Electric Acupuncture Mimics Exercise to Promote Myogenesis, Angiogenesis and Neurogenesis
Zhen Su1,2#, Manshu Yu2,3#, Ying Huang2, Janet D Klein2, Shuyang Simon Bian2,5, Yijin Huang2,5, Faten Hassounah2, Xinwang Chen4, Xiyan Gao4, Hui Cai2*, Xiaonan H Wang2*
1Department of Nephrology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, 325000, China
2Renal Division, Department of Medicine, Emory University, Atlanta, GA, 30322, USA
3Renal Division, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210029, China
4College of Acupuncture, Moxibustion and Tuina, Henan University of Traditional Chinese Medicine, Zhengzhou, China
5Emory College of Arts and Sciences, Emory University, Atlanta, GA 30322, USA
#Both authors contributed equally to this work
*Corresponding author: Xiaonan H Wang, Renal Division, Department of Medicine, Emory University, Atlanta, GA, 30322, USA.
Hui Cai, Renal Division, Department of Medicine, Emory University, Atlanta, GA, 30322, USA.
Received: 22 November 2023; Accepted: 28 November 2023; Published: 05 January 2024
Article Information
Citation: Zhen Su, Manshu Yu, Ying Huang, Janet D Klein, Shuyang Simon Bian, Yijin Huang, Faten Hassounah, Xinwang Chen, Xiyan Gao, Hui Cai, Xiaonan H Wang. Electric Acupuncture Mimics Exercise to Promote Myogenesis, Angiogenesis and Neurogenesis. Journal of Biotechnology and Biomedicine 7 (2024): 01-14.
DOI: 10.26502/jbb.2642-91280122
View / Download Pdf Share at FacebookAbstract
Our previous study demonstrated that acupuncture with low frequency electrical stimulation (Acu-LFES) attenuates skeletal muscle atrophy by improving muscle progenitor cell regeneration. The present study examines whether Acu-LFES improves revascularization, innervation, and protein anabolism in muscle of chronic kidney disease (CKD) mice. CKD was induced by the 5/6 nephrectomy in mice. Acu-LFES treatment was applied in hindlimbs of CKD mice. Pro-teins from hindlimb (gastrocnemius), forelimb (triceps brachii) and back (longissimus) muscles were isolated and Pro-tein synthesis was measured by the surface-sensing of translation (SUnSET) assay. Exosomes were isolated using serial centrifugation and concentration and size of the collected exosomes were measured using a NanoSight instrument. The mature microRNA library was validated using a High Sensitivity DNA chip. Protein synthesis was enhanced in the Acu/LFES-treated gastrocnemius; however, in non-Acu/LFES treated muscles, triceps brachii and longissimus, protein synthesis was also significantly increased. These increases were accompanied with increased myogenesis markers myoD and myogenin. The mRNA expression of PDGF and ENO2 were enhanced by Acu/LFES. The protein amount of Igf-1, Igf-1 receptor, VEGF (a protein that stimulates the formation of blood vessels), and peripherin (expressed mainly in the nervous system) were also increased by Acu/LFES. Deep sequencing revealed that miR-5107-5p and miR-30-5p were sharply decreased in serum exosomes of Acu/LFES mice. Using a luciferase reporter assay, we demonstrated that miR-5107-5p directly inhibits VEGF, and miR-30-5p inhibits ENO2. Conclusions: Acu-LFES treatment increases myogenesis, angiogenesis and neurogenesis, as well as protein synthesis. Acu/LFES inhibits miR-5107 and miR-30, resulting in increased VEGF and ENO2 contributing to these processes.
Keywords
<p>Acu/LFES; Exosome; ENO2; microRNA; PDGF; VEGF</p>
Article Details
Introduction
Muscle atrophy or loss of skeletal muscle mass occurs in many diseases and disuse conditions as well as in the chronic kidney diseases (1-4) . Skeletal muscle is very susceptible to improvement through interventions such as exercise, improved nutrition and mechanical stimulation, which are often recommended to prevent atrophy (5). Lack of these interventions can have the opposite effect. In particular, exercise training increases muscle strength that is a very efficient method to avoid muscle loss (6). Unfortunately, this can be challenging for those who are already frail or who have severe medical conditions and are too sick to engage in physical activity. An alternative nonpharmacological intervention is required. Acupuncture with low frequency electrical stimulation (Acu/LFES) is one of these interventions. Previous research, including ours, has revealed that acupuncture can correct muscle atrophy in human and animals with various diseases, including hindlimb suspension induced muscle loss (7), amyotrophic lateral sclerosis (8), catabolic diseases (diabetes or chronic kidney disease) with muscle wasting (9, 10), or sciatic nerve injury-caused muscle atrophy (11, 12). Low-frequency electrical stimulation alone has been shown to have beneficial effects on disuse muscle atrophy by maintenance of protein synthesis (13). In addition, some studies have found that manual acupuncture (without electrical stimulation) prevented muscle atrophy by suppressing hindlimb suspension-induced upregulation of atrogin-1 and MuRF1 (7). However, the precise mechanisms by which these treatments improve muscle mass needs to be explored. Successful muscle regeneration includes increase in protein synthesis, restoration of the blood supply and innervation. Insulin-like growth factor 1 (Igf-1) is a major anabolic hormone that stimulates the growth of muscle and other tissues in the body (14). One important function of this pathway is to increase protein synthesis. Activation of Igf-1 will upregulate IRS-PI3K-Akt leading to phosphorylation of mechanistic target of rapamycin complex 1 (mTORC1) and subsequent downstream 4E-binding protein-1 (4E-BP1) (15), which initiates protein synthesis at the ribosome (16). The phosphorylation of mTORC1 and 4E-BP1 have been used as a hallmark of protein synthesis. Restoration of the blood supply in newly generating muscle tissue is critical to rebuilding muscle function. Without revascularization, muscle regeneration is incomplete, and could result in significant fibrosis (17, 18). Secretion of angiogenic factors such as vascular endothelial growth factor (VEGF) or platelet-derived growth factor (PDGF) play significant roles in new blood vessel formation as well as new growth of already-existing blood vessels (19-21). In addition, muscle innervation is essential for maturation and functional activity of regenerating muscles (22). Peripherin is an innervation marker that is expressed mainly in neurons of the peripheral nervous system, where it plays a role in neurite elongation during development and axonal regeneration after injury (23). Furthermore, neuron-specific enolase (NSE or ENO2) serves as a molecular marker of axon injury, regeneration, and reinnervation (24).
