Drug Delivery: Bactericidal Effect of Manganese Oxide-Loaded Carbon Nanotubes Enhances Drug Efficiency
Md Sydur Rahman1*, Al-Nakib Chowdhury2, Mahabubur Rahman3, Md. Manwarul Islam4
1Graduate Student, Department of Chemistry, Mississippi State University, USA
2Professor, Department of Chemistry, Bangladesh University of Engineering and Technology, Bangladesh
3Senior scientist and Head of the Laboratory, Department of Bacteriological Research, ICDDR, Bangladesh
4Professor, Department of Chemistry, Bangladesh University of Engineering and Technology, Bangladesh
*Corresponding author: Md Sydur Rahman, Graduate Student, Department of Chemistry, Mississippi State University, USA.
Received: 06 June 2022; Accepted: 20 June 2022; Published: 23 June 2022
Article Information
Citation: Md Sydur Rahman, Al-Nakib Chowdhury, Mahabubur Rahman, Md. Manwarul Islam. Drug Delivery: Bactericidal Effect of Manganese Oxide-Loaded Carbon Nanotubes Enhance Drug Efficiency. Journal of Biotechnology and Biomedicine 5 (2022): 137-147
View / Download Pdf Share at FacebookAbstract
Nanoparticles increase their activity due to their large surface-to-volume ratio. It has promoted research to check the antibacterial activity of the synthesized manganese oxide nanoparticles of actual size. Carbon Nanotube (CNT) shows excellent potential as a biomedical substrate based on its high chemical stability, elasticity, mechanical strength, electrical conductivity, and nano-level attachment of CNT with microbiologically susceptible manganese oxide materials that opens new possibilities to enhance the antibacterial delivery system. Functionalized CNTs with nanoactive materials realize nanoscaled containers in which the active content is encapsulated by a protecting carbon shell. CNT itself doesn't have any antibacterial activity and can frequently release the carrying drugs to the target places in specific chemical environments. The ability to functionalize the sidewalls of CNT also leads to biomedical applications such as neuron growth and regeneration. This research focuses on the amplified application of CNT as a nano-carrier based delivery vehicle and their appropriate design for desired drug delivery results in different areas of infectious diseases.
Keywords
<p>Drug delivery; Carbon nanotube; Manganese oxide; Antibacterial susceptibility; Antimicrobial activity</p>
Article Details
1. Introduction
Antibacterials are among the most commonly used drugs. For example, 30% or more patients admitted to the hospital are treated with one or more courses of antibacterial.However, antibacterial is also among the drugs commonly misused by physicians. Among inorganic antibacterial agents, manganese can be employed most extensively to fight infections and control spoilage next after silver [1-3]. Catalytic oxidation by metallic manganese and reaction with dissolved monovalent manganese ion probably contribute to its bactericidal effect. As yet, with all of the progress, many medications, even those discovered using the most advanced molecular biology strategies, have unacceptable side effects due to the drug interacting with parts of the body that are not the target of the drug. Side effects limit the ability to design optimal medications for many diseases such as cancer, neurodegenerative diseases, and infectious diseases.Scientists now use nanotechnology to approach classical and novel drug delivery applications [4,5]. Controlled, and targeted deliveries are the most enviable requirements expected from a carrier, which involves a multi-disciplinary site or targeted approach. Pharmaceutical nanoparticles are sub-nanosize-based structures that contain drugs or bioactive substances constituted of several tens or hundreds of atoms or molecules with a variety of sizes within them [6]. CNTs have become the strongest candidates mainly in the field of biomedical engineering, biotechnology, and pharmaceutical nanotechnology after their discovery in 1991 [7]. Various biomedical applications of nanomaterials have been proposed in the last few years leading to the emergence of a new field in diagnostics and therapeutics. Most of these applications involve the administration of nanoparticles into patients. CNTs are enjoying increasing popularity as building blocks for novel drug delivery systems as well as for bioimaging and biosensing [8-12]. This research focuses on the application of CNTs as nano carrier-based delivery systems and their appropriate design for achieving the desired drug delivery results in the different areas of infectious diseases. In this research work, the antibacterial activity of Mn3O4 nanoparticles was investigated againstsome bacterial microorganisms. Mn3O4 nanoparticles were synthesized by solution-phase routes, and their interactions withthese bacterial microorganisms were studied. To observe the activity of the CNT as a drug delivery agent, the test for antibacterial activity of the Mn3O4 nanoparticles loaded CNTs was successfully accomplished.
