Recovery of Proliferative Fibroblast Cells from Refrigerated Sheep Skin after Different Postmortem Time Intervals and their Characterization
Mahipal Singh*, Xiaoling Ma
Animal Biotechnology Laboratory, Agricultural Research Station, Fort Valley State University, Fort Valley, GA 31030, USA
*Corresponding author: Mahipal Singh, Animal Biotechnology Laboratory, Agricultural Research Station, Fort Valley State University, Fort Valley, GA 31030, USA.
Received: 26 September 2023; Accepted: 04 October 2023; Published: 01 February 2024
Article Information
Citation: Mahipal Singh, Xiaoling Ma. Recovery of Proliferative Fibroblast Cells from Refrigerated Sheep Skin after Different Postmortem Time Intervals and their Characterization. Journal of Biotechnology and Biomedicine. 7 (2024): 93-100.
DOI: 10.26502/jbb.2642-91280130
View / Download Pdf Share at FacebookAbstract
Background: Postmortem tissues are a potential source of stem/progenitor cells for cellular therapies, preservation of germplasm and revival of endangered and/or dead species by cloning. How long they can be recovered after animal death, however, is not known precisely. The objective of this study was to evaluate the window of postmortem interval (PMI) within which live and proliferative cells can be recovered from refrigerated sheep skin. Ear skin was procured from animals from slaughterhouse and stored at 4°C in the lab. Small explants (2-3 mm2) were then cultured in DMEM media supplemented with 10% FBS, 50 units/mL of penicillin, 50 µg/mL of streptomycin, and 2.5 µg/mL of fungizone after different PMI. Outgrowth of cells around the explants was scored after 10-12 days of culture at 37°C in a CO2 incubator and cells from selected PMI were sub-cultured for 3-5 times and characterized with respect to their growth profiles, genetic stability, cryopreservation ability and gene expression.
Results: A total of 474 explants adhered to dish surface, of which 369 (77.89%) exhibited outgrowth in various PMI including 34.79% of 65-days postmortem (dpm) interval. We observed recovery of proliferative cells up to a maximum of 65 days of PMI. Percent of explants exhibiting outgrowth as well as relative confluence of outgrowing cells decreased with increasing PMI. Comparative Growth Curves and GFP expression patterns, upon transfection with a GFP plasmid, were not significantly different in 0-dpm and 65-dpm cell populations (p<0.05). Recovered cells cryopreserved with >80% post-freezing cell-viability and were passaged up to 35 times in in vitro cultures. The cytogenetic analysis of 65-dpm tissue derived cells exhibited a normal female sheep karyotype without any genetic aberrations.
Conclusions: These results show that normal proliferative cells can be recovered from sheep skin up to about 2 months postmortem, if tissues are kept refrigerated. To our knowledge this is the first report of recovering proliferative cells from mammalian tissues up to such a long time of >2 months after death. The discovery has potential applications in preserving veterinary and livestock germplasm after death to revive in future by cloning as well as in cellular therapies in human and veterinary medicine.
Keywords
<p>Sheep; Fibroblasts; Postmortem; Skin; Cell therapy; Stem cells; Cryopreservation; Skin banking; Animal cloning</p>
Article Details
Abbreviations:
PBS: Phosphate Buffered Saline; DMEM: Dulbecco's Modified Eagle Medium; FBS: Fetal Bovine Serum; PMI: Postmortem Time Interval; SD: Standard Deviation; GFP: Green Fluorescent Protein; GC: Growth Curve; DPM: Days Postmortem; SCNT: Somatic Cell Nuclear Transfer; P: Passage
Background
Recovery of proliferative cells from postmortem tissues is an emerging area of research. It is considered potential source of stem and progenitor cells for cellular therapies in human and veterinary medicine [1,2]. It is also an important source of preservation of domestic, wild, and endangered animal species to prevent permanent loss of genetics, especially at a time when effects of climate change on reproduction and food production systems are being experienced worldwide [3-6]. Collection of tissues from live animals present an ethical issue, however, accidentally dead animals (domestic or wild) and slaughterhouse is a potential alternate to harvest tissues. Death, however, brings successive cessation of metabolic activities and ultimately carcass decomposition compromising nuclear DNA integrity of the individual cells in postmortem tissues [7] and ultimately cell death. Cold storage of biological tissues has been shown to delay decomposition and cell death [8]. However, how long the individual cells can live in postmortem tissues stored in refrigerating conditions remains under explored. Earlier research on muscle stem cells reported cell survival up to 17 days postmortem in human beings and up to 16 days in mice [9]. Neural stem and progenitor cells from postmortem rat brains were recovered up to a week, when stored at 4°C [10]. Skin tissues stored at 4°C postmortem were cultured up to 14 days in rabbits and pigs [11], 12 days in goats and sheep [12], 41 days in goats [13] and 49 days in bovine [14]. Here we show recovery of live and proliferative cells from refrigerated sheep skin up to 65 days after animal death and their characterization with respect to cytogenetic stability, growth profile, longevity in culture, post freezing cell-viability and GFP gene expression.
