Metabolic Shifting Probiotic in Type 2 Diabetes Mellitus Management: Randomized Clinical Trial
Gissel García1a, Josanne Soto1b, Lays Rodríguez1c, Maricela Nuez4, Noraika Domínguez1d, Emilio F. Buchaca1c, Duniesky Martínez2, Rolando J Gómez1b, Yohanka Ávila1c, Martha R. Carlin3, Raúl J. Cano3,4*
1aPathology Department, Clinical Hospital “Hermanos Ameijeiras”. Calle San Lázaro No 701, Esq.a Belascoaín, Centro Habana, La Habana, P.C. 10400, Cuba
1bClinical Laboratory Department, Clinical Hospital " Hermanos Ameijeiras ". Calle San Lázaro No 701, Esq.a Belascoaín, Centro Habana, La Habana, P.C. 10400, Cuba
1cInternal Medicine Department, Clinical Hospital " Hermanos Ameijeiras " . Calle San Lázaro No 701, Esq.a Belascoaín, Centro Habana, La Habana, P.C. 10400, Cuba
1dEndocrinology Department, Clinical Hospital " Hermanos Ameijeiras ". Calle San Lázaro No 701, Esq.a Belascoaín, Centro Habana, La Habana, P.C. 10400, Cuba
2Research and Development Department. Center for Genetic Engineering and Biotechnology of Sancti Spíritus (CIGBSS),. Circunvalante Norte S/N, Olivos 3, Apartado Postal 83, Sancti Spíritus, P.C 60200, Cuba
3The BioCollective, LLC, 5650 N. Washington St., Ste C9, Denver, CO 80216
*4Professor Emeritus, Biological Sciences Department, California Polytechnic State University, San Luis Obispo, CA 93407 USA
*Corresponding author: Raul J. Cano, Professor Emeritus, Biological Sciences Department, California Polytechnic State University, San Luis Obispo, CA 93407 USA.
Received: 16 May 2023; Accepted: 23 May 2023; Published: 30 June 2023
Article Information
Citation: Gissel García, Josanne Soto, Lays Rodríguez, Maricela Nuez, Noraika Domínguez, Emilio F. Buchaca, Duniesky Martínez, Rolando J Gómez, Yohanka Ávila, Martha R. Carlin, Raúl J. Cano. Metabolic Shifting Probiotic in Type 2 Diabetes Mellitus Management: Randomized Clinical Trial. Journal of Biotechnology and Biomedicine. 6 (2023): 270-280.
DOI: 10.26502/jbb.2642-91280090
View / Download Pdf Share at FacebookAbstract
Objective: The objective of this study is to assess the efficacy of BiotiQuest™ Sugar Shift, a probiotic formulated to transform monosaccharides and rehabilitate human gut microbiota, in stabilize HbA1c and blood glucose, improve insulin resistance, and reduce inflammation.
Methods: A double-blind, placebo-controlled study was carried out over 12 weeks with 64 Cubans, aged 30 to 65 with Type 2 Diabetes Mellitus diagnosed, 18 of whom being treated with insulin. Participants were randomly assigned to take either two capsules of the probiotic supplement or of placebo. Clinical measures were evaluated at 28-day intervals, included fasting and post-prandial glucose, HbA1c, lipid panel, insulin, creatinine, and serum lipopolysaccharide levels were assessed.
Results: The treated group demonstrated a stabilization in their fasting blood glucose and postprandial glucose levels in treated group respect to the Placebo cohort. The HbA1c levels did not show significant changes in the treated group. The insulin levels decreased significantly in the treated group by day 84 compared to day 1 and to day 84 of the Placebo cohort (p=0.024 and p=0.015, respectively). Serum LPS levels also decreased significantly in the treated group (p=0.001).
Conclusions: BiotiQuest™ Sugar Shift is suitable as adjunct therapy for the control of T2D. However, the 12-week trial period was not sufficient to detect significant reductions in all clinical parameters measured and a longer study is recommended.
Keywords
<p>Diabetes, Probiotics, clinical trials, inflammation, insulin resistance, fasting glucose, lipopolysaccharides (LPS)</p>
Article Details
Abbreviations:
T2D: Type 2 Diabetes Mellitus; SS: BiotiQuest™ Sugar Shift; LPS: Lipopolysaccharides
Introduction
Type 2 diabetes mellitus (T2D) is a chronic metabolic disorder characterized by hyperglycemia, insulin resistance, and chronic inflammation [1]. T2D affects a significant number of people worldwide, with the prevalence increasing in all countries regardless of their level of development [2]. Despite treatment options, patients do not always achieve glycemic control, leading to the need for alternative therapies [3]. Certain formulations of probiotics can improve glucose levels in individuals suffering from T2D [4]. Probiotics are mixtures of living microorganisms that improve, or restore the intestinal microbiota or impact health benefits [5]. Not all probiotic formulations are balanced or effective. BiotiQuest™ Sugar Shift (SS) is a novel probiotic consisting of eight strains of bacteria with Generally Recognized as Safe (GRAS) classification designed with the aid of a community-based flux balance analysis algorithm [6]. to produce mannitol, short chain fatty acids (SCFA) and reduced glutathione. Mannitol production by the consortia is achieved through the conversion of dietary glucose and fructose [7]. We hypothesize that SS can enhance the human gut microbiota to utilize intestinal glucose and fructose, and produce anti-inflammatory metabolites, leading to stabilized HbA1c and blood glucose, improved insulin sensitivity, and reduced inflammation. To test our hypothesis, a 12-week double-blind, placebo-controlled study employing SS was conducted in a population of Cuban subjects with T2D.
