Therapeutic Potential of “Smart” Exosomes in Peripheral Nerve Regeneration
Rajiv Supra1, Daniel R. Wilson2, Devendra K Agrawal2*
1College of Osteopathic Medicine, Touro University, Henderson, Nevada, New York
2Department of Translational Research, College of Osteopathic Medicine of the Pacific, Pomona, California, USA
*Corresponding author: Devendra K Agrawal, Department of Translational Research, College of Osteopathic Medicine of the Pacific, Pomona, California.
Received: 24 March 2023; Accepted: 27 April 2023; Published: 04 May 2023
Article Information
Citation: Rajiv Supra, Daniel R Wilson, Devendra K Agrawal. Therapeutic Potential of “Smart” Exosomes in Peripheral Nerve Regeneration. Journal of Biotechnology and Biomedicine 6 (2023): 189-196.
DOI: 10.26502/jbb.2642-91280082
View / Download Pdf Share at FacebookAbstract
Peripheral nerve injury results in severe loss of motor and sensory function in the affected limb. The gold standard for peripheral nerve repair is autologous nerve grafts, but their inherent drawbacks limit their use. Satisfactory clinical data are yet to be obtained using tissue engineered nerve grafts with neurotrophic factors introduced in these grafts for nerve repair. Therefore, peripheral nerve regeneration still remains a challenge for clinicians. Exosomes are secreted nanovesicles from the extracellular membrane. They are critical for communication within the cell and play a crucial role in the pathologic process of the peripheral nervous system. Recent research supports the role of exosomes in exhibiting neurotherapeutic effects through axonal growth, Schwann cell activation, and regulating inflammation. Indeed, the use of “smart” exosomes by reprogramming or manipulating the secretome contents and functions are rising as a therapeutic option for treating peripheral nerve defects. This review provides an overview on the promising role of exosomes in the process of peripheral nerve regeneration.
Keywords
<p>Exosomes; Inflammation; Microvesicles; Nerve Regeneration; Nerve repair; Neurotherapeutic effect; Schwann cell</p>
Article Details
Introduction
Peripheral nerve injury result in lifelong disability that reduces the quality of life of more than one million people worldwide [1,2]. The peripheral nervous system is able to regenerate to a certain degree after peripheral nerve injury. The preferred method of peripheral nerve repair is through surgery using nerve grafts. Autologous nerve graft is the gold standard therapy for peripheral nerve injury [4,5]. The clinical use of autologous nerve grafts, however, has many drawbacks such as mismatch of length, neuroma formation, and availability of donor nerves [6]. Additionally, less than 50% of patients that undergone peripheral nerve graft surgery achieved sensory and motor recovery [7]. Natural synthetics and biomaterials have also been developed as potential substitutes for autologous nerve grafting, however, these substitutes fail to achieve satisfactory clinical results [8]. Although major advances have been made in the field of nerve regeneration, peripheral nerve repair still remains a challenge and exploring novel factors becomes critical to further improve the therapeutic effects of peripheral nerve injury. Exosomes are nano-sized vesicles that are secreted into the extracellular environment by neurons, osteocytes, and mesenchymal stem cells (MSCs) [9]. Exosomes regulate intercellular signaling and communication by delivering nucleic acids, proteins, and lipids to the recipient cell [10]. Exosomes are ubiquitous in body fluids such as urine, amniotic fluid, blood, and saliva. Their ability to function as modulator of target cells makes it crucial for maintaining homeostasis in multicellular organisms [11,12]. Exosomes play a critical role in intercellular communication, maintaining homeostasis, and regeneration of the nervous system [13]. Axonal regrowth involves a highly intricate process involving the activation of Schwann Cell (SC) and regulation of inflammatory processes [14]. Recent research reveals exosomes participating in these regenerative processes while exerting neuroprotective effects [15]. Exosomes are under investigation as a promising therapy for treating peripheral nerve injury. In this review, we provide an overview of the current research about the function of exosomes as well as their emerging role in peripheral nerve repair.
