Elevated Antibody Titers in Abdala Vaccinees Evaluated by Elecsys® Anti-SARS-Cov-2 S Highly Correlate with UMELISA SARS-Cov-2 ANTI RBD, ACE-2 Binding Inhibition and Viral Neutralization Assays
Gilda Lemos-Perez1*, Sheila Chavez-Valdes1, Hany Gonzalez-Formental1, Giselle Freyre-Corrales1, Amalia Vazquez-Arteaga1, Beatriz Alvarez-Acevedo2, Lismary Avila-Díaz1, Ricardo U Martínez-Rosales1, Yahima Chacon-Quintero1, Edelgis Coizeau-Rodriguez1, Ariel Palenzuela-Diaz2, Enrique Noa-Romero4, Gerardo E Guillen-Nieto1
1Center for Genetic Engineering and Biotechnology, Ave. 31 No. 15802 e/ 158 y 190, Cubanacán, Playa, Havana, C.P. 10600, P.O. Box 6162, Cuba
2International Health Center “La Pradera” , 15th Street No. 22210 e/222A y 234, Siboney, Playa, Havana, Cuba
3Immunoassay Center. Calle 134 y Ave. 25, Cubanacán, Playa, CP 6653, Havana, Cuba
4Civilian Defense Scientific Research Center, Carretera de Jamaica y Autopista Nacional, San José de las Lajas, Mayabeque, Cuba
*Corresponding author: Gilda Lemos Perez, Center for Genetic Engineering and Biotechnology, Ave. 31 No. 15802 e/ 158 y 190, Cubanacán, Playa, Havana, CP. 10600, PO. Box 6162, Cuba.
Received: 06 May 2022; Accepted: 19 May 2022; Published: 02 September 2022
Article Information
Citation: Gilda Lemos-Perez, Sheila Chavez-Valdes, Hany Gonzalez-Formental, Giselle Freyre-Corrales, Amalia Vazquez-Arteaga, Beatriz Alvarez-Acevedo, Lismary Avila-Díaz, Ricardo U Martínez-Rosales, Yahima Chacon-Quintero, Edelgis Coizeau-Rodriguez, Ariel Palenzuela-Diaz, Enrique Noa-Romero, Gerardo E Guillen-Nieto. Elevated Antibody Titers in Abdala Vaccinees Evaluated by Elecsys® Anti-SARS-Cov-2 S Highly Correlate with UMELISA SARS-Cov-2 ANTI RBD, ACE-2 Binding Inhibition and Viral Neutralization Assays. Journal of Biotechnology and Biomedicine 5 (2022): 151-157
DOI: 10.26502/jbb.2642-91280055
View / Download Pdf Share at FacebookAbstract
SARS-CoV-2 a coronavirus that jumped the human barrier almost high morbidity and mortality worldwide since December 2019, posing an enormous health, social and economic problem. Obtaining effective treatments that can diminish deaths and sequelae and vaccines to slow or prevent viral transmission, and reduce disease severity and/or death are of utmost importance. Abdala is a Cuban vaccine based on the recombinant RBD subunit of the spike protein expressed in Pichia pastoris yeast. It demonstrated high efficacy (92.28 %) in phase III clinical trials for reducing transmission, and more than 90% effectiveness in reducing disease severity and mortality. Antibody titers were evaluated in 42 Abdala vaccinees using the Elecsys® Anti-SARS-CoV-2 S test. Fifteen days after immunization, sera from vaccinees showed high antibody titers (median of 1595 U/mL). The results obtained in this study also demonstrate correlation between the Cuban test UMELISA SARS-CoV-2 ANTI RBD used during the clinical trials and Elecsys® test results.