Early microRNA (miRNA) studies demonstrated that miRNAs have significant roles in the regulation of protein function by binding on the 3’-UTR of mRNA resulting in inhibition of protein translation (25). miRNAs have been recognized for regulation of muscle mass through altering proteins related with muscle metabolic processes such as Igf-1 signaling proteins (26, 27). Our microRNA deep sequencing of serum exosomes revealed thirty-four miRs that were altered by Acu/LFES, including miR-30, let-7 and miR-5107 (GEO accession number GSE176530). We identified that lethal-7 (Iet-7) binding on Igf-1 3’-UTR lead to inhibition of Igf-1 signaling (28). In agreement with our finding, Zhu et al. reported that overexpression of let-7 in mice resulted in insulin resistance and impaired glucose tolerance (29). These phenomena occurred in part through let-7-mediated repression of multiple components of the insulin-PI3K-mTOR pathway, including Igf-1 receptor, insulin receptor and insulin receptor substrate-1 (29). There are no accounts in the literature that examine the impact of miR-5107 or miR-30 on skeletal muscle recovery. In the current study we explore how Acu/LFES prevents skeletal muscle atrophy in CKD-induced muscle wasting. We hypothesize that Acu/LFES promotes revascularization, innervation and protein synthesis not only in Acu/LFES-treated muscle, but also in distant Acu/LFES-untreated muscle, through communication via serum-derived exosome-encapsulated microRNA. For proof of this hypothesis, we measured protein synthesis, revascularization and innervation markers in three different skeletal muscles of CKD mice. The expression of miR-5107 or miR-30 miRNAs in circulating exosomes were analyzed. The purpose of study is to identify the mechanisms through which Acu/LFES acts as a promising nonpharmacological treatment to prevent skeletal muscle atrophy.
Methods
Animals and CKD model
The 5/6 nephrectomy animal model and Acu/LFES experiment were approved by the institutional animal care and use committee of Emory University (PROTO201800117). The C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and were housed with a 12-hour light/12-hour dark cycle. Power analysis was used to calculate the minimum sample size requirement. To reduce the s.d., we minimized physiological variation by using animals with same sex (male) and same age (two month). 5/6 nephrectomy was induced by removing the right kidney and two poles of the left kidney in anesthetized mice (xylazine 12 mg/kg, ketamine 60 mg/kg). Initially, mice were fed with low protein chow (Harlan Teklad: 14% protein, 3.5% fat, 49% carbohydrate) and special water (0.45% NaCl) for two weeks, then normal protein (23% protein) food with 0.2% adenine until the end of experiments. Mice muscles were harvested at 0.5-, 6-, 24, 48- and 72-hours after Acu/LFES treatment. Blood was collected before terminating mice, and blood urea nitrogen (BUN) was determined by measurement of the rate of conversion of NADH to NAD monitored at 340 nm using BUN Kinetic Procedure Kit (Thermo Electron, Louisville, CO.).
Acu-LFES treatment
The mice were kept in specially designed restraint without anesthesia so that they would remain in a recumbent position during Acu-LFES treatment. Acupuncture points selected were according to the World Health Organization Standard Acupuncture Nomenclature (30). The positive point (GB34, Yang Ling Quan) is under the front head of the fibula about 6 mm (20-g mouse) from the superficial fibular nerve and deep fibular nerve. The negative point (ST36, Zu San Li) is outside of the knee joint under the head of the fibula about 7 mm from the fibular nerve. The needles were connected into an SDZ-II Electronic Acupuncture Instrument using a consistent pulse, an electric frequency of 20 Hz, and electric current of 1mA. Disposable sterile needles with a diameter of 0.25 mm (Shen Li Medical & Health Material Co., Ltd., Wujiang, China) were used (9).
Determination of protein synthesis by puromycin incorporation
To determine the rate of protein synthesis we utilized surface sensing of translation (SUnSET) methodology (31). In vivo, 0.04μmol/g puromycin (Calibiochem, Catalog #: 540222) was injected intraperitoneally into mice 30 minutes before harvesting skeletal muscle. Muscle was harvested at 0, 6, 24, 48 and 72 hours after Acu/LFES treatment and homogenized in Mueller’s Buffer (50mM HEPES, 0.1% Triton-X100, 4mM EGTA, 10mM EDTA, 15mM Na4P2O7, 100mM β-glycerophosphate, 20mM NaF, 5mM NaVO4 and 1% protease inhibitor cocktail). Puromycin-containing proteins were analyzed by Western blot. Proteins were separated on 10% SDS-PAGE gels. Anti-puromycin antibody was purchased from Millipore (Catalog #: MABE343; Burlington, MA).
Western blot and antibodies
Skeletal muscle or cells were homogenized in Gentle Lysis Buffer (10 mM Tris-HCl, 10 mM NaCl, 2 mM EDTA, 0.5 % NP-40, 1 % glycerol, and fresh added: 1 mM Na3VO4; 10mg/ml PMSF; 5mg/ml Aprotinin; 1mg/ml Leupeptin and phosphatase inhibitors cocktail 1 and 2 (Sigma). Equal amounts of protein were loaded on the acrylamide/bis SDS-PAGE gel. Protein was transferred to a PVDF membrane and blotted with a specific primary antibody. Primary antibodies: mTORC1 (cat# 2972), p-mTORC1 (Ser2448; cat# 2481), 4E-BP1 (53H11; cat# 9644); phospho-4E-BP1 (Thr37/46; Cat# 2855) and VEGF-A (E9X8Q; Cat# 50661) were from Cell Signaling; GAPDH (SC-365062), Peripherin (A-3) and IgfR-β (SC-713) from Santa Cruz. Anti-Igf-1 antibody (ab9572) was purchased from Abcam. MyoD, Myogenin, eMyHC are from DSHB (University of Iowa, lows, IA). GAPDH is from Millipore (Burlington, MA). All antibodies were used at 1:1000 dilution. Protein bands were scanned and quantified using the LI-COR Odyssey infrared scanning system (Li-COR Biosciences, Lincoln, Nebraska).
Isolation of exosomes
Exosomes were harvested from mouse serum. Mice sera were obtained from heart puncture. 0.5 ml serum from each mouse was diluted 5-times with PBS before isolation of exosomes. For purification and characterization of exosomes from serum or conditional medium, cell debris and organelles were eliminated by centrifugation at 1,000 g for 10 min, 4oC. The supernatant fraction was further centrifuged at 16,000 g for 30 min. The second supernatant was sterile filtered through a 0.22 μm filter. Exosomes were pelleted at 120,000 g for 90 min at 4°C (L8-70M ultracentrifuge, Beckman-Coulter, Indianapolis IN). Finally, the exosome pellet was re-suspended in 100-400 μl RNA stabilization reagent (Qiagen, Germantown, MD) for RNA extraction. Exosomal size and concentration were verified using a NanoSight instrument (Malvern, Westborough, MA) (32).
Reverse transcription and quantitative PCR (q-PCR) for microRNA, mRNA, and miRNA-Seq Library Preparation and Sequencing
Total RNA was extracted using Tri-Reagent (Molecular Research Inc., Cincinnati, OH). The details for measurement of miRNAs, mRNA and miRNA-Seq Library Preparation and Sequencing are in the supplementary methods and previous description (28). Expression of miRNAs of serum exosomes were standardized to the mouse miR103. Individual mRNA expression was standardized to the 18S gene. The primers were listed in the Supplementary table 1.
|
(mg/dl) |
Mean |
SD |
Number |
P value vs control* |
|
Control |
11.17105 |
0.291757 |
4 |
|
|
CKD |
36.77961 |
1.943946 |
4 |
P < 0.01 |
|
CKD-0.5hr |
38.13651 |
1.485584 |
4 |
P < 0.01 |
|
CKD-6hr |
28.92599 |
9.674348 |
4 |
P < 0.01 |
|
CKD-24hr |
31.51645 |
3.424477 |
4 |
P < 0.01 |
|
CKD-48hr |
37.93092 |
1.567599 |
4 |
P < 0.01 |
|
CKD-72hr |
30.92434 |
3.253809 |
4 |
P < 0.01 |
|
*A 2-tailed Student’s t test was used for comparisons to control. |
||||
Table 1: BUN level in mice
Cell culture
C2C12 cells (ATCC, Manassas, VA) were cultured in growth medium (Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum, 100 u/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine). For transfection, C2C12 cells in growth medium were seeded in 6-well plates and transfected using Effectene transfection reagent (Qiagen, Valencia, CA). Cells were harvested 48 hours after transfection and assayed for luciferase.