2. Materials and Methods
All chemicals employed in the experiment were of analytical grade and were obtained from Sigma Aldrich, England, with a minimum purity of 99.5%. The water used in all experimental work was double distilled. The aliphatic-aromatic mixture was prepared by using an analytical balance with a precision of ±o.1 µg. Special care was taken to prevent evaporation and the introduction of moisture into the experimental samples. CNT was prepared following early established procedure using organic bulk method [13]. In this type of preparation, first, a solution of 40 ml of equal aliphatic alcohol mixture (Hexanol + Octanol) and 60 mL of the equal aromatic compound mixture (Benzene + m-Xylene) were prepared. 7g (about 15% by weight of carbon sources) of Benzalkonium chloride (BZK) and 1g of FeCl3 was then added and the mixture was stirred for 12 hours. BZK is a cationic surfactant that plays the role of stabilizing nanoparticles to be formed. 0.5g of hydrazine hydrate was added as a reducing agent to the above-obtained solution, and the mixture was stirred again for 24 hours to get a dense solution. In the meantime, iron nanoparticles were formed by the reduction of FeCl3 present in this combined dense solution. Then forced pyrolysis reaction was carried out by introducing the obtained solution into a tube furnace with an inert atmosphere with argon gas at 600 oC for 20 minutes. CNT (MWCNT) thus prepared is very identical in size and shape. Preparation of Mn3O4 nanoparticles followed the modified way of forced hydrolysis of hydrated manganese (ll) acetate. About 500 ml of 0.2 molar analytical reagent graded hydrated manganese (ll) acetate [Mn(CH3COO)2.H2O] solution was heated in the oven for 3 hours at 80 oC. Brown precipitates of Mn3O4 nanoparticles thus synthesized were washed several times with double distilled water and then with ethanol. After drying them at 100 oC for 10 hours, they were preserved. The synthesized CNTs and Mn3O4 nanoparticles were then purified and characterized by Energy Dispersive X-Ray spectroscopic (EDX), and Scanning Electron Microscopic (SEM) analysis.
2.1 Loading of CNTs with Mn3O4 nanoparticles
Loading of Mn3O4 nanoparticles in CNTs was done following aqueous in-situ CNT’s cavity stabilization that prevents pollution and agglomeration of metal oxide nanoparticles and can lead to nanomaterial attached CNTs. For this, at the time of formation of these metallic oxides, CNTs were incorporated into the preparation system and then heated in the overall solution for 2 hours at 100 oC. The loaded CNTs were precipitated down, and the unattached nanoparticles were dispersed in the water. After separation, finally, metal oxides combined CNTs were dried at room temperature.
2.2 Antibacterial susceptibility testing
Of the many media available, National Committee for Clinical Laboratory Standards (NCCLS) [14] recommends Mueller-Hinton agar due to its results in good batch-to-batch reproducibility; it results in satisfactory growth of most bacterial pathogens. The agar medium should have a pH of 7.2 to 7.4 at room temperature. The surface should be moist but without a droplet of moisture. The antibiotic disks should be maintained at 8 °C or lower or freeze at -14 °C or below until needed, according to the manufacturer's recommendations. At first, well-isolated colonies were selected from agar plates. Then each colony of bacteria was transferred with a wire loop to a test tube containing 4 to 5 mL of a suitable broth medium, such as tryptic-soy broth. The broth culture was allowed to incubate at 35 °C until it achieved or exceeded the turbidity of 0.5 McFarland standard. The turbidity was adjusted with sterile saline or broth in adequate light, and to aid in the visual comparison, read the tube against a white background with contrasting black lines. Within 15 minutes after adjusting the turbidity of the inoculum suspension, a sterile, nontoxic swab with an applicator was dipped into the adjusted suspension. The swab was rotated several times and pressed firmly on the inside wall of the tube above the fluid level to remove excess inoculum from the swab. The dried surface of Mueller-Hinton agar plates was inoculated by streaking the swab over the entire sterile agar surface. This procedure was repeated two more times and rotated the plate 60° by angle each time to ensure an even distribution of inoculum. Then the plates were replaced on the top and allowed 3 to 5 minutes, but no longer than 15 minutes, for any excess surface moisture to be absorbed before applying the antibiotic materials.Instead of a disk, an appropriate well (no closer than 35 mm from center to center) was cut on the surface of the agar plate by using sterile forceps to get the best diffusion of the antibacterial nanomaterials. No more than six wells should be cut on a 150 mm plate. The previously prepared suspension of Mn3O4 was poured by 50 µL, 100 µL, and 120 µL or sometimes 150 µL in the well. The plates were then placed in an incubator at 37 °C for 15 minutes after the wells were cut properly. The plates should be incubated aerobically (no CO2). After 16-18 hours of incubation, each plate was examined, and measured the diameters of the zones of complete inhibition, including the diameter of the well, using a ruler. Then the zone sizes were analyzed and reported to the organisms to be either susceptible, intermediate, or resistant.