Keywords
<p>COVID-19; Abdala vaccine; Elecsys® Anti-SARS-CoV-2 S test; UMELISA SARS-CoV-2 ANTI RBD test; Antibody titers</p>
Article Details
Abbreviations:
ACE2: Angiotensin converting enzyme 2; AU: Arbitrary units; BAU: Binding antibody units; CECMED: Cuban Center for State Control of Medicines and Medical Devices; CIE: Immunoassay Center; CIGB: Cuban Center for Genetic Engineering and Biotechnology; CIGBSS: Cuban Center for Genetic Engineering and Biotechnology/Sancti Spiritus branch; CIM: Center for Molecular Immunology; CI: Confidence interval; COVID-19: Coronavirus disease 2019; CPE: Cytopathogenic effect; GMT: Geometric mean titer; hFc-RBD-HRP: Human Fc-RBD conjugated with horse radish peroxidase; IC50: Inhibitory concentration; IQR: Interquartile range; MEM: Eagle minimal essential medium; MN50: Microneutralization assay; OD: Optical density; RBD: Receptor-binding domain; S protein: Virus spike protein; SARS-CoV: Severe acute respiratory syndrome coronavirus; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; SD: Standard deviation; sVNT: Surrogate viral neutralization test; VNT: Viral neutralization test
1. Introduction
COVID-19 is a severe disease produced by a new coronavirus (SARS-CoV-2), whose first victims were reported in Wuhan, China, in late 2019. This disease rapidly spread to other provinces in China and to the rest of the world [1]. By the end of January 2020, the World Health Organization (WHO) declared this disease a global health emergency [2], and it was recognized as a pandemic in March 2020 [3]. On March 23, confirmed SARS-CoV-2 cases exceeded 300,000 globally; the disease was present in 185 territories in six continents. The death total was 14,509. Together with the well-known containment measures for respiratory infection control, testing of suspected cases and contacts (with or without symptoms) by rRT-PCR was highly recommended [4]. On March 27, 2020, the USA became the country with the most confirmed cases, surpassing China (241,703 cases and 82,875 deaths) A few days later (on April 3), the number of confirmed cases globally exceeded one million and global deaths surpassed 56 thousand [5]. (August 21, 2022), although mortality is continuously decreasing, 593 million cases, and 6,4 million deaths have been reported globally [6]. The rapidly growing infection rate of SARS-CoV-2 and the appearance of virus variants worldwide have taken an enormous health, social and economic toll, which triggered a race for developing diagnostics, therapeutic agents and vaccines that may ultimately slow or prevent viral transmission. The entire genome structure of SARS-CoV-2 (the COVID-19 etiologic agent) was made available since early January 2020 [7]. It is almost identical to SARS-CoV except for some additional protein genes and its host receptor, the ACE-2 (angiotensin-converting enzyme 2) protein that allows entry into the cell. Among the structural proteins of SARS-CoV-2, the Spike (S) and nucleocapsid are the main immunogens used in diagnosis. The S protein contains two subunits, S1 and S2. The S1 subunit contains a Receptor Binding Domain (RBD), which is responsible for recognition and binding to the cell surface receptor, making it the main target for vaccine development. The S2 subunit contains other basic elements necessary for membrane fusion [8, 9]. Several approaches have been used for COVID-19 vaccine development: the classic approach of inactivated virus: Sinopharm (BBIBP-CorV) and Sinovac (CoronaVac) Chinese vaccines and virus vector vaccines, such as the Oxford/AstraZeneca (AZD1222, UK), Johnson & Johnson’s Janssen (Ad26.COV2.S, USA) and Gamaleya’s Sputnik V (Gam-COVID-Vac, Russia). The most recent type are mRNA vaccines, used by the Moderna (mRNA-1273, USA) and Pfizer/BioNTech (BNT162b2, Germany) vaccines, approved for human use for the first time. Protein subunit vaccines like Novavax (NVX-CoV2373, UK) have also entered the market [10].