Transfection and luciferase reporter assay
The luciferase report vectors (pMIR-REPORT Luciferase) were purchased from Applied Biosystems (Cat#: AM5795; Waltham, MA). The construct was made by Emory Integrated Genomics Core. The two custom-made the vectors contained the firefly luciferase gene with the 3’-UTR (1401-1450) of VEGF (pLuc-VEGF) or the 3’-UTR (439-496) of ENO2 vector (pLuc-ENO2). The insert was cloned between the Spe I and Hind III of the multiple cloning sites. For transfection, C2C12 cells in growth medium were seeded in 6-well plates on day-1. Vector pLuc-VEGF (or pLuc-ENO2) was co-transfected with miR5107 mimic (or miR5107 inhibitor or miR-ctrl) using Effectene transfection reagent (Qiagen, Valencia, CA) on Day 2. Renilla luciferase was used for transfection efficiency control. Cells were harvested 48 hours after transfection and Luciferase activities were measured by dual-luciferase assays (Promega) using TD-20/20 Luminometer (Turner designs, Sunnyvale, CA). The miRNA mimics, inhibitors and miRNA mimic negative control were purchased from Hanheng Biomedical Technology (Shanghai, China).
Statistical analysis
All data are presented as mean ± SEM. A 2-tailed Student’s t test was used for comparisons of two groups. For a comparison of more than two groups, we used one-way ANOVA with a post hoc analysis by Bonferroni test. To analyze an interaction between two cohorts, we used two-way ANOVA. Statistical differences with P<0.05 were considered significant. N represents the number of animals per condition in an experiment.
Results
Acu/LFES in hindlimb increased protein synthesis in gastrocnemius, triceps brachii and longissimus muscles
Our previous studies found that Acu/LFES improves skeletal muscle regeneration capacity and improves muscle function following denervation and in CKD mice (9, 12). To investigate the mechanism by which Acu/LFES enhances muscle function in CKD, 5/6 nephrectomy was used to generate CKD mice. The blood urea nitrogen (BUN) was significantly increased in 5/6 nephrectomy mice (Table 1). Acu/LFES mice received needles in point GB34 and S36 using a consistent pulse, an electrical frequency of 20 Hz, and electrical current of 2 mA for 30 min. Sham mice had acupuncture needles inserted near the LFES insertion positions, and needles were connected to the LFES device, but electrical stimulation was not applied. Blood and gastrocnemius (leg), triceps brachii (arm) and longissimus (back) muscle were harvested at six different time points: immediately after Acu/LFES (0.5-hour), and 6-, 24-, 48- until 72-hours after Acu/LFES.
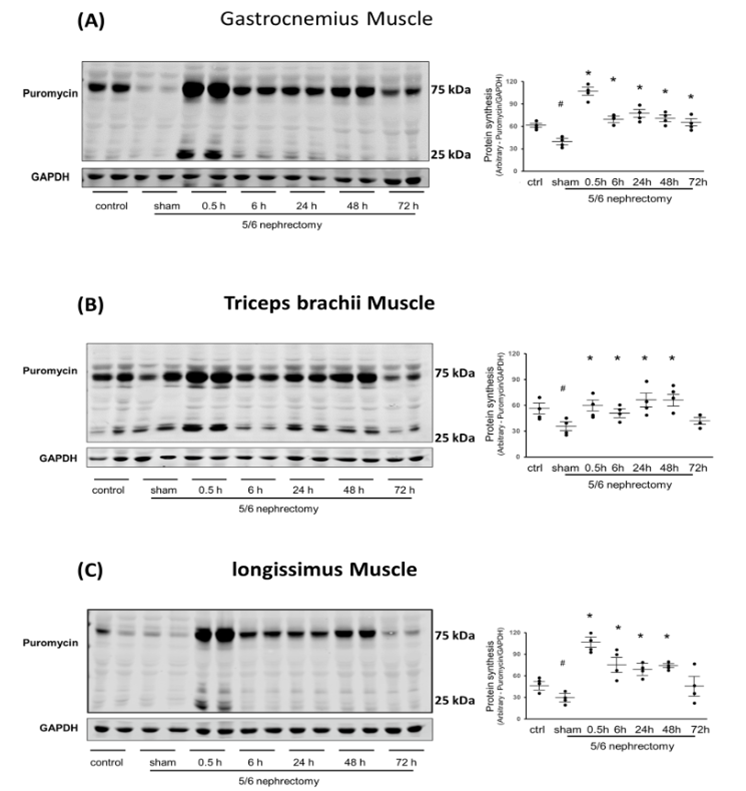
In all three muscles, protein synthesis was significantly decreased in the muscle of untreated CKD compared with normal control muscle (Figure 1). However, in gastrocnemius muscle, Acu/LFES increased protein synthesis by 2.7-fold immediately after treatment (0.5-hour) and an elevated protein synthesis was maintained up to the last experimental reading at 72-hour (Figure 1A).
Remarkably, protein synthesis exhibited a substantial increase in the triceps brachii muscle, reaching 1.7-fold at 0.5 hours, 1.5-fold at 6 hours, 2.0-fold at 24 hours, and 2.1-fold at 48 hours. Notably, these enhancements surpassed those observed in the electrically stimulated muscle. This heightened protein synthesis was sustained for up to 48 hours, as depicted in Figure 1B. To further validate the impact of Acu/LFES on overall muscle function, we examined protein synthesis in the longissimus muscle, located on either side of the vertebral bodies. In this muscle group, protein synthesis demonstrated a significant increase of 3.1-fold at 0.5 hours, 1.9-fold at 6 hours, 1.7-fold at 24 hours, and 2.1-fold at 48 hours, as illustrated in Figure 1C. These findings suggest that Acu/LFES treatment in the hindlimb not only enhances protein synthesis in the treated muscle but also exerts positive effects on untreated forelimb and back muscles.
|
Body weight (g) |
Soleus (mg) |
EDL (mg) |
TA (mg) |
Gastrocnemius (mg) |
|
|
Control |
34.2±1.2 |
10.3±0.9 |
11.3±0.9 |
53.3±2.1 |
189.7±7.3 |
|
CKD |
25.6±1.1* |
7.6±0.7* |
6.5±0.9* |
35.6±1.3* |
111.0±6.1* |
|
CKD-0.5h |
26.9±1.5* |
7.6±0.9* |
6.8±0.6* |
36.6±1.1* |
115.0±8.3* |
|
CKD-6h |
25.7±1.3* |
7.1±0.4* |
6.7±0.6* |
34.5±0.8* |
121.3±9.1* |
|
CKD-24h |
27.2±0.8* |
7.4±0.8* |
6.8±0.9* |
35.6±1.2* |
125.5±8.1* |
|
CKD-48h |
27.7±1.4* |
7.9±0.7* |
6.9±0.7* |
37.9±1.5* |
130.2±7.1* |
|
CKD-72h |
28.1±1.1* |
8.1±0.9* |
7.2±0.8* |
37.6±1.2* |
132.0±6.9* |
|
*P<0.05 by A 2-tailed Student’s t test for comparisons of control (n=4) |
|||||
Table 2: Body or muscle weights.