3. Results and Discussion
Scanning Electron Microscope (SEM) resolves features from the optical regime down to the sub-nanometer length scale. It uses a focused beam of high-energy electrons to generate a variety of signals at the surface of solid specimens. The signals that derive fromelectron-sample interactions reveal information about the sample, including external morphology (texture), to confirm the size and shape of nanoparticles. The SEM photographs were taken with an average magnification of up to ×100000 at room temperature. Figure 1, 2 shows dense and clean nanotubes. Distinguishable CNTs are visible at high resolution, and the cross-section confirms CNTs' specific diameter. Obviously, the quality is much better. The diameter of the nanotubes is about 70-95 nm on average (Figure 2).

Figure 1: SEM image confirms the uniform tube shape of synthesized CNTs

Figure 2: Average tube diameters were found in the range of 70-95 nm for synthesized CNTs.
The elemental analysis was successfully confirmed by EDX characterization. From Figure 3, it can be observed that there is a clear abundance of carbon elements which strongly supports that CNTs contain only carbons. The abundance is simply detected by the k(alpha) shell electrons at 0.277 KeV. The carbon percentage by mass and percentage by atomic abundance is 99.34%. Very little amount (0.66%) of unwanted oxygen might come from alcohols.
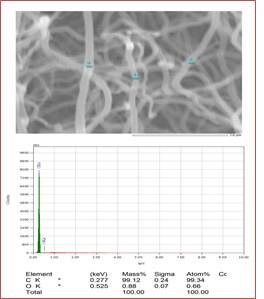
Figure 3: Elemental confirmation of synthesized CNTs that describes the almost full percentage of carbon.
SEM images of synthesized Mn3O4 nanoparticles also reveal a specific presentation of their authentic granular sizes (Figure 4). They were aggregated as common nature of oxides but still provide clear information about their sizes. These aggregated particles are loosely attached to each other and can be easily separated by making suspension. Mn3O4 was found with average sizes of 2-3 nm for every granules.
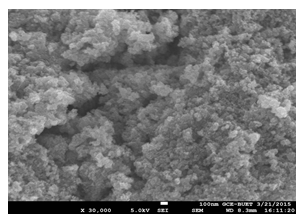
Figure 4: Uniform size and shape of the synthesized Mn3O4 nanoparticles
Figure 5 confirms that Mn3O4 nanoparticles were successfully prepared. Mn was abundantly present at 0.6 KeV, 5.9 KeV, and 6.5 KeV, comprising L and k shells, where O is confirmed at 0.52 KeV by K(alpha) shell electrons. As the energy of the X-rays is characterized by the difference in energy between the two shells and by the atomic structure of the element from which they were emitted, this allows the accurate elemental identification of the specimen to be measured.
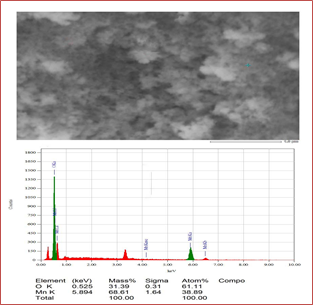
Figure 5: Elemental analysis of synthesized Mn3O4 that confirms the presence of manganese and oxygen elements.
As SEM images can only provide the outer morphology of any experimented substances so the inside particles can not be seen. This study reveals CNTs with a high-rated attachment (both coating and filling) yield, and this was confirmed by the weighing difference of two separate experiments one for only Mn3O4 and another for insitu loading. From Figure 6, it can be understood that nanoparticles were perfectly coated in the side wall of CNTs. As the size of the synthesized metal species was uniform with nano dimension, so percentages of loading were very good.
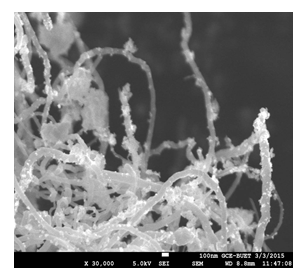
Figure 6: SEM image for loading state where Mn3O4 nanoparticles are attached noncovalently with the inside and outside walls of CNTs.