Human Experiments
Report of the Scientific Research Ethics Committee
The members of the Scientific Research Ethics Committee (CEI) certify that: The clinical trial entitled “Effect of BiotiQuest TM and Kesto Mix dietary supplements in the treatment of Type 2 Diabetes Mellitus” was evaluated on June 22, 2021, from an ethical, scientific, and methodological perspective, and in the absence of conflicts of interest.
It was considered that:
- The research project meets the requirements of suitability in relation to the study objectives, justification of risks, measures to be taken for the treatment of adverse effects in case they arise, as well as the rights, safety, and welfare of the subjects or patients.
- Both the informed consent text and the procedures for obtaining it are adequate.
Based on the above, this committee approves the conduct of this clinical trial without modifications to the trial protocol.
Materials
Test substance
The test substance, SS, was manufactured by BlisterPak Pro, LLC in Lafayette, Colorado. Each capsule contained 96 mg (18 billion CFU) of a bacterial consortium consisting of eight strains of GRAS-classified bacteria, including Bacillus subtilis De111™, Bifidobacterium bifidum, Bifidobacterium longum, Lactobacillus paracasei, Lactobacillus plantarum TBC0036, Lactobacillus reuteri, Leuconostoc mesenteroides TBC0037, and Pediococcus acidilactici. In addition, each capsule contained 370 mg of prebiotics and fillers such as inulin, microcrystalline cellulose, D-mannitol, and stearic acid [5].
Placebo Control
Placebo capsules (BlisterPak Pro) were visually indistinguishable from the test substance, contained 370 mg of the same prebiotics and fillers as the test substance but lacking the bacterial consortium.
Methods
Study design
A 12-week clinical study was conducted at Hospital Clínico Quirúrgico Hermanos Ameijeiras (HHA) in La Habana, Cuba to investigate the effect of SS as a supplementary therapeutic approach for T2D. The study was randomized, double-blind, placebo controlled. Patient were enrolled from June 2021 to April 2022 Patients meeting the inclusion criteria were enrolled by an attending physician. The study enrolled 64 patients and randomly assigned to either the SS cohort or the Placebo group. The SS cohort, consisting of 32 patients receiving the Sugar Shift (SS) test substance, while the Placebo group, consisted of 32 patients who were given a placebo. Table 1 provides an overview of the demographic and other baseline characteristics of the study population. Patients were randomly assigned to treatment groups using EpiData 3.1 software The software generated a list of random numbers for each group, which was used by pharmacy to assign patients to their respective groups based on their compliance with the inclusion and signature criteria for informed consent. To ensure blinding, the products were dispensed in identical packaging.
|
Demographic Variables |
SS cohort (n = 30) |
Placebo (n = 27) |
P Value |
|||
|
No. |
% |
No. |
% |
|||
|
Sex |
Female |
18 |
60 |
14 |
51.9 |
0.725a |
|
Male |
12 |
40 |
13 |
48.1 |
||
|
Age |
Media ± SD |
56.3 ± 6.7 |
53.2 ± 7.6 |
0.120b |
||
|
Nutritional Assessment |
Normal weight |
3 |
10 |
5 |
18.5 |
0.722a |
|
Overweight |
16 |
53.3 |
8 |
29.6 |
||
|
Obesity |
11 |
36.7 |
14 |
51.9 |
||
|
Kind of Treatment |
Diet |
3 |
10 |
2 |
7.4 |
0.549a |
|
Diet plus oral hypoglycemic agents |
19 |
63.3 |
15 |
55.6 |
||
|
Insulin |
0 |
0 |
2 |
7.4 |
||
|
Combined treatment |
8 |
26.7 |
8 |
29.6 |
||
Table 1: Distribution of Patients according to Demographic Characteristic (sex and age), Nutritional Assessment and Kind of Treatment.
Power and sample size consideration
The minimum required sample size for the study was estimated using a two-sided alpha of 0.05, a 95% confidence level, a standardized mean difference of 0.75, and a test power of 80%.
Eligibility
Patients aged 30-65 with T2D who attended HHA diabetes consultations and consented for inclusion in the study regardless of race, sex or skin color. Individuals with kidney disease, cancer, and pregnancy or were taking dietary supplements were excluded.
Ethical Considerations
The trial was approved by the Hermanos Ameijeiras Ethics Committee for Clinical Investigation in accordance with the World Medical Association Declaration of Helsinki and Good Clinical Practice [8].