Experiments were performed in the normal control, CKD sham-Acu/LFES (sham) and CKD Acu/LFES treated mice. Puromycin was injected 30 minutes before harvest. Muscle was harvested from the immediately (0hr), and 6-, 24-, 48- and 72-hours after Acu/LFES. Puromycin in tissue lysates of gastrocnemius muscle (A), triceps brachii muscle (B) and longissimus muscle (C) was measured by Western blots. The point graphs show the change in incorporated puromycin normalized to their corresponding GAPDH protein. Data is provided in arbitrary units (n = 4/group; * = p<0.05 vs. sham-Acu/LFES; # = p<0.05 vs. normal control by two-way ANOVA analysis).
Muscle protein synthesis markers were increased by Acu/LFES in gastrocnemius, triceps brachii and longissimus muscle
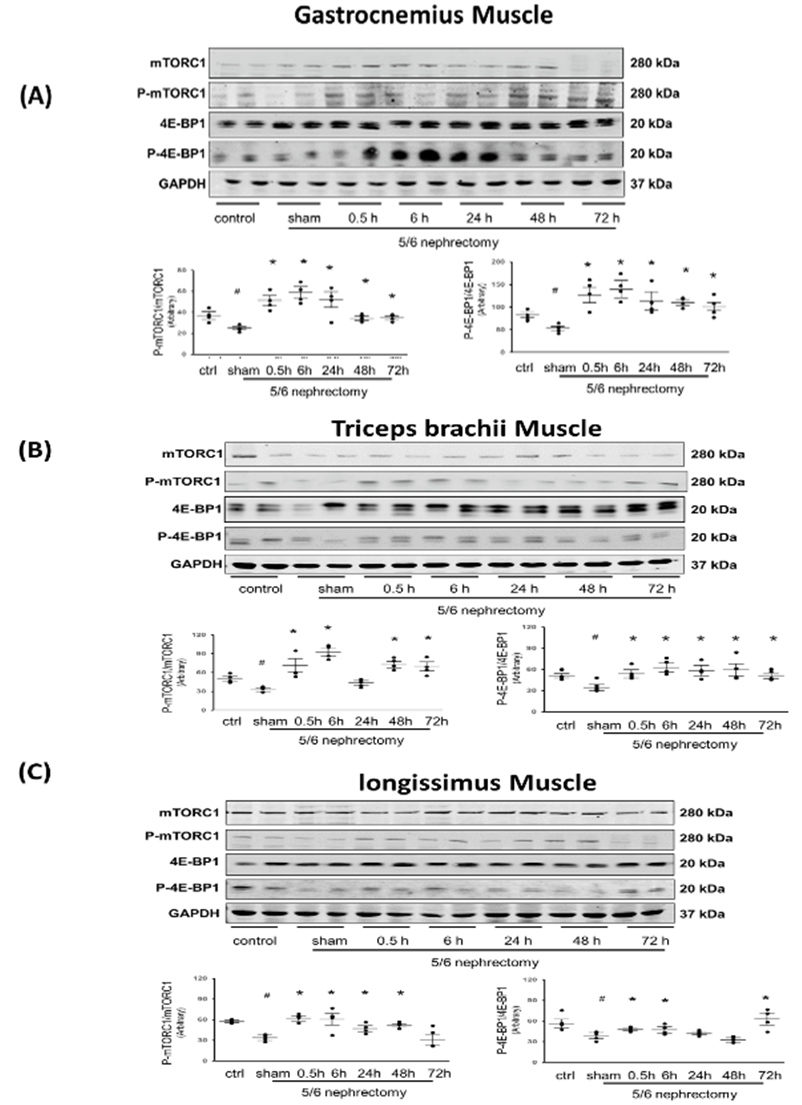
Measures of protein synthesis by puromycin incorporation are relatively common. However, some investigators consider that this technique is not accurate in energy-starved cells (33). To prove that CKD (atrophy) muscle does not have the same problem, it was critical to measure protein synthesis signaling markers (phosphorylated mTORC1 and 4E-BP1) to verify our results. Phosphorylated mTORC1 was depressed in CKD muscle without Acu/LFES treatment, but in the Acu/LFES-treated gastrocnemius muscle, it was significantly increased immediately (0.5-hour) after treatment and remained elevated for up to 72 hours. The same changes were also found in phosphorylated 4E-BP1 (Figure 2A). In triceps brachii muscle, which was not Acu/LFES treated, the increase of phosphorylated mTORC1 was apparent immediately after the gastrocnemius muscle was treated (0.5-hr) and at 6-, 12-, 48-, and terminal 72-hours. No change was observed at the 24-hour time point. The 4E-BP1 phosphorylation were increased at all time points (Figure 2B). In longissimus muscles, these two protein synthesis markers increased at 0.5-hours after Acu/LFES treatment (of the gastrocnemius) until 6- or 48-hour (Figure 3C). These results support our previous observation [13], showing that Acu/LFES treatment in hindlimb improves protein synthesis not only in local muscle, but also in distant muscles.
The total and phosphorylated proteins of mTORC1 and 4EBP1 were measured by Western blotting in gastrocnemius muscle (A), triceps brachii muscle (B) and longissimus muscle (C) in normal and CKD mice with or without Acu/LFES treatment. The point graphs show the ratio of phosphorylated protein to total protein and normalized to the GAPDH from the same sample. Data is provided in arbitrary units (n = 4/group; * = p<0.05 vs. sham; # = p<0.05 vs. normal control by two-way ANOVA analysis).