Antibacterial activity measurement of individual material of pure Mn3O4 and Mn3O4 nanoparticles loaded CNTs was done by Mueller Hinton agar disk diffusion susceptibility testing method according to NCCLS and international guidelines. Experiments were done in single and doublet from two different areas of drug-loaded wells to find uniform distribution. The antimicrobial activity of Mn3O4 nanoparticles was evaluated based on the diameters of the clear inhibition zone surrounding the wells in the dishes. (Figure 7-9) shows representative disk diffusion plates with different bacteria after 24 hours of incubation. The diameter of the inhibition zone of Escherichia coli, Streptococcus pneumonia, Pseudomonas aeruginosa, Vibrio cholerae, Enterobacter cloacae indicating that they are susceptible to the synthesized Mn3O4 nanoparticles solution. This experiment suggests a highly precise antibacterial activity of Mn3O4 against these bacterial microorganisms.
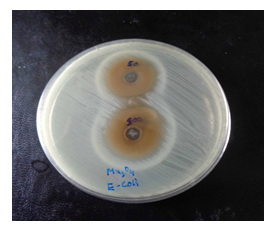
Figure 7: Image of zone inhibition for Escherichia coli by the pure Mn3O4 nanoparticles. These zones are for the specific amount of 50 µL and 100 µL
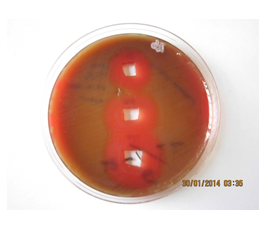
Figure 8: Image of zone inhibition for Streptococcus pneumoniae by a specific amount of 50 µL, 100 µL, and 120 µL of pure Mn3O4 nanoparticles
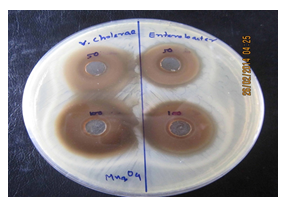
Figure 9: Image of zone inhibition for Vibrio cholerae (left) and Enterobacter cloacae (right) by the specific amount of 50 µL and 100 µL of pure Mn3O4 nanoparticles
(Figure 10-11) and table 1 display the effectivity of the Mn3O4 when they are attached to the side walls of CNTs. As there was proper diffusion of Mn3O4 nanoparticles, so the overall experiment of loaded states showed very good results, and CNTs didn't resist the antibacterial activity of Mn3O4. CNT is a well-established drug delivery agent, and it is necessary to maintain many terms and conditions for any substance to act as a drug delivery agent; and CNT satisfies all the required conditions, [15, 16] and from these antibacterial experiments, the effectivity of drugs is comparatively unchanged so using CNT as drug delivery vehicle in the field of antibacterial treatment would be possible in the near future.
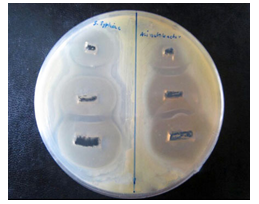
Figure 10: Image of zone inhibition for SalmonellaTyphi (left) and Acinetobacter baumannii (right) by the specific amount of 50 µL, 100 µL, and 120 µL of pure Mn3O4 loaded CNTs
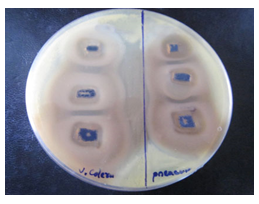
Figure 11: Image of zone inhibition for Vibrio cholerae (left) and Pseudomonas aeruginosa (right) by the specific amount of 50 µL, 100 µL, and 120 µL of Mn3O4 loaded CNTs
|
Name of Bacteria |
Comparing the diameter of zone of inhibition of different volumes of Mn3O4 encapsulated CNTs with pure Mn3O4 (mm) |
|||||
|
Pure Mn3O4 |
Mn3O4 encapsulated CNTs |
|||||
|
50 μL |
100 μL |
150 μL |
50 μL |
100 μL |
150 μL |
|
|
Pseudomonas aeruginosa |
29 |
35 |
38 |
27 |
34 |
35.5 |
|
Vibrio cholerae |
37 |
45 |
- |
35 |
42 |
48 |
|
Salmonellatyphi |
35 |
44 |
49 |
32 |
40 |
45 |
|
Acinetobacter baumannii |
30 |
38 |
45 |
27 |
35 |
42 |
Table 1: Antibacterial activity of Mn3O4 loaded CNTs comparing with the individual Mn3O4 itself to check the drug delivery efficiency of CNT.