Variables Measured
The variables evaluated in the study are summarized in Table 2.
|
Variables |
Cohort |
Study Days |
p value |
|||
|
Day 1 |
Day 28 |
Day 56 |
Day 84 |
|||
|
(Range of Quality Control Standard)1 |
Descriptive Statistics (mean ± SD) |
|||||
|
Primary Outcome |
||||||
|
HbA1c(≤ 6%) |
SS cohort |
7.2 ± 1.1 |
ND |
ND |
7.2 ± 1.1 |
0.262b |
|
Placebo |
7.3 ± 1.6 |
ND |
ND |
7.4 ± 1.9 |
0.387b |
|
|
Secondary Outcomes |
||||||
|
FBG (4.2 - 7 mmol/L) |
SS* cohort |
8.4 ± 2.7 |
8.7 ± 4.2 |
8.5 ± 3.4 |
8.4 ± 3.0 |
0.942a |
|
Placebo |
8.3 ± 2.9 |
8.9 ± 4.5 |
8.2 ± 4.4 |
9.4 ± 4.8 |
0.001a |
|
|
Post Prandial-2h (≤ 10 mmol/L) |
SS cohort |
11.8 ± 4.5 |
11.4 ± 4.1 |
11.2 ± 4.6 |
11.6 ± 4.0 |
0. 646a |
|
Placebo |
11.6 ± 4.2 |
11.4 ± 5.1 |
10.2 ± 3.7 |
12.4 ± 5.3 |
0.013a |
|
|
Cholesterol (3.6 – 5.2 mmol/L) |
SS cohort |
4.3 ± 0.8 |
ND |
ND |
4.4 ± 0.8 |
0.181b |
|
Placebo |
4.4 ± 0.6 |
ND |
ND |
5.1 ± 1.3 |
< 0.001b |
|
|
HDL-c (≥ 0.9 mmol/L) |
SS cohort |
1.2 ± 0.4 |
ND |
ND |
1.1 ± 0.3 |
0.008b |
|
Placebo |
1.2 ± 0.3 |
ND |
ND |
1.1 ± 0.3 |
0.416b |
|
|
LDL-c (2.6–3.35 mmol/L) |
SS cohort |
2.9 ± 0.8 |
ND |
ND |
3.2 ± 0.9 |
0.001b |
|
Placebo |
2.8 ± 0.6 |
ND |
ND |
3.5 ± 1.1 |
< 0.001b |
|
|
Triglycerides (0.5–1.85 mmol/L) |
SS cohort |
2.1 ± 0.8 |
ND |
ND |
1.7 ± 0.6 |
0.005b |
|
Placebo |
2.4 ± 1.2 |
ND |
ND |
1.6 ± 1.4 |
0.002 b |
|
|
Insulin (2.6–24.9) mUI/mL |
SS cohort |
23.8 ± 22.0 |
ND |
ND |
19.8 ± 13.1 |
0.0496c |
|
Placebo |
26.4 ± 20.5 |
ND |
ND |
26.5 ± 19.6 |
0.4897c |
|
|
Serum LPS |
SS cohort |
0.45 ± 0.13 |
ND |
ND |
0.30 ± 0.04 |
0.0009c |
|
Placebo |
0.42 ± 0.16 |
ND |
ND |
0.36 ± 0.14 |
0.0681c |
|
|
HOMA-IR Index |
SS cohort |
8.85 ± 9.87 |
ND |
ND |
7.32 ± 5.36 |
0.0007a |
|
Placebo |
11.26 ± 11.48 |
ND |
ND |
12.34 ± 13.14 |
0.2472a |
|
|
Creatinine (65.4-119.3 µmol/L) |
SS cohort |
83.6 ± 21.50 |
ND |
ND |
77.17 ± 17.96 |
0.1745c |
|
Placebo |
77.17 ± 18.48 |
ND |
ND |
80.29 ± 15.51 |
0.2099c |
|
|
1Values in parenthesis represent thew Quality Standards used with the Immunochemical Autoanalyzer Cobas 6000. *SS = Sugar Shift treated cohort (n=30); Placebo cohort (n=27), ND = measurement was not done; a. Friedman Test, b. Wilcoxon signed rank test, c. t-Test: Paired Two Samples for Means |
||||||
Table 2: Base line and End-of-Study Outcomes of the Study.
Trial intervention
The trial intervention consisted of subjects taking 2 capsules daily, approximately 12 hours apart, of either the Sugar Shift (SS) test substance or a placebo control. Sample packages containing 56 capsules each were distributed every 28 days over the 12-week study period.
Sample Collection, Processing, and Data Management
Participants underwent sample collection, supplement delivery, and clinical evaluation every 28 days. Blood samples were collected by venipuncture, then properly identified with the inclusion number, processed, and aliquoted within one hour for storage and future use. Blood samples were collected by venipuncture and properly identified with the inclusion number, processed, and aliquoted within one h for storage and future use. All records were maintained in a dedicated database. Access to these records was limited to study and clinical staff responsible for patient care. The HHA was responsible for managing the security of the information technology infrastructure.
Adverse events (AE)
All participants in the study were administered either the test substance or a placebo to assess any impact on kidney function as measured by serum creatinine levels.
Clinical Determinations
Clinical determinations were performed using a Cobas 600 modular immunochemical autoanalyzer (Roche Diagnostics). The analysis was conducted in accordance with manufacturer’s recommendations.
LPS Determinations
The participating patients’ serum LPS levels were measured using the ToxinSensor™ Endotoxin Detection System (Version 11242021), with results expressed in Endotoxin Units (EU) in accordance with the manufacturer's instructions (www.genscript.com).