Material and Methods
Ethics, sample collection and storage
All experimental protocols for animal tissues in this study were approved by Institutional Animal Care and Use Committee # SP-R-01-2016. Ears of individual sheep (Katahdin breed; age 2-3 years) were collected from university slaughterhouse. Ears excised from the animal head were brought to the laboratory within an hour, cleaned with 70% alcohol swabs, wrapped in clean sterile paper towels, and stored in plastic bags in a refrigerator set at 4°C.
Explant preparation and culture
Ear skin explants were prepared and cultured as described [15]. In brief, inner side of the stored ears were cleaned with 70% ethanol swabs, and the skin explants (2-3 mm2) excised aseptically after 0, 10, 20, 27, 30, 35, 38, 41, 45, 50, 55, 60, 65 and 70 days of postmortem storage. Excised explants were adhered onto 60 mm diameter dishes (Falcon, B. D. Biosciences, Oxnard, CA, USA) pre-scratched using a scalpel at 5 uniform spots to facilitate adherence. Adhered explants were cultured in DMEM media supplemented with 10% FBS, 50 units/mL of penicillin, 50 µg/mL of streptomycin, and 2.5 µg/mL of fungizone at 37°C, 5% CO2 in a humidified environment. The media in dishes was changed twice a week and any floating explant and/or contaminated dishes removed from the study. Outgrowth of cells around explants was observed under an inverted microscope. Presence or absence of the outgrowth of cells around explants (clusters of > 50 cells) was recorded and the level of confluence captured on day 10-12 of culture for different postmortem intervals (PMI).
Recovery of primary cells and cryopreservation
Primary outgrowing cells around the explants were recovered by trypsinization on reaching around 80-90% confluence as described [15]. Briefly, the cells in dishes were washed twice with 2.0 mL of the balanced salt solution without calcium and magnesium (Gibco@ Life Technologies, Grand Island, NY, USA), and incubated with 2.0 mL of 0.125% trypsin for 5-10 min at 37°C. The trypsinized cells were neutralized with five volumes of growth media and counted to assess cell-viability using Trypan Blue Dye Exclusion Method [16]. The cells were pelleted at 200 X g for 7 min. and suspended in Synth-a-Freeze® (Life Technologies Corp., Carlsbad, CA) media. Cells were aliquoted into cryogenic storage vials (1.0 X 105 cells / vial) and frozen at -80°C deep freezer overnight using Nalgene™ Cryo 1°C Freezing Container (Nalgene, Rochester, NY). Next day the vials were transferred to liquid nitrogen tank and stored until used for further experiments. To study cell characteristics, the frozen vials were quickly thawed at 37°C, mixed slowly with 9.0 mL of the media, pelleted at 200 X g for 7 min, dissolved in complete growth media, and cultured in appropriate culture flasks/dishes. To determine post cryopreservation cell-viability, representative vials in triplicate thawed and viable cells counted manually using a hemocytometer as well as plated for in-vitro culture. Cell-viability was expressed as percentage of live cells from total cell count. The cells passaged at least twice before using for an assay. For mycoplasma testing of the cells used for further characterization, the cells were cultured for a total of 10 d, and the culture supernatants were tested for the contamination at day 4 as well as day 10 of the culture using a MycoFLUOR TM Mycoplasma Detection Kit (Molecular Probes) using the manufacturer's instructions.
Immunofluorescence
An aliquot of actively growing 65-dpm cells (p3) was cultured overnight in chambered polystyrene slides (BD Falcon, Bedford, MA). The cultured cells were labeled with mouse monoclonal anti-vimentin-FITC antibodies (Abcam Inc., Cambridge, MA) to test for their true fibroblastic nature using immunofluorescence technique as per the manufacturer’s instructions.
Growth profiles
Growth curves were generated from passage 3-4 cultures using a 24-well micro titer plate format as described [17]. Briefly, 20, 000 live cells were inoculated in each well in 0.5 mL of DMEM media supplemented with 10% FBS. The wells were trypsinized (in triplicate) after 2, 4, 6, 8, 10, 12, 14 and 16 days of culture. The viable cells were counted in each well using a hemocytometer. Mean and standard deviation of the cells / mL in triplicate wells were plotted against time using Excel program (Microsoft) to generate growth curves.