4. Conclusion
CNTs hold great promise for use in biomedical fields [17, 18]. Among numerous potential applications, including DNA and protein sensors, separators, biocatalysts, and tissue scaffolds, this research work emphasizes the use of CNTs loaded with nano-active species as drug delivery vehicles in the field of antibacterial treatment. Microbes are unlikely to develop resistance against conventional and narrow-target antibiotics because these antibiotics attack a broad range of targets in the organisms, which means that microbes would have to develop a host of mutations simultaneously to protect themselves. It was found that well-functionalized CNTs are nontoxic to cells for limited doses [19-21], and this work may promote a synergy of techniques and approaches that strongly increases the effectiveness of the antibacterial drugs by deploying them to a target site to limit side effects.
Acknowledgement
This work was the partial fulfillment of academic degree of Master of Philosophy of Md Sydur Rahman, financially supported by department of chemistry of Bangladesh University of Engineering and Technology (BUET). Microbiological part of this research work was technologically supported by International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR, B). Teachers and students of the department of chemistry of BUET supported in many ways during this research work to make it successful.
References
- Chen Y, Liu C, Li F, et al. Preparation of single-crystal α-MnO2 nanorods and nanoneedles from aqueous solution. J Alloys Compd 397 (2005): 282-285.
- Yuan A, Wang X, Wang Y, et al. Textural and capacitive characteristics of MnO2 nanocrystals derived from a novel solid-reaction route. J Electrochim Acta 54 (2009): 1021-1026.
- Zhang X, Duan Y, Guan H, et al. Effect of doping MnO2 on magnetic properties for M-type barium ferrite. J Magn Mater 311 (2007): 507-511.
- Pastorin G. Crucial Functionalizations of Carbon Nanotubes for Improved Drug Delivery. JPharmaceutical Research 26 (2009): 746-769.
- Hilder, Tamsyn A, Hill James M. Modeling the Loading and Unloading of Drugs into Nanotubes 5 (2009): 300-308.
- Davalapalli H. Role of nanotechnology in pharmaceutical product development. J pharm sci 96 (2007): 2547-2565.
- Iijima S. Helical microtubules of graphitic carbon. J Nature 354 (1991): 56-58.
- Kam NWS, Jessop TC, Wender PA. Nanotube molecular transporters: internalization of carbon nanotube-protein conjugates into mammalian cells. J Am Chem Soc 126 (2004): 6850-6851.
- O’Connell M, Wisdom JA, Dai HJ. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci 102 (2005): 11600-11605.
- Kang B, YU DC, Chang SQ, et al. Intracellular uptake, trafficking and subcellular distribution of folate conjugated single walled carbon nanotubes within living cells. J Nanotechnology 19 (2008): 375103-375110.
- Kim SN. Carbon nanotubes for electronic and electrochemical detection of biomolecules. J Adv Mater 19 (2007): 3214-3228.
- Kolosnjaj-Tabi. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to Swiss mice. J ACS Nano 4 (2010): 1481-1492.
- Rahman MS. The Systematic Synthesis of Carbon Nanotubes from Aliphatic-Aromatic Compound Mixture Resolves Growth Uniformity and Production Complexity. Journal of Nanotechnology Research 2 (2020): 001-009.
- NCCLS. National Committee for Clinical Laboratory Standards.
- Pastorin G. Crucial Functionalizations of Carbon Nanotubes for Improved Drug Delivery”.J.Pharmaceutical Research 26 (2009): 746-769.
- Hilder, Tamsyn A, Hill James M. Modeling the Loading and Unloading of Drugs into Nanotubes 5 (2009): 300-308.
- Harris PJF. Carbon nanotube science: Synthesis, Properties and Applications. Cambridge University Press, Cambridge 2009.
- Liu Z, Davis C. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc Natl Acad Sci USA 105 (2008): 1410-1415.
- Khalid P, Hossain MA. Toxicology of Carbon Nanotubes - A Review. Int J of Appl Eng Res 11 (2011): 148-157.
- Sun Z. Carbon Nanotubes release Cytotoxicity Mediated by Human LymphocytesIn Vitro J Plos one (2011): 0021073.
- Elkin T, Jiang X, Taylor S, et al. Immuno carbon nanotubes and recognition of pathogens. J ChemBiochem 6 (2005): 640-643.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 