Statistical Analyses
The data collected were analyzed using Statistical Package for Social Sciences (SPSS) version 23.0 and R for Windows - R: The R Project for Statistical Computing, version 4.2.0. Descriptive statistics were used to characterize the samples. Qualitative variables were summarized in absolute numbers and percentages, while quantitative variables were summarized as mean and standard deviation for normally distributed data. The Kolmogorov-Smirnov test was used to test for normality. To compare differences between groups according to qualitative variables, the chi-square test (χ2) was used, while the Student’s t-test was used for age (a quantitative variable). The Friedman test was used to compare medians of variables that provided information on the effect of therapeutics between different timepoints (Day 1, 28, 56, and 84), with the Wilcoxon signed range test used for initial (Day 1) and final (Day 84) comparisons. The F-Test Two-Sample for Variances was used to compare variance between both groups for information on the effect of the probiotic at different timepoints. Additionally, analysis of covariance (ANCOVA) was employed to compare differences observed at the end of treatment between both groups. To detect differences in quantitative LPS and insulin determinations between and within the groups from Day 1 to Day 84, the study employed the paired sample t-test and Pearson's correlation. Additionally, an analysis was performed assuming unequal variance to detect differences between day 1 and day 84 with a significance level of α = 0.05.
Results
Participants, Demographic and Clinical Characteristics:
A total of 27 participants/cohort were required to achieve a power of 0.8. At the beginning of the study, the two randomized groups had comparable demographic and clinical features (Table 1). The study enrolled a total of 64 individuals, with 32 participants assigned to each group. Seven patients, two from the SS cohort and five from the Placebo cohort, withdrew from the study after the first visit due to personal or work-related reasons. Thus, the final analysis consisted of 57 participants, with 30 in the SS cohort and 27 in the Placebo cohort, as depicted in Figure 1 of the CONSORT 2010 flowchart [9,10]. Table 1 displays similar demographic characteristics of two cohorts. The SS cohort and Placebo cohort had a higher proportion of females, 60.0% and 51.9% respectively, with a mean age of 55years. The groups did not differ significantly in terms of sex (p = 0.725) or age (p = 0.120). The proportion of overweight or obese patients was slightly higher in both groups, with no significant difference (p = 0.722). Most patients in both groups were treated with a combination of diet and oral hypoglycemic agents, and there was no significant difference in treatment modality between the groups (p = 0.549).
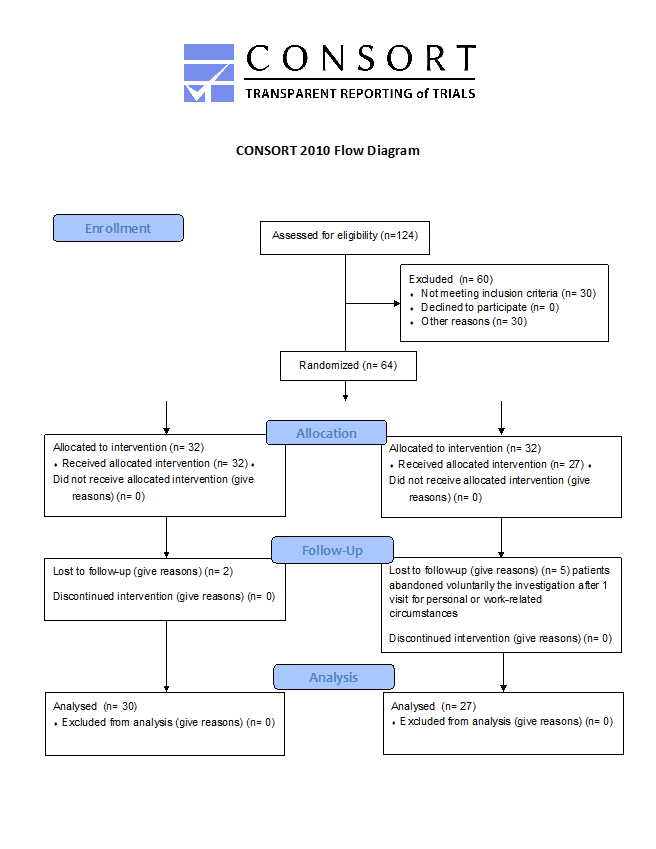
Figure 1: CONSORT Diagram of Recruitment and Retention Throughout the Study.
Safety of the Probiotic Product
During the study, participants in the SS cohort did not report any adverse events, suggesting the probiotic product was safe for use. One participant in the Placebo cohort, reported bloating during their second visit on Day 28.
Kidney Function
Creatinine levels were monitored to assess kidney function (Table 2). The mean creatinine levels were within the normal range and not significantly different (p = 0.240 and p = 0.210, respectively). It was concluded that the test substance did not have an adverse effect on kidney function.
Clinical Chemistry
Glycosylated Hemoglobin (HbA1c)
The results of all primary and secondary outcomes in this study are summarized in Table 2. No significant changes were observed in HbA1c levels in either the two cohorts. The average glycosylated hemoglobin was 7.2% in the two measurements made (Day 1 and Day 84) in the SS cohort and 7.3% in the Placebo cohort (Table 2).