In Cuba, the first COVID-19 case was confirmed on March 11 [11]. According to PAHO on April 13, 2022) in Cuba there were 1 096 837 confirmed cases and 8518 deaths for a very low lethality of 0.09%, which decreased to 0.01% in the first days of March 2022 [12]. Cuba worked on five vaccine candidates [13]. The Cuban Center for Genetic Engineering and Biotechnology (CIGB) obtained and manufactures Abdala®, a Cuban vaccine also named CIGB-66, based on the recombinant RBD subunit of the spike protein produced in Pichia pastoris yeast [14]. Its first clinical trial, a Phase I/II trial, began on December 7, 2020 [15] and the Phase III trial was started on March 22, 2021 [16] with a 92.28% efficacy result [15, 16]. At the same time, since the city of Havana became the epicenter of community transmission in Cuba, a vaccine intervention with Abdala was carried out in this city from March 29 to September 30, 2021 [17]. The Abdala vaccine was approved for emergency use on July 9, 2021 [18, 19]. Abdala phase I and II clinical trial results have recently been published in The Lancet [20]. Reports have shown that it was more than 90% effective against disease severity and death, despite the prevalence of the Delta variant of SARS-CoV-2 at the time [20, 21], likewise against the Omicron variant, now predominant in the country [12]. It has been approved for emergency pediatric use in children from 2 to 18 years old [22, 23]. Mexico and several other countries have considered Abdala suitable for emergency use [24]. Results from two trials, one with Mambisa (CIGB-669, Cuba), one of the world’s eleven using the nasal route of administration, for emergency use authorization [25]. The second involves Mambisa, as well as Abdala for emergency use in convalescents, in whom the immune response is boosted with a single dose of either vaccine [26]. Both trials have shown good results, so authorization is expected soon in both cases. The WHO prequalification process has been initiated for the Abdala vaccine [27]. After the largest ever massive vaccination campaign in Cuba with home-grown vaccines, the number of cases is rapidly declining, as well as the number of cases requiring hospitalization and on most days no deaths are reported. Results that prove without doubt the quality of Cuban vaccines [12]. Cuba has ranked consistently among the first three countries in number of vaccinations according to its population. On April 29, 2022, 94.16% people (87.89% with complete initial protocol and 6.37% with at least one dose) had been vaccinated in Cuba. The number of doses applied per 100 people was 315.68; therefore, 10.66 million inhabitants had been vaccinated in Cuba [28]. (To see these results in the Our World in Data charts, it is necessary to add Cuba to them.) .High antibody titers, primarily IgG, correlate with good virus neutralizing levels and thus protective immunity [29,30]. The objective of this study was to evaluate serum samples from the Abdala trial vaccinees using the Elecsys® Anti-SARS-CoV-2 S system (Roche Diagnostics, Switzerland) and correlate the results with those previously obtained with the in-house test and the Cuban UMELISA SARS-CoV-2 ANTI RBD test manufactured by the Immunoassay Center (CIE).
2. Materials and Methods
2.1 Vaccine
The Cuban Abdala vaccine (CIGB-66) developed by the CIGB (Center for Genetic Engineering and Biotechnology, Cuba) was administered as immunogen [14, 18, 20, 21].
2.2 Serum samples
The study was carried out using sera collected from 42 individuals participating in clinical trials for the Abdala vaccine candidate. In total 126 serum samples were used: prior to immunization (pre), and fifteen days post both the second and the third (last) immunizations. Collected sera were stored at -20oC until evaluation.
2.3. Ethics
Subjects gave their consent after being informed that the immediate use of the samples was to determine post vaccination antibody titers and there were possibilities of their use in related research. GLP (Good Laboratory Practices) were followed throughout the study. Confidentiality of the donors’ personal data was ensured. The initial study was approved by the Research Ethics Committee of the Saturnino Lora Provincial Hospital in Santiago de Cuba; this one, by the CIGB ethics committee in Havana, Cuba.
2.4. SARS-CoV-2 antibody quantifications
2.4.1 Elecsys® Anti-SARS-CoV-2 S assay (Roche Diagnostics, Switzerland).
SARS-CoV-2 antibodies against the Spike RBD viral protein were determined using the quantitative Elecsys® Anti-SARS-CoV-2 S test [31] on the Cobas e411 Analyzer (Roche Diagnostics). The test is a double-antigen sandwich electro-chemiluminescence immunoassay, which uses streptavidin-coated micro particles to separate bound from unbound substances prior to applying voltage to the electrode. This assay has a measuring range of 0.40-250 U/mL (up to 2500 U/mL with on-board 1:10 dilution), with a concentration ≥0.80 U/mL considered positive. The manufacturer specific U/mL of the Elecsys® Anti-SARS-CoV-2 S assay can be considered equivalent to the BAU/mL of the first WHO International Standard for anti-SARS-CoV-2 immunoglobulin.