GFP transfection of primary cells
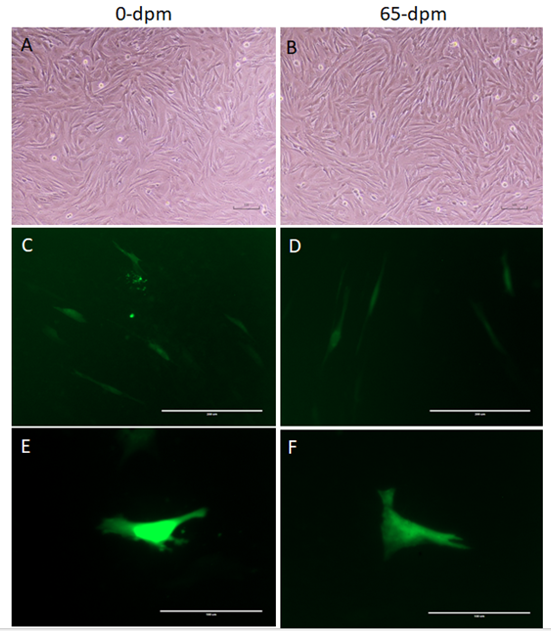
Plasmid pcDNA™3.1/NT-GFP containing GFP gene was prepared using EndoFree Plasmid purification kit (Qiagen Inc.) and DNA was quantified using NanoDrop 2000. We transfected fibroblast cells prepared from 0-dpm and 65-dpm cell-lines at p5 level as per the manufacturer’s instructions, using a Transfectamine 3000 kit from Invitrogen (Life Technologies Inc., CA). GFP gene expressing cells were compared in 0-dpm and 65-dpm cell populations by observing under UV light in EVOS fluorescent inverted microscope using GFP filter. GFP positive cells were counted under inverted microscope in at least 10 visible areas for each sample.
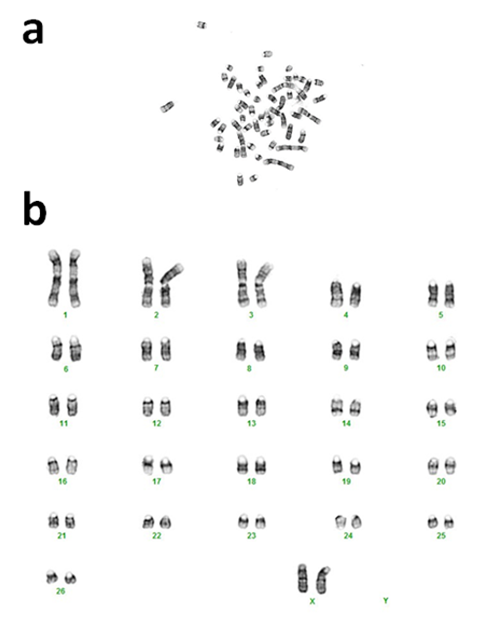
Cytogenetic analysis
Sheep cells recovered from 65-dpm interval at p3 were processed for karyotyping using GTL Banding technique as previously established [18] at Cell-line Genetics (Madison, WI; www.clgenetics.com). Chromosome assignments were made according to the Atlas of Mammalian Cytogenetics [19].
Statistical analysis
Growth curve data were expressed as mean ± standard deviation of cells/mL. Post-freezing cell viability data expressed as % of live cells from total cell count. GFP data were expressed as mean ± SD of GFP expressing cells in at least 10 visible areas. All experiments performed in triplicate and all data analyzed using Excel program (Microsoft). Statistical significance was assessed using unpaired Student’s t-test at p < 0.05.