Fasting Blood Glucose (FBG)
The results reported in Table 2 indicate that after three months, the SS cohort demonstrated a stabilization in their FBG levels, whereas the Placebo cohort did not show a similar stabilization in this parameter. (Figure 2a). The SS cohort had a mean FBG of 8.4 mmol/L at the first dose, which remained relatively stable at the second (Day 28) and third doses (Day 56), and ultimately resulted in a value of 8.4 mmol/L at the end of treatment. There were no significant differences between FBG levels at different time points (p = 0.942). In contrast, the Placebo cohort showed a different pattern, with a significant (p = 0.001) increase in median FBG value between the first and last day of treatment (8.3 mmol/L) vs. 9.4 mmol/L) (Table 2). After comparing FBG levels between the two groups at the end of treatment (day 56 and day 84) an analysis of variance was conducted. The results showed that the Placebo cohort had significantly higher variability in FBG levels (23.88) than the SS cohort (8.26) after 12 weeks of treatment (84 days), as presented in Table 2 and Figure 2a. These findings were further supported by the Analysis of Covariance (ANCOVA) results, which showed a negative covariance between the two groups (z= -6.85, p(z) = 7.62257E-12) at 84 days, indicating an increase in fasting glucose levels in the Placebo cohort and a decrease in the SS cohort.
Postprandial glucose – 2h (PPG)
Mean PPG levels observed in the SS cohort was 11.8 mmol/L at baseline, with a slight decrease (11.6 mmol/L) at the end of the study of the study. This decrease, however, was not significant (p = 0.646). In contrast, the Placebo cohort demonstrated a significant increase in PPG at the end of treatment, compared to the stable values of the SS cohort (p = 0.013) (Table 2). Although there were no significant differences between both groups at the end of the study (p = 0.62), a declining trend in postprandial glucose from day 1 to day 84 was observed (Figure 2b). ANCOVA analysis revealed a negative covariance between the Placebo cohort (26.817) and the Sugar Shift group (-0.0289), indicating that postprandial glucose increased in the Placebo cohort and decreased in the SS cohort at the end of 12 weeks. The kinetics of cohort variances throughout the study for both FBG and postprandial glucose are illustrated in Figure 2a and 2b.
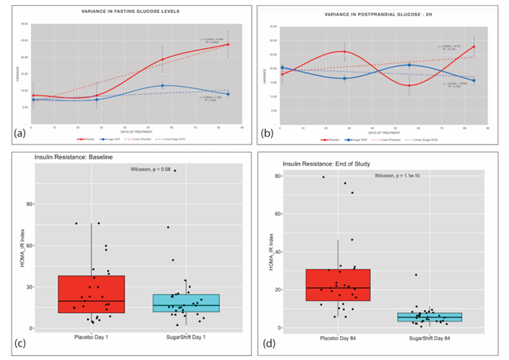
Figure 2: Dynamics of Serum Glucose and Insulin Resistance Measurements and Variances Throughout the 84-day Study. (a) Variance in Fasting Glucose of Placebo and Treatment (SS) groups showing trends over the 84-day study. (b) Variance in 2-hour Post Prandial glucose of Placebo and Treatment (SS) groups showing trends over the 84-day study. (c) Baseline boxplots with jitter showing distribution and medians in HOMA-IR indices with statistical significance (Wilcoxon) for Placebo and Treatment groups. (d) End of Study (84 days) boxplots with jitter showing distribution and medians in HOMA-IR indices with statistical significance (Wilcoxon) for Placebo and Treatment groups.
Cholesterol
Mean cholesterol levels in the SS cohort did not show significant differences (p = 0.181), but there was a significant (p = 0.0001) increase in mean cholesterol values in the Placebo cohort. The F-test Two-Sample for Variance analysis showed a significant increase (p = 0.0001) in variance from day 1 to day 84 for the Placebo cohort but not for the SS cohort (p = 0.391). Mean cholesterol values for the Placebo cohort increased from 4.41 mmol/L at day 1 to 5.14 mmol/L at day 84, while for the SS cohort, mean cholesterol values remained steady at 4.31 mmol/L at day1 and 4.42 mmol/L at day 84. The variances for the SS cohort were 0.645 and 0.584, respectively, and for the Placebo cohort, they were 0.311 and 1.644, respectively (Table 2).
HDL and LDL Cholesterol
The means of the HDL-c values of day 1 and day 84 remained practically unchanged for both groups. However, in the study group, the discrete variability was significant (p = 0.008).
Triglycerides
On average, triglyceride levels decreased from 2.1 to 1.7 mmol/L in the SS cohort and from 2.4 to 1.6 mmol/L in the Placebo cohort, and these results were significant in both cases (SS cohort: p = 0.005; Placebo cohort: p = 0.002). The analysis of variance between groups did not show significant differences (Table 2).
Insulin
Insulin levels were measured for all study participants and analyzed using both paired t-tests and F-tests for analysis of variance. The average level in SS group decreased from 23.8 mUI/mL to 19.8 mUI/mL. In the Placebo cohort however, the mean value did not change between Day 1 and Day 84 (26.4mUI/mL) (Table 2). Considering that the movement of insulin in the SS cohort was significant (p=0.049) (Table 2), an ANOVA analysis was conducted. After 84 days of treatment, participants in the Placebo cohort (Days 1 and 84) and SS cohort (Day 1) showed higher insulin concentrations compared to those in the SS cohort on Day 84. The paired t-tests revealed that the p-values between the Placebo cohort (Day 1 and Day 84) and Placebo cohort Day 1 and SS cohort Day 1 were 0.120 and 0.100, respectively. However, the p-value for the comparison between SS cohort Day 1 and Day 84 was significant (p = 0.024). The mean insulin level for SS cohort Day 1 participants was 9.01 μU/L with a variance of 92.24, whereas the SS cohort Day 84 group showed a mean of 7.09 μU/L and a variance of 29.05. The mean values for Placebo cohort Day 1 and Placebo cohort Day 84 were 11.27 μU/L and 12.34 μU/L, respectively, with corresponding variances of 131.44 and 170.74. Similarly, the F-test showed that the insulin serum levels for both groups were not significantly different on Day 1 (p = 0.192), whereas on Day 84, the insulin levels were significantly different between the control and SS participants (p = 0.000), with means of 12.5 μU/L for the Placebo cohort and 8.64 μU/L for the SS cohort. This represented a 30.9% reduction in insulin levels in the patients receiving the probiotic formulation.