2.4.2. UMELISA SARS-CoV-2 ANTI RBD assay
IgG class antibodies were quantified by the UMELISA SARS-CoV-2 ANTI-RBD test, approved by CECMED (Cuban Center for State Control of Medicines and Medical Devices) and commercialized by the Immunoassay Center (CIE, Cuba) [32]. The test is an automated fluorescence-based quantitative assay that uses ultramicro-ELISA plates coated with the RBD fragment of the virus S protein as solid phase. Briefly, 10 µL of 1:100 pre-diluted samples are incubated in the wells of the strips for 30 minutes. After a wash step, a biotin-bound monoclonal antibody against human IgG is then added, which will bind to the antibodies fixed in the previous step. A further wash removes the unreacted biotinylated antibodies. A streptavidin/alkaline phosphatase conjugate is then added, which will bind to the biotin molecules. Another wash removes excess conjugate. A fluorogenic substrate (4-methylumbelliferyl phosphate) is added, which will be hydrolyzed and the intensity of the emitted fluorescence will allow quantification of the IgG antibody levels to the SARS-CoV-2 RBD protein in the samples. Titers are given in arbitrary units per milliliter (AU/mL) with a cut-off value of 1.95 AU/mL.
2.4.3. sVNT assay
Antibody neutralization properties were determined using an “in-house” surrogate virus neutralization test (sVNT) measuring RBD-ACE2 (receptor binding-angiotensin converting enzyme 2) inhibition (CIGB, Cuba) and confirmed by a live viral neutralization test (see 2.4.4 below) at the Civilian Defense Scientific Research Center (Cuba). In the surrogate neutralization test, vaccinee samples were pre-incubated with peroxidase-conjugated recombinant RBD protein at different dilutions and subsequently the mixture is incubated with ACE-2 protein fixed in the solid phase. Results are given in inhibition percentage. The assay positivity threshold is 20%. The live neutralization assay is based on the standard virus microneutralization assay. Vero E6 cells were incubated in absence or presence of the diluted sera and overlaid with viral suspension. At 96 h post-infection, the cytopathogenic effect (CPE) was evaluated by optical microscopy and the cells stained with neutral red. After three washes neutral red was dissolved in lysis solution and optical density (OD) was measured at 540 nm. The viral neutralizing titer (VNT50) is calculated as the highest serum dilution at which 50 % of the cells remain intact, using neutral red incorporation in the control wells (no virus added) for comparison. Costar 3591 plates were coated with hFc-ACE2 protein (provided by the Center for Molecular Immunology, CIM, Cuba) at 250ng per well in phosphate-buffered saline (PBS) pH 7.4 for three hours at 37oC. After a wash step with 0.1% (v/v) Tween-20 in distilled water, the plates were blocked with 2% (w/v) nonfat dry milk powder in PBS for 1h at 37°C. During the coating incubation period, the samples and assay controls were pre-incubated with hFc-RBD-HRP (CIGB, Cuba) conjugate diluted to 1:100,000 in 0.2% (w/v) skim milk in PBS, for 1h at 37 oC. A Mab, CBSSRBD-S.8 (CIGBSS, Cuba), with significant neutralization activity against SARS-CoV-2 was used as positive control in this surrogate assay. Human AB Serum (Sigma, USA) was used as a negative control. After the pre-incubation period, 50µL of sample-conjugate mixture is added to the ACE-2 blocked plate and incubated for 1h at 37oC. Unbound reactants are removed by four washes with 0.1% (v/v) of Tween-20 in distilled water. After washing, 50 µL of 3,3',5,5'-tetramethylbenzidine at 10µg/mL dissolved in phosphate-citrate buffer (0.2M phosphate, 0.1M citrate, pH 5.0) and 0.006% (v/v) hydrogen peroxide were added per well, followed by incubation at room temperature for 10 min. The reaction was stopped by adding Stop solution (2M H2SO4). Microtiter plates were read at a 450 nm wavelength.
