. Clin. Invest 59 (1977): 633-640.
- Ranathunga S, Rajapakse N, Kim SK. Purification and characterization of antioxidative peptide derived from muscle of congereel (Conger myriaster). Eur Food Res Technol 222 (2006): 310-315.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 