Insulin Resistance
Insulin resistance was measured using the HOMA-IR index, an indicator of insulin sensitivity [11], with lower values indicating better insulin sensitivity. The study used the F-Test Two-Sample for Variance to compare the differences in insulin resistance between the two groups. The analysis found no significant difference in variance between the two groups at baseline. However, after 84 days of treatment, there was a significant reduction in variance in insulin resistance in the SS group compared to baseline. The results showed that the mean HOMA-IR index for the Placebo group increased from 11.26 at baseline to 12.34 on Day 84, while the mean HOMA-IR index for the SS group decreased from 8.85 at baseline to 7.32 on Day 84. Additionally, the variance in HOMA-IR index for the Placebo group increased from 131.74 at baseline to 172.74 on Day 84, while the variance in HOMA-IR index for the SS group decreased from 92.24 on Day 1 to 28.13 on Day 84. After 84 days of treatment, the SS group showed a significant improvement in both mean HOMA-IR index and variance in insulin resistance compared to the Placebo group. This is shown in Figure 2c and 2d, which illustrate the differences between the two groups.
Serum Lipopolysaccharides (LPS)
Serum LPS was measured for all participants in the two cohort groups (Table 2). There were observed significant differences in the levels of serum LPS between the Control and Treated groups after 84 days of treatment (p = 0.012). Similarly, there was a significant difference between day 1 and day 84 in the SS cohort (p=0.0009).
Discussion
Serum glucose (FBG and Postprandial glucose and HbA1c)
To analyze the effects of SS on glycemic control as evaluated by FBG, postprandial glucose, and HbA1c levels, it is important to consider that the median values indicate that both groups consisted of individuals with stable glucose and HbA1c parameters, likely due to the hypoglycemic treatments that most patients in both cohorts were taking before the study. Towards the latter part of the study period (days 56 and 84), however, it was observed that the FBG values of individuals in the Placebo cohort increased significantly (as shown in Fig 2). In contrast, glucose levels in the SS cohort remained relatively stable, with a tendency to decrease towards the final day of the study (day 84). These data showed a significant difference between the two study groups, with F-test data indicating a p-value of 0.006 when comparing the variance between the Placebo and the SS cohort at the end of the study Covariance studies [12] showed a negative covariance (-0.6086977), that is, are moving in opposite directions. These results indicate that while levels of FBG are increasing the Placebo cohort, they are decreasing in the SS cohort, and for the 84-day data, these values remained stable in the SS cohort. The control of postprandial glucose is crucial in the management of T2D and involves various factors such as the macronutrient composition of the meal, gastric emptying and intestinal glucose absorption, gastrointestinal hormones, insulin and glucagon secretion and action, de novo lipogenesis, and glucose disposal [13-15]. The vast variety of factors associate to postprandial glucose regulation could explain the behavior of this parameters and suggest evaluating some of them in a long period of time. Measuring HbA1c is an accepted and valued method for estimating long-term glycemic control [6]. In this study, HbA1c levels were found to be similar between the two groups. A meta-analysis conducted by Hu Y-Meng et al. [17] on the effects of different probiotic supplements in patients with T2D revealed that, regardless of the study design, duration of treatment (6-12 weeks), and doses of supplements, many studies showed a slight reduction in FBG and postprandial glucose, and a negligible change in HbA1c [17]. These results are consistent with the findings presented in this study. However, to assess the stabilization trend of these parameters over a longer period, studies evaluating probiotics for at least 6 months are needed [18-20]. Such studies have shown significant decreases in HbA1c, FBG, and PPG, although some have reported variations in the values of HbA1c and FBG [18,21], while others have shown no improvements [13]. The use of probiotics in the management of T2D has been associated with various mechanisms of action, including the promotion of a nonimmunologic gut defense barrier [22,23], normalization of increased intestinal permeability [24], and improved gut microecology [24]. Another potential mechanism of probiotic therapy is the improvement of the intestinal immunologic barrier, which could be achieved through intestinal immunoglobulin A responses and alleviation of intestinal inflammatory responses. These effects can contribute to a gut-stabilizing effect and ultimately help regulate hyperglycemia in T2D patients [22,25]. SS is a unique probiotic bacterial consortium that was specifically designed to endogenously convert glucose and fructose into mannitol within the gastrointestinal tract. No other probiotic consortium has been reported to have a similar mechanism of action. The primary producers of mannitol in the SS consortium are Leuconostoc mesenteroides and Lactobacillus reuteri, while the primary glucose consumer is Bifidobacterium spp. Furthermore, the SS consortium includes Pediococcus acidilactici, Lactobacillus paracasei, Bifidobacterium longum, and Lactobacillus reuteri, which are the primary butyrate producers, with a predicted net production of 4.5 x 105 mmol/h [7,26,27]. Overall, the use of probiotics, such as SS, may have a significant impact on the metabolism of sugars in the gut microbiome and ultimately help regulate hyperglycemia in T2D patients [26,28]. Further research is needed to understand the full extent of the mechanisms of action and long-term effects of probiotic therapy in T2D management.