Inhibition values were determined as follows:
Inhibition (%) = (1 − sample optical density value/negative control optical density value) × 100.
Results are given in inhibition percentage. The assay threshold for positivity is 20%. Inhibition values were plotted using GraphPad Prism 8.0.2 and the neutralization titer was defined as the concentration that showed 50% inhibition (inhibitory concentration, IC50) as determined by log (inhibitor) vs. normalized response – variable slope model.
2.4.4 VNT50 assay
Neutralization antibody titers were detected by a standard virus microneutralization assay (MN50) using SARS-CoV-2 (CUT2010-2025/Cuba/2020 strain) [33]. Vero E6 cells (2×104 per well) were seeded in 96-well plates one night before use. Human sera were inactivated at 56 °C for 30 min. The serum samples were prepared by two-fold serial dilutions in Eagle Minimal Essential Medium (MEM, Gibco, UK) containing 2 % (v/v) fetal bovine serum (Capricorn, Germany). SARS-CoV-2 strain at 100 TCID50 was incubated in absence or presence of diluted sera for 1 h at 37 °C. Afterwards, Vero E6 cell were overlaid with virus suspension. At 96 h post-infection, the cells were inspected for signs of cytopathogenic effects (CPE) by optical microscopy and stained with neutral red (Sigma, USA). After three washes neutral red was dissolved in lysis solution (50 % ethanol, 1 % acetic acid) for 15 min at 25 °C, and optical density (OD) was detected at 540 nm. The highest serum dilution showing an OD value greater than the cut-off was considered the neutralization titer. The cut-off value is calculated as the average OD of the cell control wells divided by two. Viral neutralizing titers (VNT50) were calculated as the highest serum dilution at which 50 % of the cells remain intact compared to neutral red incorporation in the control wells (no virus added).
2.5. Statistical analysis
All statistical analyses were performed by GraphPad Prism v. 8.0.2. In line with common conventions of descriptive statistics, standard deviation (SD) was calculated for mean values and interquartile range (IQR) for median values, geometric mean titer (GMT) and 95% confidence intervals (CIs) were also estimated. We used Wilcoxon matched-pairs signed rank test to compare the pre- and post-immunization titers of serum samples and Spearman non-parametric correlation coefficient (Spearman's rho, r) test to perform inferential analysis among test results with the categories: none or very weak r < 0.3, weak 0.3 < r <0.5, moderate 0.5 < r < 0.7 and strong r > 0.7. Cohen’s kappa (k) [9] was calculated to assess the agreement between the test assays with the categories of none (below 0.00), slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80) and almost perfect (0.81–1.00).
3. Results
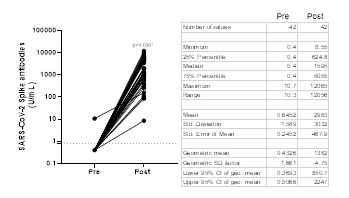
Antibody titers obtained with the Elecsys® Anti-SARS-CoV-2 S test in Abdala vaccinees are shown in Figure 1. Prior to immunization, only one sample showed detectable anti-RBD antibodies (≥ 0.80 U/mL) by the Roche test. Post-vaccinated sera showed a GMT of 1382 U/mL (95% CI: 850.7- 2247), that represents a 3191.7-fold increase compared to the baseline value of 0.43 U/mL obtained before immunization. In Abdala vaccinees, the seroconversion rate was 100% using the Roche test, 15 days after the last dose.
Figure 1: Before-after graph showing SARS-CoV-2 Spike antibody titers obtained by Elecsys® Anti-SARS-CoV-2 S in Abdala vaccinees. Statistical differences between Pre- and Post-vaccination sera (P < 0.0001) were determined by Wilcoxon matched-pairs signed rank test. The dotted line represents the cut-off of the test (³ 0.8 U/mL). The table shows the descriptive statistics of these results.