Results
Recovery of proliferative cells up to 65 days of postmortem interval
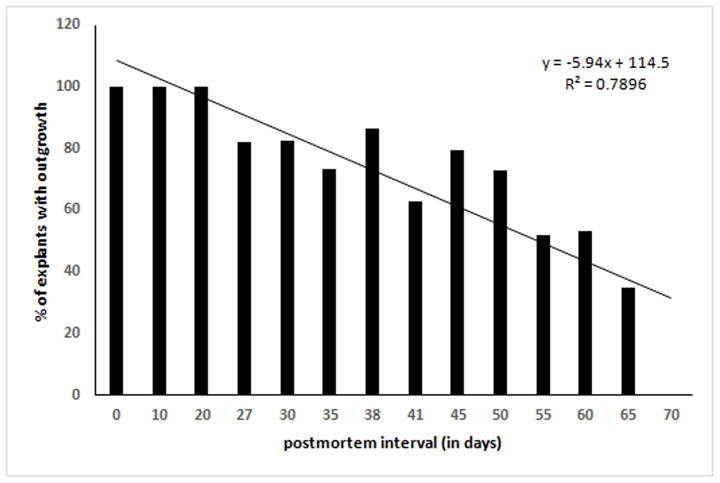
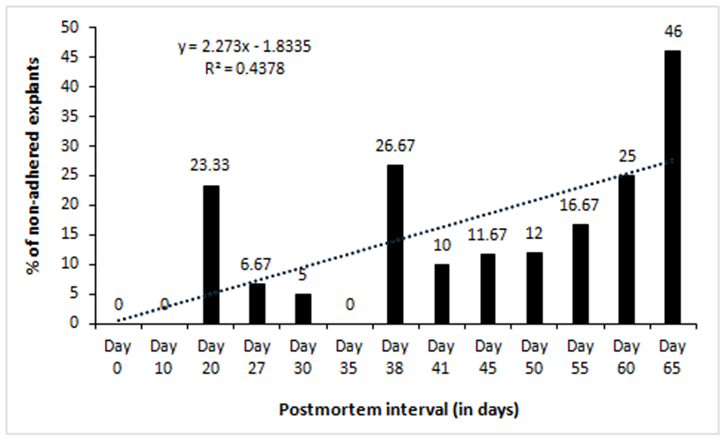
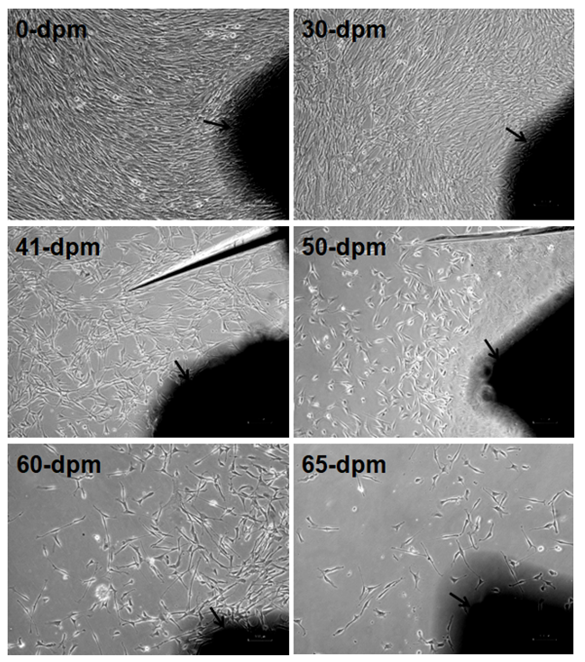
We cultured 60 explants (2-3 mm2) each after different days of PMI from tissues stored at 4°C. Twelve dishes (60 mm diameter) were used for each time point (2 dish/animal, 5 explants/dish). After 10-12 days of culture in a CO2 incubator, the outgrowth of fibroblast-like cells around the explants was scored. Out of 474 explants that adhered to dish surface, 369 (77.85%) exhibited outgrowth in different PMI. Results show outgrowth of cells in all the time points tested except 70-days postmortem (Table 1). In general, percent of explants exhibiting outgrowth was inversely correlated with PMI (Figure 1). Additionally, the confluence of outgrowing cells around the explants on a given day also decreased with increasing PMI (Figure3). On the other hand, the percentage of non-adhering (floating) explants increased with increasing PMI, especially after day 41 (Figure2), although during initial days of storage the pattern is not consistent.
Table 1: Outgrowth of fibroblast cells around skin explants after different postmortem time intervals.
Note: Ten explants used for each animal for each PMI. Age of animals was 7-24 months. n/d = not done; (.) = dish contaminated during experiment and thus removed from the study; (-) = no outgrowth. *explants not adhering to dish surface, never exhibited outgrowth in our experience.
Figure 3: Comparative confluence of outgrowing cells in different PMI on day 10-12 of culture. Arrow marked dark black areas are skin explants adhered to dish surface. Images captured by Nikon Eclipse TS100 inverted microscope. Magnification, x100. dpm = days postmortem. Note the progressive reduction of cell numbers with increasing PMI.