Lipids
The results of the lipid profile analysis indicate that cholesterol levels remained stable within the normal range in the SS cohort, whereas a significant increase was observed in the Placebo cohort. However, further studies with a longer-term follow-up are necessary to fully assess the impact of SS on blood cholesterol levels. It is hypothesized that the probiotic bacteria, such as Bifidobacterium and Lactobacillus, may influence the regulatory mechanisms of cholesterol conversion into bile acids and their elimination in feces, which could potentially explain the observed differences between the two groups [29]. These probiotics can incorporate cholesterol into their plasma membrane, convert it into coprostanol and deconjugated bile acids via the activity of the enzyme bile salt hydrolase (BSH) [30,31]. With long-term colonization of the gut by these probiotics, the activity of BSH increases, which promotes a higher degree of BSH activity and thus, the production of deconjugated bile acids [29]. The decrease in cholesterol by this metabolic pathway could explain the slight increase in HDL-c in the SS cohort since excess cholesterol elimination would not occur through the main pathway. Both groups showed a significant decrease in triglyceride levels, which could be partly attributed to probiotic bacteria regulation linked to diet and increased physical activity [32]. The study revealed that the Placebo cohort had a higher level of physical activity compared to the SS cohort. This suggests that incorporating physical exercise into the probiotic intervention may enhance its regulatory mechanism.
Insulin resistant and LPS
Insulin resistance is a condition where the body tissues do not respond properly to insulin during glucose metabolism, and it has been linked to a range of factors, including genetic predisposition, aging, obesity, and a sedentary lifestyle [33,34]. Recent studies have also highlighted the potential role of the gut microbiota in the development of insulin resistance [35]. While the exact mechanisms by which gut microbial communities influence insulin secretion remain unclear, it’s thought that certain microbial components, such as muropeptides and LPS, may penetrate the gut barrier and interact with receptors within the pancreas or insulin-responsive tissues, ultimately leading to compromised endocrine control of metabolism and promoting insulin resistance in the liver and periphery [36]. LPS, in particular, has been shown to interact with Toll-like receptor 4 and promote inflammation in metabolic tissue, and this innate immune response could play a role in poor blood glucose control [36]. Probiotic supplementation, however, may offer a promising approach to improving insulin sensitivity by modifying the gut bacterial composition and reducing intestinal endotoxin concentrations, thereby reducing inflammatory signaling and decreasing insulin resistance [36]. By favorably altering the gut microbial community, probiotics could potentially improve blood glucose regulation and help prevent the development of insulin resistance.
Conclusions
The findings of this study suggest that BiotiQuest Sugar Shift, when taken twice daily for 12 weeks in combination with standard medical care, can improve biomarkers associated with T2D. These results indicate that BiotiQuest Sugar Shift may be a beneficial adjunct nutritional supplement for managing biomarkers associated with T2D, as well as potentially reducing inflammation in other metabolic syndrome-associated diseases. However, longer-term studies are needed to confirm these findings and to determine optimal dosages and treatment duration beyond the 90-day period studied. Additionally, an in-depth analysis of the gut microbiome’s diversity and composition and functional biomarkers is needed to better understand the role of the microbiome in T2D control.
Research in context
What is already known about this subject?
- Certain formulations of probiotics can improve glucose levels in individuals suffering from. Diabetes Mellitus type 2
- BiotiQuest™ Sugar Shift is a symbiotic formulation rationally designed for the conversion of monosaccharides and the restoration of human gut microbiota to produce anti-inflammatory metabolites.
What is the key question?
- Is BiotiQuest™ Sugar Shift able to stabilize blood glucose, improve insulin resistance, and reduce inflammation?
What are the new findings?
- This is the first 12-week double-blind, placebo-controlled study employing BiotiQuest™ Sugar Shift conducted in a population of Cuban subjects with Diabetes Mellitus Type 2.
- The probiotic formulation stabilizes fasting glucose response and reduces insulin resistance insulin as measured by the HOMA-IR index in T2D patients. However, HbA1c and postprandial glucose did not show significant differences compared to the Placebo cohort.
- The probiotic formulation reduced serum LPS, an inflammatory biomarker associated with T2D and present in other disease conditions as well.
- In order to detect significant reductions in all clinical parameters measured a six month period of study are needed.
How might this impact on clinical practice in the foreseeable future?
- The results improve our understanding in the control mechanism of Diabetes mellitus type 2 based in the reduction of inflammation by lowering LPS and gut microbiota modification.
Acknowledgments
We would like to express our heartfelt gratitude to Dr. Miguel H. Estévez del Toro, Director of the Hermanos Ameijeiras Hospital, and to Yurailis Reyes, Eng., for their invaluable support in initiating and conducting this study. Additionally, we extend our thanks to Edward Chan, MD, for his expert review of this manuscript, which has significantly improved its quality.
Trial registration
ISRCTN registry number ISRCTN48974890
RPCEC registry number RPCEC00000413
Funding
This work was supported by The BioCollective, LLC and Environmental Diagnostic Consultants, LLC.