The degree of agreement between both tests was analyzed in the 126 sera samples evaluated. Results are shown in Table 1. The concordance value (k) obtained was 0.873 (95% CI, 0.781 to 0.964).
|
UMELISA SARS-CoV-2 ANTI RBD assay |
Elecsys Anti-SARS-CoV-2 S test |
||
|
Positive |
Negative |
Total |
|
|
Positive |
82 |
4 |
86 |
|
Negative |
3 |
37 |
40 |
|
Total |
85 |
41 |
126 |
Observed agreements: 119 (94.44% of the observations)
Agreements expected by chance: 71.0 (56.37% of the observations)
Kappa= 0.873 (95% CI, 0.781 to 0.964) SE of kappa = 0.047
Table 1: Two-way contingency table for anti-RBD antibody detection using UMELISA SARS-CoV-2 ANTI RBD and Elecsys® Anti-SARS-CoV-2 S (internally considered the gold standard test).
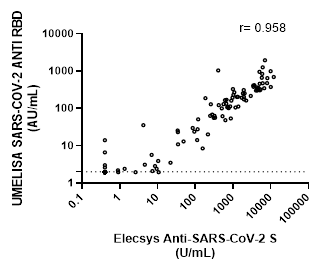
Correlation between the Roche and UMELISA tests is shown in Figure 2. A positive value of r=0.9589, (CI: 0.9415-0.9711), suggests a strong correlation between both assays.
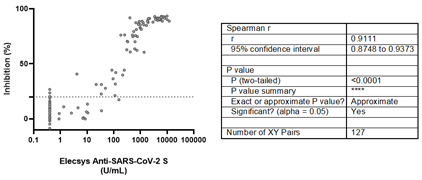
Although 10 positive samples by Elecsys® Anti-SARS-CoV-2 S showed inhibition values below 20% by the sVNT, assay, a rho value of 0.9111 (95% CI: 0.8748-0.9373) was estimated, suggesting a strong positive correlation between both assays (Figure 3).
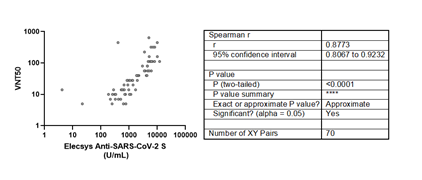
The live viral micro-neutralization test also showed a strong positive correlation with the Roche assay (Figure 4). Although five anti-RBD positive samples by the Roche test showed no neutralization titers, there is a positive strong correlation between both tests (r =0.8773).
4. Discussion
Since the beginning of the COVID-19 pandemic, many efforts have been displayed to obtain highly effective vaccines capable of eliciting protective immune response, which are essential for the prevention and mitigation of the morbidity and mortality caused by the virus. Therefore, vaccines are expected to be decisive in the strategies for stopping, controlling, or slowing the pandemic, fighting the virus, and limiting worldwide transmission of SARS-CoV-2. Currently, there are several commercially available vaccines, based on different approaches and varying efficacies, such as the mRNA-based BioNtech-Pfizer and Moderna (95% and 94% respectively) [34,35], using an adenovirus vector as AstraZeneca (70% efficacy) [36] and Sputnik V (91.6% efficacy) [37] and inactivated virus as CoronaVac (50% efficacy) [38]. With over 10 billion doses of COVID-19 vaccines already administered [27]; we are witnessing the largest global vaccine deployment in history. As this study shows, Abdala vaccinees exhibited high antibody titers by the Roche test (antibody titer median of 1595 U/mL). This result was not a surprise, since it has been recognized that mannosylated proteins secreted in Pichia pastoris as a host, have the potential to function as adjuvants, favoring in this case an RBD subunit vaccine. In addition, there is enhanced antigen presentation and T-cell activation properties upon interaction with receptors in antigen-presenting cells [39,40]. The antibody titers obtained in Abdala vaccinees were similar to antibody titers previously reported for a commercial vaccine: BNT162b2 from Pfizer-BioNTech using the same test [41]. During serological studies of the Cuban COVID-19 vaccine candidates, throughout the preclinical and clinical phases, the abovementioned Cuban commercial and in-house tests have been used. It is important to note that the proteins used for IgG quantification or the inhibition assays were obtained in mammalian cells, a heterologous expression system with respect to the one used for obtaining the vaccine antigen. Although the antibody quantification assays used in this study have different principles, and moreover, the Roche sandwich test detects total high affinity antibodies and UMELISA detects IgG type antibodies, both had an almost exact agreement (k= 0.873) and a strong positive correlation (r=9589). The sVNT and VNT50 assays also showed a positive and strong correlation with the Elecsys® Anti-SARS-CoV-2 S test (rho value of 0.911 and 0.8773 respectively). The main limitation of the study is the small number of samples studied, but the results were validated by their consistency.