Muscle regeneration proteins were raised by Acu/LFES along with inflammatory markers in the muscle of CKD mice
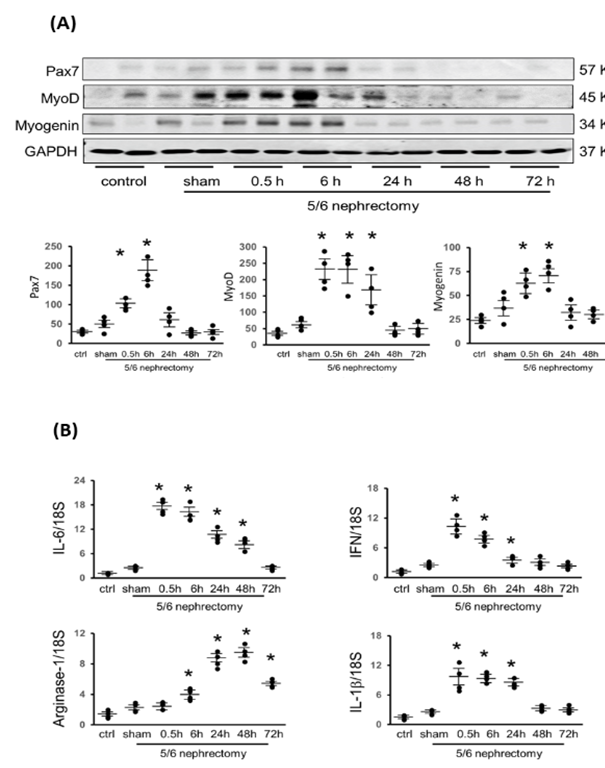
To identify the impact of Acu/LFES on myogenesis, pax7, myoD and myogenin proteins were analyzed in the CKD muscles. In gastrocnemius muscle, pax7, a transcription factor that plays a role in activation of muscle precursor cells, was increased immediately after Acu/LFES through to 6-hours. MyoD, a protein that promotes myoblasts proliferation, was increased from 0.5- to 24-hours. Myogenin, a transcription factor involved in myoblasts differentiation, was increased from 0.5- to 6-hours (Figure 3A). These three proteins were also upregulated in triceps brachii (supplementary Figure 3) and longissimus muscle (supplementary Figure 4). A transient increase in local pro-inflammatory cytokine expression mediates the repair and regeneration of damaged myofibers through myogenesis. Inflammatory factors were analyzed by PCR. IL-6 and interferon gamma (IFN) were increased from 0 to 48-hours or 0 to 24-hours, respectively (Figure 3B). IL-1 beta, a type-1 macrophage marker, was increased from 0 to 24-hours. Arginase-1, a type-2 macrophage marker, was increased from 6 hours through 72-hours, which is the end point of measurement. These results support the concept that Acu/LFES treatment improves myogenesis with a transient increase in pro-inflammatory cytokines.
(A) Myogenesis related proteins Pax-7, myoD, and myogenin were measured by Western blotting in gastrocnemius muscle in normal and CKD mice with or without Acu/LFES treatment. The point graphs show the change of each protein normalized to the GAPDH from the same sample. Data is provided in arbitrary units (n = 4/group; * = p<0.05 vs. sham by two-way ANOVA analysis). (B) The mRNA expressions were assayed using total RNA isolated from gastrocnemius muscle of normal control and CKD mice with sham Acu/LFES or at the 0.5-, 6-, 24-, 48- and 72-hours after Acu/LFES. The inflammation related mRNAs IL-6, Interferon gamma (IFN), arginase-1 and IL-1β were assayed by real time qPCR. The point graph shows the fold changes of individual mRNA expression in each group normalized with 18S and compared with the sham group (n = 4/group; mean ± s.e.; * = p<0.05 vs. sham by one-way ANOVA analysis).
Acu/LFES upregulated the expression of mRNA and proteins involved in revascularization and innervation in skeletal muscles.
It is widely recognized that Igf-1 exerts growth-promoting effects on nearly every cell in the body, and the closely intertwined relationship between protein synthesis and the Igf-1 signaling pathway has been established (34). To investigate the potential association between Igf-1 and Acu/LFES-induced protein synthesis, Western blot analysis was employed to measure the protein levels of Igf1 and IgfR.
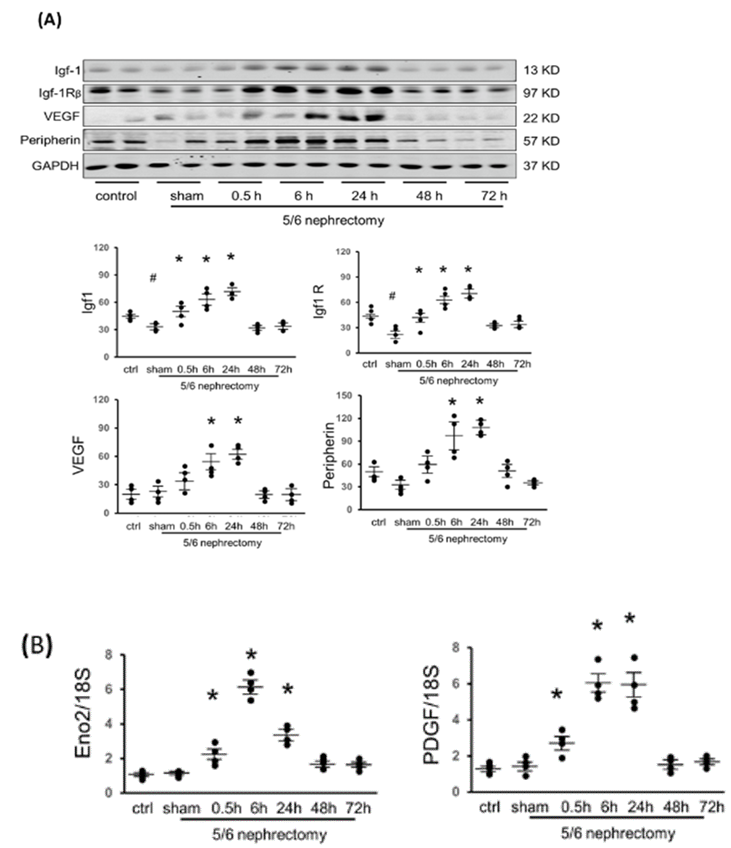
In CKD mice (without Acu/LFES), the protein amount of Igf-1 was significantly reduced in all three different muscles when compared to normal control muscle. However, upon Acu/LFES treatment of the gastrocnemius muscle, noticeable increases in Igf-1 and IgfR were observed from 0.5 to 24 hours in CKD mice (Figure 4A). In the triceps brachii, the rise in Igf-1 was evident from 0.5 to 6 hours, while IgfR showed an increase at 0.5, 48, and 72 hours (Supplementary Figure 5). Longissimus muscles exhibited an increase in Igf-1 from 0.5 to 48 hours, and IgfR showed elevation from 0.5 to 72 hours, except for the 48-hour time point (Supplementary Figure 6).
Fully functional muscles necessitate adequate vascularization and innervation. Vascular endothelial growth factor (VEGF), a signaling protein stimulating blood vessel formation, displayed an increase from 6 to 24 hours in the gastrocnemius (Figure 3), triceps brachii, and longissimus muscle (Supplementary Figure 3 & 4). Peripherin, a protein related to neurogenesis, was upregulated by Acu/LFES at 6 to 24 hours in the gastrocnemius (Figure 3), triceps brachii (Supplementary Figure 3), and longissimus muscle (Supplementary Figure 4). Additionally, the expression of another innervation marker, ENO2, and revascularization marker PDGF were upregulated in CKD gastrocnemius muscle (Figure 3B). These findings strongly support the notion that Acu/LFES treatment stimulates muscle angiogenesis and neurogenesis-related molecules in the muscles of CKD mice.
(A) Protein was isolated from normal control, CKD with sham-Acu/LFES and CKD with Acu/LFES treated mice. Igf-1, IgfR, VEGF and peripherin were measured by Western blots in gastrocnemius muscle. The point graph showed the change of the density of each protein normalized to their corresponding GAPDH protein (n = 4/group; * = p<0.05 vs. sham; # = p<0.05 vs. normal control by two-way ANOVA analysis). (B) The mRNA expression of ENO2 and PDGF were assayed by real time qPCR. The point graph shows the fold changes of individual mRNA in each group compared with the sham group (presented as one-fold) and normalized with 18S (n = 4/group; mean ± s.e.; * = p<0.05 vs. sham by one-way ANOVA analysis).