References
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116 (2006): 1793-1801.
- Corrigendum to: 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. European heart journal 41(2020): 4317.
- Singer-Englar T, Barlow G, Mathur R. Obesity, diabetes, and the gut microbiome: an updated review. Expert Review of Gastroenterology & Hepatology 13 (2019): 3-15.
- Xourgia E, Papazafiropoulou A, Papanas N, et al. Anti-diabetic treatment leads to changes in gut microbiome. Frontiers in bioscience (Landmark edition) 24 (2019): 688-699.
- Colarusso AV, Goodchild-Michelman I, Rayle M, et al. Computational modeling of metabolism in microbial communities on a genome-scale. Current Opinion in Systems Biology 26 (2021): 46-57.
- Corb Aron RA, Abid A, Vesa CM, et al. Recognizing the Benefits of Pre-/Probiotics in Metabolic Syndrome and Type 2 Diabetes Mellitus Considering the Influence of Akkermansia muciniphila as a Key Gut Bacterium. Microorganisms 9 (2021):618.
- Carlin MR, Kazemi SK, Sangwan N, et al. inventorProbiotics and Methos of Use. USA patent WO2019/113023A9.
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 310 (2013): 2191-2194.
- Antes G. The new CONSORT statement 340 (2010):28-55.
- Cuschieri SJSjoa. The CONSORT statement 13 (2019): 27.
- Hanley AJ, Williams K, Stern MP, et al. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes care 25 (2002): 1177-1184.
- Rice JA. Mathematical statistics and data analysis: Cengage Learning (2006).
- Kocsis T, Molnár B, Németh D, et al. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Scientific Reports 10 (2020): 11787.
- Dimitriadis GD, Maratou E, Kountouri A, et al. Regulation of postabsorptive and postprandial glucose metabolism by insulin-dependent and insulin-independent mechanisms: an integrative approach. Nutrients 13 (2021): 159.
- Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutrition Research Reviews 31 (2018): 35-51.
- American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes care 44 (2021): S15-S33.
- Hu Y-Meng, Zhou F, Yuan Y, et al. Effects of probiotics supplement in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. Medicina Clínica (English Edition) 148 (2017): 362-370.
- Mohammad-Shahi M, Veissi M, Haidari F, et al. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 4 (2014): 83-88.
- Beltran del Rio M, Tiwari M, Amodu LI, et al. Glycated hemoglobin, plasma glucose, and erythrocyte aging. Journal of diabetes science and technology 10 (2016): 1303-1307.
- Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, et al. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes & metabolic syndrome 12 (2018): 617-624.
- Asemi Z, Zare Z, Shakeri H, et al. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Annals of nutrition metabolism 63 (2013): 1-9.
- Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, et al. Beneficial Effects of Probiotic Consumption on the Immune System. Annals of Nutrition and Metabolism 74 (2019): 115-124.
- Min Q, Wang Y, Jin T, et al. Analysis of Intestinal Short-Chain Fatty Acid Metabolism Profile After Probiotics and GLP-1 Treatment for Type 2 Diabetes Mellitus. Front Endocrinol (Lausanne). 13 (2002): 892127, https://www.frontiersin.org/articles/10.3389/fendo.2022.892127
- Sato J, Kanazawa A, Azuma K, et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Scientific Reports 7 (2017): 1-10.
- Gurung M, Li Z, You H, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51 (2020): 102590.
- Gérard C, Vidal H. Impact of Gut Microbiota on Host Glycemic Control. Front Endocrinol (Lausanne) 10 (2019): 29.
- Hansson C. The Link Between Diet, Gut Microbiota And Type 2Diabetes/Pre-diabetes In Humans: - A systematic review (2019). http://urn.kb.se/resolve?urn=urn:nbn:se:oru:diva-76217 DiVA database.
- Sikalidis AK, Maykish A. The gut microbiome and type 2 diabetes mellitus: discussing a complex relationship. Biomedicines 8 (2020): 8.
- Sivamaruthi BS, Fern LA, Rashidah Pg Hj Ismail DSN, et al. The influence of probiotics on bile acids in diseases and aging. Biomedicine & Pharmacotherapy 128 (2020): 110310.
- Salamone D, Rivellese AA, Vetrani C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: the possible role of dietary fibre. Acta Diabetologica 58 (2021): 1131-8.
- Wang C, Li S, Xue P, et al. The effect of probiotic supplementation on lipid profiles in adults with overweight or obesity: A meta-analysis of randomized controlled trials. Journal of Functional Foods 86 (2021): 104711.
- Chomani S. The effect of exercise training at different intensities on blood glucose regulation and cardiorespiratory fitness in patients with Type 2 Diabetes: a randomized controlled trial. VIREF Revista de Educación Física 10 (2021): 148-157.
- Yang B, Li M, Wang S, et al. Lactobacillus ruminis Alleviates DSS-Induced Colitis by Inflammatory Cytokines and Gut Microbiota Modulation. Foods 10 (2021): 1349.
- Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest 126 (2016): 12-22.
- Jang HR, Lee HY. Mechanisms linking gut microbial metabolites to insulin resistance. World journal of diabetes 12 (2021): 730-744.
- Schertzer JD, Lam TKJAJoP-E, Metabolism. Peripheral and central regulation of insulin by the intestine and microbiome. American Physiological Society Rockville, MD (2021): E234-E9.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 