5. Conclusions
The different tests used (the in-house sVNT and the UMELISA SARS-CoV-2 ANTI RBD assay, Immunoassay Center, Cuba) showed strong positive correlation with the Elecsys® Anti-SARS-CoV-2 S test (Roche, USA) for antibody detection. Since the last is considered the WHO reference standard, the conclusion is that both the in-house and the Cuban UMELISA system are equally accurate for detecting anti-SARS-CoV-2 antibodies. The VNT using live virus also strongly correlates with them, but must be carried out under more stringent biosafety conditions. Indirectly, the results also show the high immune stimulating capability of the Abdala vaccine, whose vaccinees reach antibody titers by the Elecsys® Anti-SARS-CoV-2 S test similar to those of other approved vaccines, like the Pfizer-BioNTech when evaluated with the same commercial test.Highlights
- Fifteen days after immunization with the Cuban Abdala vaccine, sera from vaccinees showed high antibody titers (median of 1595 U/mL).
- There was high correlation between the Cuban test UMELISA SARS-CoV-2 ANTI-RBD used during the vaccine clinical trials and Roche’s Elecsys® test results.
- Abdala vaccinees reached antibody titers measured by the Elecsys® Anti-SARS-CoV-2 S test comparable to those of Pfizer/BionTech vaccine using the same test.
Acknowledgements
The authors would like to express their gratitude to Dr. Loida Torres from the International Health Center “La Pradera” for enabling the use of its facilities and the equipment necessary for the evaluation of the samples.
Funding
Research reported here was supported with funds from the BioCubaFarma Enterprise and the Center for Genetic Engineering and Biotechnology in Cuba.
Conflict of Interest
There was no conflict of interest, since there was no intervention from the funding or other organizations.
References
- Statement regarding cluster of pneumonia cases in Wuhan, China. . (January 9, 2020).
- COVID-19 Public Health Emergency of International Concern (PHEIC) Global research and innovation forum. . (Febuary 12, 2020)
- WHO-Europe Coronavirus disease (COVID-19) outbreak-WHO announces COVID-19 outbreak a pandemic. (March 12, 2020).
- Coronavirus disease 2019 (COVID-19) Situation report 63. (March 23, 2020)
- WHO Coronavirus disease 2019 (COVID-19) Situation report 75. (April 4, 2020)
- COVID-19 Weekly Epidemiological Update Edition 89. (August 24,2022)
- Gen Bank. Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1, complete genome (NCBI Reference Sequence: NC-045512.2).
- Ahn DG, Shin HJ, Kim MH, et al. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J Microbiol Biotechnol 30 (2020): 313-324.
- Huang Y, Yang C, Xu XF, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin 41(2020): 1141-1149.
- Kyriakidis NC, López-Cortés A, González EV, et al. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines 28 (2021).
- Protocolo vs COVID-19. MINSAP Cuba 4 de abril de 2020.
- OPS/OMS. COVID-19 Reporte 108 (13 abril 2022). Equipo de gestión de incidentes Oficina de OPS/OMS en Cuba.
- Yaffe H. Cuba’s five COVID-19 vaccines: the full story on Soberana 01/02/Plus, Abdala, and Mambisa. London School of Economics (LSE) Latin America and Caribbean blog.