Exosomes
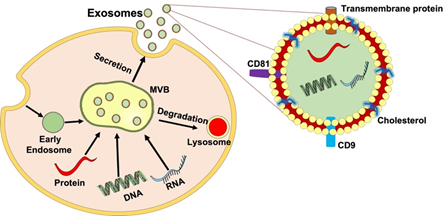
Extracellular Vesicles (EVs) are secreted by many cell types including MSCs, epithelial cells, immunocytes, and SCs [16]. EVs are membrane contained vesicles that can be divided into microvesicles (MVs) and exosomes [17]. Exosomes are created by inward budding inside endosomes, which result in the formation of multivesicular bodies (MVBs) that are able to fuse with the plasma membrane and released outside the vesicle, while MVs are budding vesicles that shed directly from the plasma membrane [18]. Additionally, exosomes can be differentiated from others by size. Exosomes are nanosized membrane vesicles that range from 40-150 nm in diameter, whereas MVs are roughly 100 to 1000 nm. The electron microscopic studies revealed the exosome morphology, which is described as “cup-shaped” [19]. Exosomes are endocytic vesicles derived from the membrane with a lipid structure consisting of phospholipids, cholesterol, ceramide, and saturated fatty-acyl chains [20,21]. The bilayer membrane enables protection and provides a controlled microenvironment allowing cargo to be moved across vast distances [22]. Secretion of these exosomes involves an intricate endocytic pathway. This begins with invagination from the plasma membrane into the cytoplasm. As the endosome buds inward to form intraluminal vesicles, they mature. At this stage in development, endosomes are called multivesicular bodies (MVBs). The MVBs then fuse with the cell membrane and released into the extracellular space. Some MVBs are transported to lysosomes where they are degraded [23]. The underlying mechanism of the biogenesis of MVB and intraluminal vesicle involves two unique molecular processes, namely the Endosomal Sorting Complex Needed for Transport (ESCRT) and the ESCRT-independent pathway [24]. As exosomes are released into the extracellular environment, they can interact with lipid ligand receptors and are subsequently internalized through fusion within the cell membrane (Figure 1) [23].
Furthermore, exosomes are abundant in a variety of specific proteins such as annexins, flotillin, and nucleotide guanosine triphosphatases (GTPases) that all play a critical role in membrane fusion and transport. Heat shock proteins (Hsp90 and Hsp70) also modulate intracellular trafficking. Tetraspanins (CD82, CD81, CD63, CD9) can regulate cell migration and signaling [25,26]. Exosomes also contain messenger RNA (mRNA), noncoding RNA (ncRNA), and micro-RNA (miRNA). These RNAs can be used and translated into recipient cells and can target genes in other cells [27]. Exosomes are emerging as a prominent form of cellular communication within the nervous system, mediating nerve remodeling, nerve protection, and synaptic plasticity [28]. Exosomes also have the potential as a therapeutic delivery system as they are able to pass the blood-brain barrier and deliver cargo to specific target cells [29]. Growing evidence also suggest the role of exosomes in promoting nerve regeneration in the peripheral nervous system [30]. The engineered exosomes, that are called “smart exosomes” by modifying the exosome-secreting cells or directly modifying isolated and/or purified vesicles, could effectively be used in the therapy following nerve injury [31]. This makes exosomal therapy a pivotal topic in the treatment of peripheral nerve injury.
Figure 1: The process and structure of exosome secretion. The inward budding of the plasma membrane forms an early endosome. As the endosome matures, intraluminal vesicles are formed, and subsequent invagination leads to the formation of multivesicular bodies (MVBs). MVBs fuse with lysosomes and plasma membranes to release intraluminal vesicles into the surrounding environment as exosomes facilitating cell-cell and cell-extracellular matrix communication via its cargo delivery of protein, lipids, mRNA, and miRNA enclosed within their bilipid layer and induce therapeutic effects in peripheral nerve regeneration.
Exosomes and Schwann Cells
Schwann cells (SCs) are the glial cells of the peripheral nervous system [32]. They provide structural and mechanical integrity through establishing myelin sheaths around axons while increasing signal transmission velocity [33]. Following peripheral nerve injury, SCs dedifferentiate into progenitor type cells that involve in repair and undergo a phenotypic transformation [34]. These cells along with macrophages terminate myelin debris and create a microenvironment that allows axon repair to take place [35]. Additionally, cytokines, neurotrophins, and growth factors are synthesized from SCs to increase the survival of neurons [36]. As newly created axons come into contact, SCs undergo redifferentiation and accomplish nerve repair [37]. Recent research reveals exosomes can promote peripheral nerve repair through upregulating SCs. One study showed adipose MSC-derived exosomes are able to enhance the myelination, migration, and proliferation of SCs after they were internalized by SCs. This was accomplished by increasing the expression of the corresponding genes in vitro. A similar in vivo experiment revealed that the group treated with exosomes optimized functionality of SCs and led to increased rates of remyelination, axonal regrowth, and muscle restoration as opposed to the control group [38]. Additionally, it has been studied that adipose MSC-derived exosomes can decrease the rate of autophagy of SCs through downregulating karyopherin subunit a2 (Kpna2) expression via miRNA-26b. This ultimately has shown to improve the repair process of peripheral nerve injury [39]. MSC-derived exosomes can also improve proliferation of SCs through upregulation of anti-apoptotic Bcl-2 mRNA levels while downregulating the pro-apoptotic Bax mRNA levels. This subsequently provides a neuroprotective function following peripheral nerve injury [40]. Furthermore, adipose MSC-derived EVs has a promotional effect on SCs and can improve SC proliferation in a dose-dependent manner. This was done via adipose MSC-derived EVs entering SCs through endocytosis as opposed to binding or fusing with the plasma membrane of SCs. The miRNAs contained within the EVs impact gene expression in SCs in response to nerve damage [41]. Additionally, adipose MSC-derived exosomes were also noted to demonstrate SC proliferation and remyelination in 10 mm nerve defects in peripheral nerve injury murine models [42]. Collectively, these studies demonstrate exosomes exerting a neuroprotective effect by influencing SCs and can be a potential therapy for peripheral nerve injury.