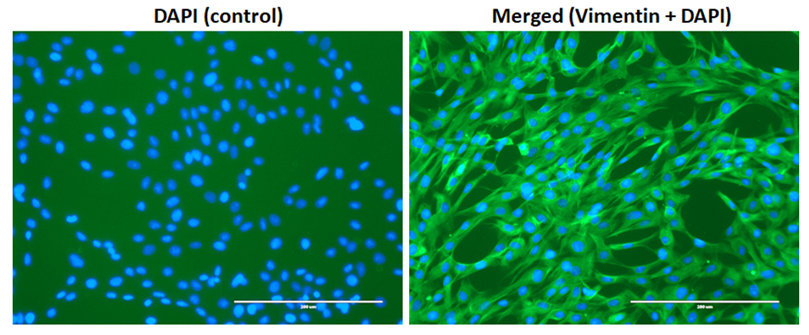
Growth characteristics of recovered cell populations
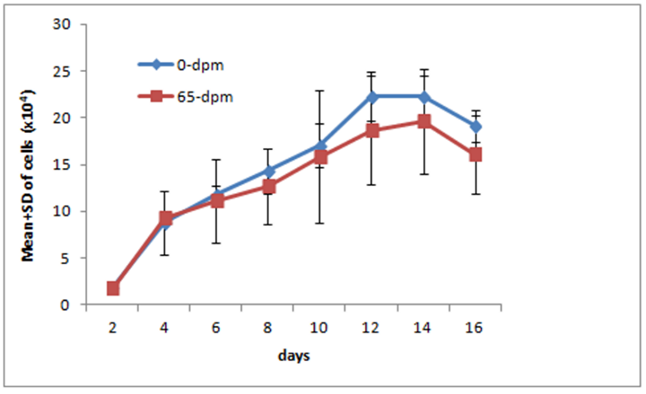
The outgrowth observed around skin explants exhibited cells with large, flat, bi, or multipolar, elongated (spindle-shaped) with flat and oval nucleus consistent with fibroblast cell morphology. To test whether they were truly fibroblasts, we labelled these cells with anti-vimentin antibodies. Vimentin is a fibroblast specific marker protein and express only on fibroblast cells. As can be seen in figure 9, all cells in our experiment stained positive, suggesting that these were indeed true fibroblasts. Occasionally epithelial cells were seen but they disappear after 2-3 sequential passages. To see the growth profile of the recovered cells, we generated Growth Curve (GC) of 0-dpm and 65-dpm tissue derived cell populations. The mean and standard deviation (SD) of 0-dpm cell populations was 1.90±0.04 x104, 8.80±3.40 x104, 11.79±1.01 x104, 14.32±4.09 x104, 17.1±7.07 x104, 22.29±2.63 x104, 22.25±2.26 x104 and 19.13±1.72 x104 for 2, 4, 6, 8, 10, 12, 14 and 16 days of culture, respectively. Similarly, Mean and SD for 65-dpm cell population was 1.78±0.16 x104, 9.32±0.78 x104, 11.14±4.43 x104, 14.32±4.09 x104, 17.10±7.07 x104, 17.69±5.85 x104, 19.62±5.67 x104, 16.11±4.16 x104 for 2, 4, 6, 8, 10, 12, 14 and 16 days of culture, respectively. The data shows that the growth of 0-dpm cells was slightly higher compared to 65-dpm cell populations but was not significantly different (p < 0.05) for any time point (Figure 4). Both cell populations were negative when tested for mycoplasma.
Chromosome stability of recovered proliferative cell population
To determine cytogenetic stability of the postmortem tissue derived cells, we tested cultured cells from 65-dpm tissues (SSF65 cell-line, p3). Cytogenetic analysis was performed on twenty G-banded metaphase cells. All 20 cells exhibited an apparently normal female karyotype. The diploid (2x) number of the chromosomes in the cell-line was determined to be 54, XX [20]. As can be seen in Figure 5, it consisted of 52 autosomes and 2 (X and X) sex chromosomes. This result is consistent with other sheep cytogenetic studies [15,20-22].
Comparative GFP gene expression in recovered cell populations
To determine, if the recovered cells have equivalent transcriptional and translational machinery, we transfected equal number of 0-dpm (control) and 65-dpm cell populations with pcDNA3.1-GFP: NT, a GFP gene containing plasmid. GFP gene was expressed in both the cell populations (Figure 6). Mean ± SD of GFP expressing cells per visible area was 31.33±3.51 and 22±4.58 for 0-dpm and 65-dpm cell populations, respectively. Although, the number of cells expressing GFP in 0-dpm (control) was slightly elevated, it was not statistically significant (p < 0.05).
Figure 6: GFP gene expression in 0-dpm (left panels) and 65-dpm (right panels) tissue derived cell populations (p5). A & B, light microscopic images of cultures (magnification, x100); C & D, GFP expression after 72 h post-transfection (magnification, x200); E & F, same as C & D but magnification, x400 to focus single cells. Light microscopic images were captured using a TS100 inverted microscope and DSL2 camera (Nikon), whereas GFP images were captured using an EVOS fluorescent microscope with a GFP filter.