- Limonta M, Chinea G, Martín A, et al. The SARS-CoV-2 receptor-binding domain expressed in Pichia pastoris as a candidate vaccine antigen.
- Registro Público Cubano de Ensayos Clínicos (RPCEC). ABDALA Clinical Study.
- Registro Público Cubano de Ensayos Clínicos (RPCEC). ABDALA Clinical Study - Phase III.
- Registro Público Cubano de Ensayos Clínicos (RPCEC). ABDALA-Intervention.
- CECMED. Aprueba el CECMED el autorizo de uso de emergencia del candidato vacunal cubano ABDALA.
- Hernández-Bernal F, Ricardo-Cobas MC, Martín-Bauta Y, et al. Safety tolerability and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: A randomised double-blind placebo-controlled phase 1-2 clinical trial (ABDALA Study). eClinical Medicine (2022): 101383.
- Más-Bermejo PI, Dickinson-Meneses FO, Almenares-Rodríguez K, et al. Cuban Abdala Vaccine: Effectiveness in Preventing Severe Disease and Death from COVID-19 in Havana Cuba; a Cohort Study. Available at SSRN.
- Emite el CECMED el autorizo de uso en emergencias de la vacuna cubana ABDALA para población pediátrica con edades entre 12 y 18.
- CECMED. Emite el CECMED el autorizo de uso de emergencia de la vacuna cubana ABDALA para población con edades entre 2 y 11 años.
- Cofepris (Comisión Federal para la Protección contra Riesgos Sanitarios). Cofepris emite autorización para uso de emergencia de vacuna Abdala.
- Cuban Public Registry of Clinical Assays. Mambisa Study.
- Cuban Public Registry of Clinical Assays. CIGB-Mambisa, Abdala in convalescents.
- Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process.
- Our World in Data. Coronavirus (COVID-19) Vaccinations. (April 29, 2022)
- Dogan M, Kozhaya L, Placek L, et al. SARS-CoV-2 specific antibody and neutralization assays reveal the wide range of the humoral immune response to virus. Commun Biol 4 (2021): 129.
- Salvagno GL, Henry BM, di Piazza G, et al. Anti-SARS-CoV-2 Receptor-Binding Domain Total Antibodies Response in Seropositive and Seronegative Healthcare Workers Undergoing COVID-19 mRNA BNT162b2 Vaccination. Diagnostics (Basel) 11 (2021): 832.
- Roche Product Information Elecsys® Anti-SARS-CoV-2 Immunoassay intended for qualitative detection of antibodies to SARS-CoV-2 in human serum and plasma.
- UMELISA SARS-COV-2 IGG (Cuban Sanitary Registry D2107-11).
- Manenti A, Maggetti M, Casa E, et al. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J Med Virol 2020.
- Evidence Assessment: Pfizer-BioNTech COVID-19 vaccine. (January 2021).
- Evidence Assessment: mRNA-1273 COVID-19 vaccine. (January 2021).
- Evidence Assessment: ChAdOx1-S [recombinant] vaccine (AZD1222) vaccine against COVID-19 developed by Oxford University and Astra Zeneca. (February 2021).
- Jones I, Roy P, Sputnik V. COVID-19 vaccine candidate appears safe and effective. Lancet 397 (2021): 642-643.
- Evidence Assessment: Sinovac/CoronaVac COVID-19 vaccine. (April 2021).
- Karbalaei M, Rezaee SA, Farsiani H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J Cell Physiol 235 (2020): 5867-5881.
- Macio?a AK, Pietrzak MA, Kosson P, et al. The Length of N-Glycans of Recombinant H5N1 Hemagglutinin Influences the Oligomerization and Immunogenicity of Vaccine Antigen. Front. Immunol. 2017: 8:444.
- Salvagno GL, Brandon H, Pighi L, et al. Total Anti-SARS-CoV-2 Antibodies Measured 6 Months After Pfizer-BioNTech COVID-19 Vaccination in Healthcare Workers SSRN (2021).


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 