Exosome carried miRNAs were altered by Acu/LFES treatment
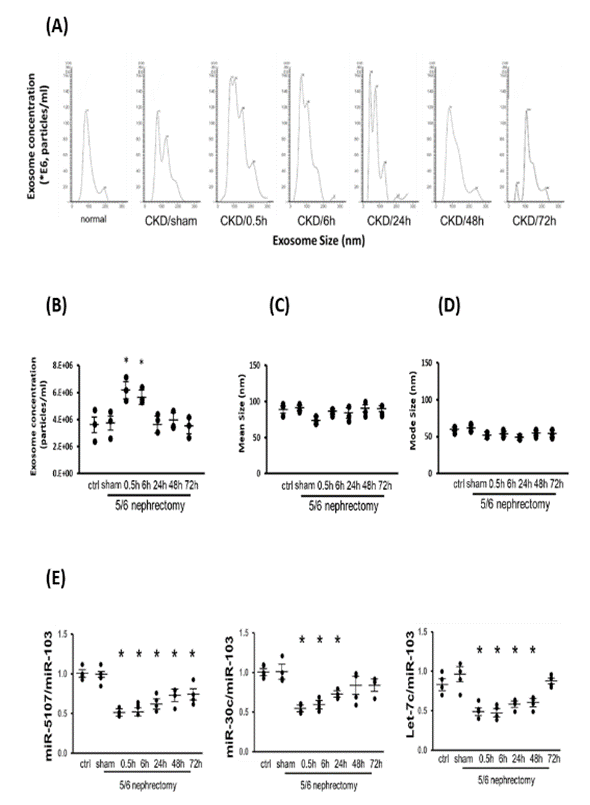
Electrical acupuncture has been associated with modifications in both circulating exosome concentrations and exosome cargo, including microRNAs (28). To delve into the role of exosomes in response to Acu/LFES, we isolated exosomes from the serum of all cohorts, employing a NanoSight instrument for quantification. The results revealed a discernible shift in exosome distribution in the serum induced by Acu/LFES (Figure 5A).
In CKD mice, Acu/LFES led to a significant 1.5-fold increase in exosome concentration at the 0.5- and 6-hour time points (Figure 5B). Notably, there were no significant differences observed in either exosome mean size or mode size within each group (Figure 5C & 5D). The miRNA deep sequencing results identified alterations in 34 miRNAs within serum exosomes. Specifically, miR-5107-5p, miR-30-5p, and four members of the let-7 miRNA family were significantly reduced by Acu/LFES in the muscles of normal mice (GEO accession number GSE176530 & Supplementary Figure 2).
To corroborate these findings, we conducted real-time qPCR for three selected miRNAs using RNA isolated from serum exosomes of Acu/LFES mice. The expression of miR-5107-5p exhibited a decrease from 0.5 to 72 hours, miR-30c-5p decreased from 0.5 to 24 hours, and let-7c-5p demonstrated a reduction at 0.5 to 48 hours (Figure 5E). These results indicate that Acu/LFES significantly increases serum exosome concentration without altering exosome size. Moreover, Acu/LFES induces changes in the miRNA composition carried by the exosome cargo.
Exosomes were isolated from the serum of each group of mice. The exosome distribution (A), concentration (B), mode size (C) and mean size (D) were measured using a NanoSight instrument (means ± SE; n = 3/group; *P < 0.05 vs. CKD/sham by one-way ANOVA analysis). (E) Total RNA was isolated from exosome of normal control and CKD mice with sham Acu/LFES or at the immediately (0hr), and 6-, 24-, 48- and 72-hours after Acu/LFES. The mRNA expression of miR-5107, miR3c-5p and Let-7c-5p were assayed by real time qPCR. The point graph shows the fold changes of individual miRNA compared with the sham group (n = 4/group; mean ± s.e.; * = p<0.05 vs. sham by one-way ANOVA analysis).
The 3’-UTRs of VEGF and Eno2 were directly targeted by miRNAs in cultured C2C12 muscle cells
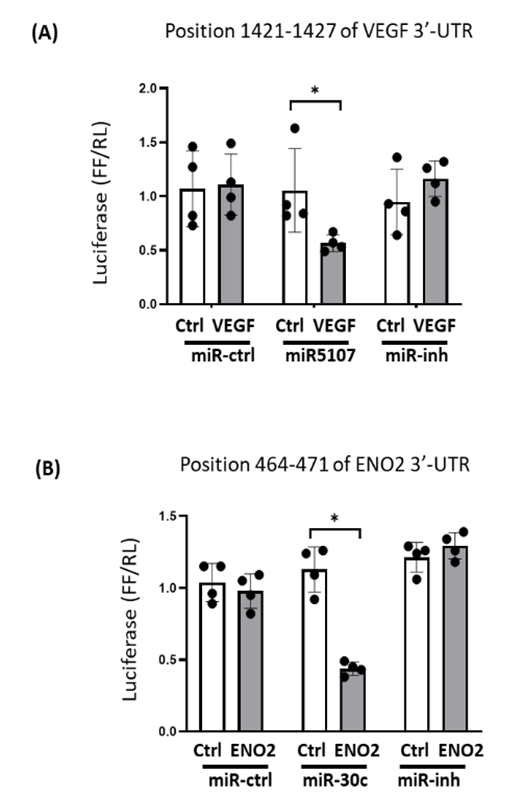
According to a miRNA databases (TargetSacn 6.0 and miRDB) search, miR-5107-5p targets VEGF mRNA. Since microRNA inhibits protein translation of their target mRNAs, a decrease of miR-5107-5p could release the inhibition of protein translation and result to increased targeted proteins, in this case, VEGF. To experimentally confirm that miR-5107-5p directly interacts with the VEGF, the VEGF target sites of miR-5107-5p (Position 1421-1427 of VEGF 3'-UTR) were cloned into a luciferase reporter construct (pLUC-VEGF/3UTR). When cells were transfected with pLUC-VEGF/3UTR along with miR-5107-5p, luciferase activity was 51% decreased (P<0.05 vs pLUC-ctrl). When a miR5107 inhibitor replaced the miR-5107 in the assay, the decreased luciferase activity vanished (Figure 6A). According to a consensus target sequence search, the 3’-UTR of ENO2 contains one conserved binding site for miR-30c-5p, which located on 464-471 at the 3’-UTR of ENO2. A luciferase reporter vector (pLUC-ENO2/3UTR) was constructed to analyze the effect of miR-30 on ENO2. When C2C12 cells were transfected with pLUC-Eno2/3UTR along with miR-30C-5p, the luciferase activity was sharply decreased, but replacement with a miR-30c-5p inhibitor abolished the increase (Figure 6B). These results confirmed that miR-5107-5p directly targets on VEGF, miR-30c-5p directly targets on ENO2, and both miRs inhibit their respective mRNAs.