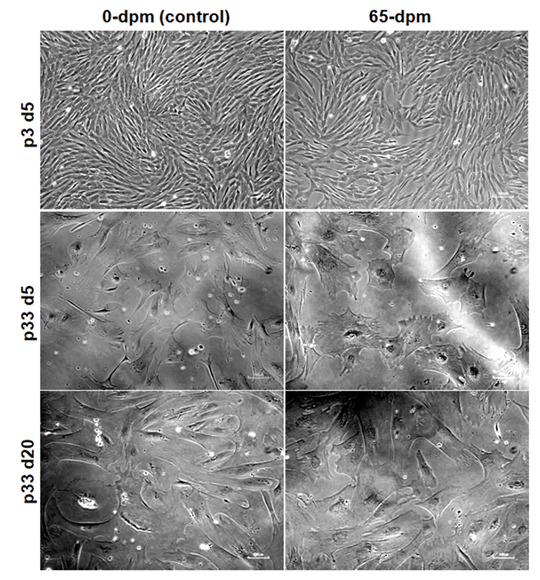
Longevity of primary cells in in-vitro cell cultures
To see as how long the cells can continue proliferating in in-vitro cultures, we performed serial passaging of the cells until they stopped growing. We observed that cell cultures in earlier passages (p1-p15) grow faster and fill the dish within 3-5 days (Figure 7, upper panel, p3 d5) whereas cultures in subsequent passages, especially after passage 15, take longer time to reach the same level of confluence. Both 0-dpm and 65-dpm cell populations exhibited slow growth after passage 20 (takes an average of 25 days to reach 80-90% confluence) and almost stopped growing at passage 32. For example, p33 cells could not expand even after 15 days of additional culture (Figure 7, middle panel vs lower panel). We observed that 0-dpm and 65-dpm cultures did not differ in number of passages they can go in in-vitro cultures.
Figure 7: Comparative cell morphology and confluence of 0-dpm and 65-dpm cell-lines at low (p3) and high (p33) passages. d5 and d20 represent 5th day and 20th day after culture initiation of the same cell cultures. Magnification, x100, note that picture correction tool of power point was used to highlight the morphology and flatness of cells in this figure.
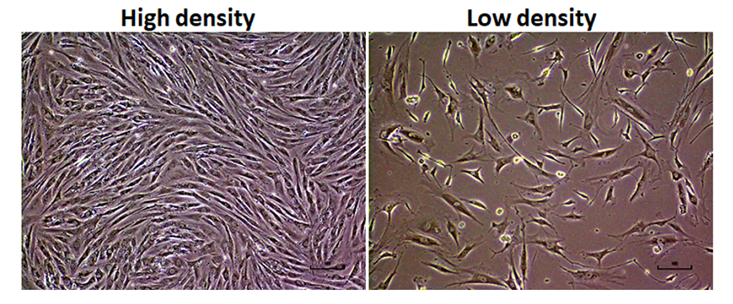
Recovery of cryopreserved cells
To test the freezing potential of PMI recovered cells for long-term storage, we cryopreserved 65-dpm cell populations. After 5 years of storage in liquid nitrogen tanks, we thawed the frozen vials and analyzed them for cell viability as well as post thaw in vitro culture potential. Post thaw cell cultures had >80% cell viability (data not shown) and exhibit normal fibroblast morphology (Figure 8).
Discussion
Earlier we developed a simple method of primary cell cultures by adhering small skin explants from postmortem tissues onto a dish surface to recover outgrowing proliferative cells and to use them as an in vitro model to study mammalian cell physiology [15]. Using that model system, here we studied the limits of postmortem cell survival in sheep skin tissues especially the effect of low temperature. We observed outgrowth of cells around skin explants up to 65 days in tissues that were stored at 4°C after animal death. Percentage of explants exhibiting outgrowth as well as the level of confluence of cells, when compared on a given day, decreased with increasing postmortem interval (Figure 1 & figure 3). It is thought that the individual cells in mammalian tissues die gradually over time suggesting gradual reduction in stem cell pool in postmortem tissues [7]. The time taken to have complete death of all cells in a tissue may depend upon several variables such as environmental temperature, chemical or radiation exposure, humidity, age, and health of an animal. When a mammalian heart stops pumping blood, oxygen is no more circulated from lungs to body tissues. Lack of oxygen leads to progressive decomposition of tissues postmortem, due to lysosomal enzyme activity, ultimately resulting in cellular death [23]. This phenomenon could explain the observed reduction in outgrowth with increasing PMI observed in this study (Figure 3). Similar reduction in cells with increasing PMI has been reported in neurosphere cell cultures in human retina [24], fibroblasts in goat ear skin [13], and pig tendon [1]. Percentage of floating explants (non-adhering explants) increased with increasing PMI, especially after about a month of tissue storage (Figure 2). We have observed that the tissue seems to dry up after storing for longer periods in refrigerator and that could have been the reason of reduced adherence of explants in later time points. The primary cultures predominantly exhibited fibroblast morphology (Figure 9). In initial passages, a few epithelial colonies were also observed, however, they disappeared in subsequent passages. It is known that fibroblasts detach quickly from dish surface compared to epithelial cells when trypsinized and thus leads to selective purification of fibroblast cells.
To study the growth profile of recovered cells, we generated a growth curve from 65 dpm cell populations and compared with 0-dpm (control). The growth curve for both cell populations was not statistically significant (p<0.05), although 65-dpm cells show slightly less growth compared to controls (Figure 4). These cells were also cytogenetically stable and exhibited normal female karyotype with 54 XX chromosomes (Figure 5). Furthermore, the GFP gene expression in 65-dpm cells was not significantly different (p < 0.05) compared to 0-dpm controls (Figure 6) suggesting the similarity of transcriptional and translational machinery in both cell populations.
To know as how long the cells can last in in-vitro cultures, we passaged 0-dpm and 65-dpm cell populations, at around 90% confluence, continuously until they stopped growing. Although, these cells lasted in cultures up to 35 passages, their growth was slowed after around 20th passage. The primary cells were also stored in Liquid nitrogen up to 5 years and recovered with >80% post freezing cell viability which exhibited normal fibroblast morphology (Figure 8), suggesting their potential use for long term preservation of germplasm which could be used in future to revive the lost elite or endangered animals by somatic cell nuclear transfer (SCNT) aka cloning. Nuclear integrity is a key requirement for successful cloning. Any damage to genetic material will lead to defective clones. In vitro culture of somatic cells ensures nuclear integrity. In vitro culture of cells from live or dead animal tissues, preserved at low temperatures, and their use for therapeutic organ reconstruction [25-27] and animal cloning [28] has been shown. However, in all these cases the tissues used to obtain viable cells as a source of nucleus were preserved within few hours of animal death. Delays in preservation of tissues may compromise in vitro culture potential, and thus the effective use of these tissues/cells.
Conclusions
This study shows that proliferative cells can be recovered from refrigerated postmortem tissues for quite long time and most importantly that these cells exhibit normal cell properties upon in-vitro expansion of cultures. To our knowledge this is the first systematic report of in vitro culture of postmortem tissues after 65 days of animal death in mammals from their carcass stored in refrigerator (4°C). These findings broaden our understanding of postmortem cellular life in mammalian tissues and may have potential applications in human and veterinary medicine for cellular therapies and preservation of germplasm after death of wild or domestic animals of agricultural value which could be revived in future by SCNT.
Declarations
Ethics approval
All experimental protocols for animal tissues in this study were approved by Institutional Animal Care and Use Committee. Approval number: SP-R-01-2016
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Competing interests
Authors declare that they have no competing interests
Funding
This work was supported by the USDA-National Institute of Food and Agriculture, Evans-Allen project number 1009701.
Authors’ contributions
MS conceived idea and designed the study, captured images, analyzed the data, wrote the paper, and supervised the experiments. XM collected samples and conducted the experiments. Both the authors read and approved the final manuscript.
Acknowledgements
Part of this work was presented in the American Society of Animal Science meeting at Salt Lake City in July 2016.
References
- Ozhathil LC, Chen Y, Nissen SD, et al. Time matters: characterization of fibroblast-like cells harvested from pig profundus tendon stored at room temperature at different postmortem time intervals. In Vitro Cell Dev Biol Anim 4 (2022).
- Haring G, Zupan J. Tissues from Post-Mortem Donors as Alternative Sources of Stem Cells for Regenerative Medicine. Adv Exp Med Biol 1288 (2020): 33-46.
- Strand J, Ragborg MM, Pedersen HS, et al. Effects of post-mortem storage conditions of bovine epididymides on sperm characteristics: investigating a tool for preservation of sperm from endangered species. Conserv Physiol 4 (2016): cow069.
- Zimmerman JK, Hogan JA, Nytch CJ, et al. Effects of hurricanes and climate oscilla-tions on annual variation in reproduction in wet forest, Puerto Rico. Ecology 99 (2018): 1402-1410.