(A) C2C12 cells were transfected with luciferase pLuc-ctrl vector (Ctrl) or the vector containing the 3’-UTR of pLuc-VEGF (VEGF). Cells were co-transfected with control miRNA (miR-ctrl), miR-5107 or miR-5017 inhibitor (miR-inh). (B) Cells were transfected with luciferase pLuc-ctrl vector (Ctrl) or the vector containing the 3’-UTR of pLuc-ENO2 (ENO2). Cells were co-transfected with control miRNA (miR-ctrl), miR-30c or miR-30c inhibitor (miR-inh). Luciferase activity in cells that received the pLuc-ctrl with miR-ctrl was designated as 1, the other bar/point show the response as a percent of this control. Triplicate determinations were made in each condition and each experiment was repeated once; the firefly luciferase (FFL) results were normalized by renilla luciferase (RL) activity. Data: mean ± s.e.; n=6; * = p<0.05 by one-way ANOVA analysis.
Discussion
Acupuncture is a form of alternative medicine. There is growing evidence of its use and acceptance for treating various health conditions. According to the World Health Organization, acupuncture is used in 103 of 129 countries that reported data (35). In the United States, data from the National Health Interview Survey showed a 50 percent increase in the number of acupuncture users between 2002 and 2012. In 2012 (the most recent year for which statistics are available) 6.4 percent of U.S. adults reported they had used acupuncture (35). In this study we have based our mechanism theory on data from scientific investigative methods (nanoscience and molecular biology). These provide detailed evidence for an underlying mechanism that supports the empirical observation that Acu/LFES is an especially useful nonpharmacological treatment to prevent skeletal muscle atrophy. Acu/LFES treatment starts the muscular recovery process similar to the manner seen in resistance exercise (28). After exercise, there are three major steps to rebuild muscle, which are the proinflammation phase (increasing cytokine and growth factors), regeneration phase (satellite cell activation) and remodeling phase (enhancing protein synthesis, revascularization and innervation) which results in recovery of muscle function. In the first step, many inflammatory cells, such as macrophages, neutrophils and lymphocytes, present at the injury site to secrete cytokines and growth factors, such as Igf-1, VEGF or PDGF, to initiate integral muscle repair and regeneration. Igf-I stimulates myoblasts proliferation and differentiation and upregulates downstream mTORC1, 4EBP1, and ribosomal protein 70-kDa S6 kinase 1 (p70S6K1), which are implicated in protein synthesis (36, 37). The increase of VEGF and PDGF contributes in regulating the normal and pathological angiogenic processes (38). We have three lines of evidence that Acu/LFES mimics the three phases of muscle regeneration ascribed to exercise. First, Acu/LFES increased the levels of Igf-1, Igf receptor, VEGF and PDGF, which is like the exercise-induced upregulation of the proinflammation phase. Second, muscular regeneration markers, such as Pax7, myoD and myogenin are all increased by Acu/LFES treatment, which supports the improving muscle regeneration phase. Third, protein synthesis is increased and the neurogenesis markers ENO2 and peripherin as well as angiogenesis markers VEGF and PDGF are upregulated supporting promotion of the remodeling phase (39).
How was the activation of protein synthesis observed not only in Acu/LFES treated muscle, but also upregulated in the untreated distant muscles? The answer is exosomes. Exosomes are small membranous vesicles that are secreted from intracellular multivesicular bodies in all cells including muscle fibers. The release of exosomes is a common cellular function in living biological systems (40). Exosomes are biological carriers that can transport their contents between cells. They are rapidly developing as a new therapy tool for treatment of multiple diseases (41). Our previous studies of exosomes found that Acu/LEFS treatment alters the expression of multiple miRNAs (GEO accession number GSE176530) that can regulate the physiology in multiple distant organs (28, 32). In the current study, we found that in response to Acu/LFES, the hindlimb muscle releases exosomes into the circulation. These exosomes were determined to carry a decreased level of miRNA, e.g. let-7, miR-5107 or miR-30, and were able to move and influence muscles distant from the treatment site. Exosome-carried microRNAs are highly stable compared with free miRNAs in circulation since these microRNAs are encapsulated inside extracellular vesicles and thus protected from outside influences. Many studies, including ours, have determined Iet-7 binding on the 3’-UTR of Igf-1 and its pathway components (Igf-1 receptor, IRS-1 and insulin receptor) leads to inhibition of Igf-1 signaling (28, 29, 42). After Acu/LFES, the lower level of circulating let-7 would remove a repressive effect resulting in activation of Igf-1/Akt/mTORC1 signaling in local and distant muscles, which results in increased levels of Igf-1 and the consequent is activation of protein synthesis. In addition to regulating muscle mass, Let-7 miRNA also targets PDGF, another growth factor, which is related with angiogenesis (43). Our miRNA deep-sequencing study demonstrated that Acu/LFES treatment significantly decreased the expression of miR-5107-5p and miR-30c-5p (supplementary Figure 2). Using a luciferase assay, we proved that miR-5107-5p binding 3’-UTR of vascular endothelial growth factor (VEGF). Conversely, a Acu/LFES-induced decline miR-5107 would lead to upregulation of VEGF protein, which could enhance skeletal muscle repair by favoring angiogenesis (19, 20). It has been reported that combined delivery of VEGF and Igf-I enhances the muscle regenerative process (39). Another interesting finding is that miR-30c-5p is significantly reduced by Acu/LFES. According to a database search, miR-30c binds 3’-UTR of enolase 2 (ENO2). Consequently, a decrease of miR-30c would increase ENO2. ENO2, also known as gamma-enolase or neuron specific enolase (NSE), is considered an innervation marker and is an enzyme present in nerve cells in both the central and the peripheral nervous systems. Additional evidence supporting Acu/LFES promoting innervation is the upregulation of peripherin. Peripherin is a type III intermediate filament protein expressed mainly in neurons of the peripheral nervous system. Peripherin is thought to play a role in neurite elongation during development and axonal regeneration after injury (44).
Conclusion
muscle atrophy is a major clinical problem since it increases morbidity and mortality in CKD as well as other catabolic diseases. Our current study has uncovered a new mechanism by which Acu/LFES mimics exercise, thus providing a useful therapeutic alternative for muscle wasting. Acu/LFES decreases let-7, miR-5107 and miR-30 in circulating exosomes. Limitation of these miRs results in the upregulation of Igf-1, VEGF and ENO2, which leads to building functional muscle by promoting myogenesis, angiogenesis and neurogenesis. Our study provides strong mechanistic insight for understanding the benefits of treating muscle atrophy with Acu/LFES.
Acknowledgements and Funding
Research reported in this publication was supported by the Department of Veteran Affairs MERIT Award 5I01BX000994 to HC; the Zhejiang Provincial Health Science and Technology Plan Foundation of China?2021KY203?and the National Natural Science Foundation of China (81671403) to ZS. AHA Career Development Grant to YW (18CDA34060053). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH, the Department of Veterans Affairs, or the US Government.
Disclosures
The authors declare no conflict of interest.
Author Contributions
XW, HC, ZS, and MY: conceptualization. MY, XC, XG and ZS: methodology. XW, ZS, MY, and FH: validation. ZS and MY: formal analysis. ZS, MY, HC, FH, YH and SB: investigation. XW and HC: resources. XW, ZS, YH, and MY: data curation. ZS and MY: writing—original draft preparation. XW and JK: writing–review and editing. XW and HC: visualization and funding acquisition. XW: supervision. All authors contributed to the article and approved the submitted version.