- Tablado Z, Revilla E. Contrasting effects of climate change on rabbit populations through reproduction. PLoS One 7 (2012): e48988.
- Rola LD, Buzanskas ME, Melo LM, et al. Assisted Repro-ductive Technology in Neotropical Deer: A Model Approach to Preserving Genetic Diversi-ty. Animals (Basel) 11(2021): 1961.
- Wei W, Michu Q, Wenjuan D, et al. Histological changes in human skin 32 days after death and the potential forensic significance. Sci Rep 10 (2020): 18753-020-76040-2.
- Forbes SL, Perrault KA, Stefanuto PH, et al. Comparison of the decomposi-tion VOC profile during winter and summer in a moist, mid-latitude (Cfb) climate. PLoS One 9 (2014): e113681.
- Latil M, Rocheteau P, Chatre L, et al. Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity. Nat Commun 3 (2012): 903.
- Xu Y, Kimura K, Matsumoto N, et al. Isolation of neural stem cells from the forebrain of deceased early postnatal and adult rats with protracted post-mortem intervals. J Neurosci Res 74 (2003): 533-540.
- Silvestre MA, Saeed AM, Cervera RP, et al. Rabbit and pig ear skin sample cryobanking: effects of storage time and temperature of the whole ear extirpat-ed immediately after death. Theriogenology 59 (2003): 1469-1477.
- Silvestre MA, Sanchez JP, Gomez EA. Vitrification of goat, sheep, and cattle skin samples from whole ear extirpated after death and maintained at different storage times and tem-peratures. Cryobiology 49 (2004): 221-229.
- Okonkwo C, Singh M. Recovery of fibroblast-like cells from refrigerated goat skin up to 41 d of animal death. In Vitro Cell Dev Biol Anim 51 (2015): 463-469.
- Brian Walcott and Mahipal Singh. Recovery of proliferative cells up to 15- and 49-day postmortem from bovine skin stored at 25°C and 4°C, respectively 3 (2017).
- Singh M, Ma X, Amoah E, et al. In vitro culture of fibroblast-like cells from postmor-tem skin of Katahdin sheep stored at 4 degrees C for different time intervals. In Vitro Cell Dev Biol Anim 47 (2011): 290-293.
- Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol Appendix 3:Appendix 3B (2001).
- Singh M, Sharma AK. Outgrowth of fibroblast cells from goat skin explants in three differ-ent culture media and the establishment of cell lines. In Vitro Cell Dev Biol Anim 47 (2011): 83-88.
- Meisner LF, Johnson JA. Protocols for cytogenetic studies of human embryonic stem cells. Methods 45 (2008): 133-141.
- SJ O'Brien, JC Menninger, WG Nash editor. Atlas of Mammalian Chromosomes. John Wiley and Sons, NJ (2006).
- Wen J, Tong Y, Zu Y. Low concentration DMSO stimulate cell growth and in vitro trans-formation of human multiple myeloma cells. British Journal of Medicine and Medical Re-search 5 (2015): 65-74.
- Fiore M, Zanier R, Degrassi F. Reversible G(1) arrest by dimethyl sulfoxide as a new method to synchronize Chinese hamster cells. Mutagenesis 17 (2002): 419-424.
- Fiore M, Degrassi F. Dimethyl sulfoxide restores contact inhibition-induced growth arrest and inhibits cell density-dependent apoptosis in hamster cells. Exp Cell Res 251 (1999): 102-110.
- Maurisse R, De Semir D, Emamekhoo H, et al. Comparative transfection of DNA into primary and transformed mammalian cells from dif-ferent lineages. BMC Biotechnol 10 (2010): 9-6750-10-9.
- Carter DA, Mayer EJ, Dick AD. The effect of postmortem time, donor age and sex on the generation of neurospheres from adult human retina. Br J Ophthalmol 91 (2007): 1216-1218.
- Palmer TD, Schwartz PH, Taupin P, et al. Cell culture. Progenitor cells from human brain after death. Nature 411 (2001): 42-43.
- Viel JJ, McManus DQ, Cady C, et al. Temperature and time interval for culture of postmortem neurons from adult rat cortex. J Neurosci Res 64 (2001): 311-321.
- Erker L, Azuma H, Lee AY, et al. Therapeutic liver reconstitu-tion with murine cells isolated long after death. Gastroenterology 139 (2010): 1019-1029.
- Hoshino Y, Hayashi N, Taniguchi S, et al. Resurrection of a bull by cloning from organs frozen without cryoprotectant in a -80 degrees c freezer for a decade. PLoS One 4 (2009): e4142.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 