Data Availability Statements
The data underlying this article will be shared on reasonable request to the corresponding author.
Data Access
The small-RNA-seq data sets from the study have been submitted to the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE176530.
References
- Wang XH, Mitch WE, Price SR. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. Nature reviews. Nephrology (2021).
- Wang XH, Price SR. Going micro in CKD-related cachexia. Nephrol Dial Transplant (2020).
- Zhang A, Li M, Wang B, et al. miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. Journal of cachexia, sarcopenia and muscle 9 (2018): 755-770.
- Huang Y, Wang B, Hassounah F, et al. The impact of senescence on muscle wasting in chronic kidney disease. Journal of cachexia, sarcopenia and muscle (2022).
- Wang XH, Mitch WE. Mechanisms of muscle wasting in chronic kidney disease. Nature reviews. Nephrology 10 (2014): 504-516
- Wang XH, Du J, Klein JD, et al. Exercise ameliorates chronic kidney disease-induced defects in muscle protein metabolism and progenitor cell function. Kidney Int 76 (2009): 751-759.
- Onda A, Jiao Q, Nagano Y, et al. Acupuncture ameliorated skeletal muscle atrophy induced by hindlimb suspension in mice. Biochem Biophys Res Commun 410 (2011): 434-439.
- Sudhakaran P. Amyotrophic Lateral Sclerosis: An Acupuncture Approach. Med Acupunct 29 (2017): 260-268.
- Hu L, Klein JD, Hassounah F, et al. Low-frequency electrical stimulation attenuates muscle atrophy in CKD--a potential treatment strategy. J Am Soc Nephrol 26 (2015): 626-635
- Su Z, Robinson A, Hu L, et al. Acupuncture plus Low-Frequency Electrical Stimulation (Acu-LFES) Attenuates Diabetic Myopathy by Enhancing Muscle Regeneration. PLoS One 10 (2015): e0134511.
- Yu J, Wang M, Liu J, et al. Effect of electroacupuncture on the expression of agrin and acetylcholine receptor subtypes in rats with tibialis anterior muscular atrophy induced by sciatic nerve injection injury. Acupunct Med 35 (2017): 268-275.
- Su Z, Hu L, Cheng J, et al. Acupuncture plus low-frequency electrical stimulation (Acu-LFES) attenuates denervation-induced muscle atrophy. J Appl Physiol (1985) 120 (2016): 426-436.
- Gibson JN, Smith K, Rennie MJ. Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet 2 (1988): 767-770.
- Mitchell HM, Hazell SL. Helicobacter pylori, gastric ulcer, and duodenal ulcer. N Engl J Med 335 (1996): 1841.
- Holz MK, Ballif BA, Gygi SP, et al. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123 (2005): 569-580.
- Chung J, Kuo CJ, Crabtree GR, et al. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 69 (1992): 1227-1236.
- Best TM, Gharaibeh B, Huard J. Stem cells, angiogenesis and muscle healing: a potential role in massage therapies? Br J Sports Med 47 (2013): 556-560.
- Ota S, Uehara K, Nozaki M, et al. Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am J Sports Med 39 (2011): 1912-1922.
- Deasy BM, Feduska JM, Payne TR, et al. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther 17 (2009): 1788-1798.
- Frey SP, Jansen H, Raschke MJ, et al. VEGF improves skeletal muscle regeneration after acute trauma and reconstruction of the limb in a rabbit model. Clin Orthop Relat Res 470 (2012): 3607-3614.
- Hannink M, Donoghue DJ. Structure and function of platelet-derived growth factor (PDGF) and related proteins. Biochim Biophys Acta 989 (1989): 1-10.
- Rantanen J, Ranne J, Hurme T, et al. Denervated segments of injured skeletal muscle fibers are reinnervated by newly formed neuromuscular junctions. J Neuropathol Exp Neurol 54 (1995): 188-194.
- Chadan S, Moya KL, Portier MM, et al. Identification of a peripherin dimer: changes during axonal development and regeneration of the rat sciatic nerve. J Neurochem 62 (1994): 1894-1905.
- Kirino T, Brightman MW, Oertel WH, et al. Neuron-specific enolase as an index of neuronal regeneration and reinnervation. The Journal of neuroscience : the official journal of the Society for Neuroscience 3 (1983): 915-923.
- Bhattacharyya SN, Habermacher R, Martine U, et al. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125 (2006): 1111-1124.
- Jung HJ, Suh Y. Regulation of IGF -1 signaling by microRNAs. Front Genet 5 (2014): 472.
- Wang XH. MicroRNA in myogenesis and muscle atrophy. Curr Opin Clin Nutr Metab Care 16 (2013): 258-266.
- Huang Y, Yu M, Kuma A, et al. Downregulation of let-7 by Electrical Acupuncture Increases Protein Synthesis in Mice. Frontiers in physiology 12 (2021): 697139.
- Zhu H, Shyh-Chang N, Segre AV, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 147 (2011): 81-94.
- Lim S. WHO Standard Acupuncture Point Locations. Evidence-based complementary and alternative medicine : eCAM 7 (2010): 167-168.
- Goodman CA, Mabrey DM, Frey JW, et al. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. Faseb J 25 (2011): 1028-1039.
- Su Z, Yuan Y, Yu M, et al. Electrically stimulated acupuncture increases renal blood flow through exosomes-carried miR-181. Am J Physiol Renal Physiol (2018).
- Marciano R, Leprivier G, Rotblat B. Puromycin labeling does not allow protein synthesis to be measured in energy-starved cells. Cell death & disease 9 (2018): 39.
- Wang XH, Mitch WE, Price SR. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. Nature reviews. Nephrology 18 (2022): 138-152.
- Health, N. N. C. f. C. a. I. Acupuncture: What You Need To Know. In https://www.nccih.nih.gov/health/acupuncture-what-you-need-to-know
- Morton RW, Oikawa SY, Wavell CG, et al. Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J Appl Physiol (1985) 121 (2016): 129-138.
- Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 1 (2011): 4.
- Melincovici CS, Bosca AB, Susman S, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 59 (2018): 455-467.
- Borselli C, Storrie H, Benesch-Lee F, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A 107 (2010): 3287-3292.
- Kim DK, Lee J, Simpson RJ, et al. EVpedia: A community web resource for prokaryotic and eukaryotic extracellular vesicles research. Seminars in cell & developmental biology 40 (2015): 4-7.
- Zhao X, Wu D, Ma X, et al. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother 128 (2020): 110237.
- Samadi P, Afshar S, Amini R, et al. Let-7e enhances the radiosensitivity of colorectal cancer cells by directly targeting insulin-like growth factor 1 receptor. J Cell Physiol 234 (2019): 10718-10725.
- Brennan E, Wang B, McClelland A, et al. Protective Effect of let-7 miRNA Family in Regulating Inflammation in Diabetes-Associated Atherosclerosis. Diabetes 66 (2017): 2266-2277.
- Foley J, Ley CA, Parysek LM. The structure of the human peripherin gene (PRPH) and identification of potential regulatory elements. Genomics 22 (1994): 456-461.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 