Exosomes and Axonal Regeneration
Axonal regrowth has been a subject of study as it is essential for functional recovery in damaged nerves. Axons must extend across damaged nerve sites and reconnect with distal nerves. Growth cones are distal tip expansions of regenerated axons that are able to guide and sense growth [43]. Filopodia are membranous protrusions that are extended by growth cones and lamellipodia interact with the surrounding environment [44]. The growth cone is able to sense the surrounding milieu through specialized structures like actin, neurofilament cytoskeleton proteins, and microtubules, all of which mediate axonal growth [45]. During peripheral nerve repair, adhesion molecules are expressed and can influence SC migration [46]. During earlier stages of this process, axons act as a guide for SC migration in the proximal nerves [47]. Axons are critical for SC repair and studies reveal when SCs lack axonal contact for long periods of time, their regenerative capacity diminishes [48]. Exosomes derived from SCs can modulate the regeneration process of axons. It has been established that exosomes from dedifferentiated SCs were internalized by axons which facilitated the survival of dorsal root ganglion neurons in vitro. Furthermore, regeneration of sciatic nerves has been researched and their ability to regenerate was further enhanced using SC derived exosomes. These exosomes contributed to a pro-regenerating phenotype of growth cones that inhibited the function of GTPase RhoA, a protein involved in growth cone collapse [49, 50]. It was also shown that the shift of SCs into a repair phenotype was accomplished by modifying the miRNA cargo of exosomes. Increased levels of miRNA-21 exhibited regenerative characteristics and are a key factor in repair SC exosomes. This regenerative capability was accomplished by downregulating PTEN and PI3-kinase activation in the nervous system [51]. Other than SC-derived exosomes, exosomes from MSCs have also shown promising data to regenerate nerves. For example, adipose MSC-derived exosomes increased rates of neuron outgrowth of dorsal root ganglion cells in vitro and enhanced repair of sciatic nerve injury [52]. Moreover, it has been studied that bone marrow MSC-derived exosomes increased axonal length and neurite growth through miRNA-mediated regulation of repair genes [53]. With these studies and their different results, it becomes imperative to explore the appropriate dosage of exosomes to achieve optimal repair in nerves. Another study revealed that fibroblast-derived exosomes modulated neurite growth and elongation of murine retinal ganglion cells by promoting Wnt10b and activating mTOR [54]. Gingival MSC-derived exosomes have also shown to increase dorsal root ganglion axon outgrowth [55]. Overall, these studies demonstrate the crucial role exosomes play in axonal regrowth and regenerative signaling.
Exosomes and Regulating Inflammation
Nerve repair and regeneration involves a pathologic process of inflammation which influences the prognosis of peripheral nerve injury [56]. SCs secrete a variety of chemokines and inflammatory cytokines during Wallerian degeneration. Macrophages are then recruited to enhance the clearance of myelin debris while initiating an inflammatory cascade [57]. Macrophages play an imperative role in coordinating inflammation and the events involved for successful nerve regeneration [58]. Macrophages possess heterogenous phenotypes which include M1 activating cells and alternatively-activated M2 cells [59]. The M1 phenotype is able to secrete IL-1b, IL-6, TNF-a, and IFN-g which results in further nerve damage. The M2 phenotype, however, is involved in the inhibition of the inflammatory response by secreting cytokines like IL-10 and IL-4 [60]. Their release of various growth factors also allows M2 macrophages to have a neuroprotective effect [61, 62]. During the earlier stages of inflammation, macrophages initially present as the M1 phenotype and later transform into M2 which decreases inflammation [63]. The inflammatory cascade aids in peripheral nerve repair, however, excess inflammation can lead to insufficient regeneration (64). With an appropriate regulation of the inflammation that follows peripheral nerve injury, neuron apoptosis and axon demyelination can decrease [65]. Therefore, targeting the inflammatory response for peripheral nerve injury has become a therapeutic intervention that has been studied. Exosomes can modulate the immune reaction to peripheral nerve injury [66]. For example, MSC-derived exosomes have been shown to harness similar anti-inflammatory effects as parent cells, which provides a favorable environment for nerve regeneration [67]. One study demonstrated umbilical cord MSC-derived exosomes to promote healing of spinal cord injury through modulating the inflammatory response in the region of damage. Their findings illustrated that exosomes can influence macrophage polarization from M1 phenotype to M2 phenotype [68]. It has been shown that exosome therapy can also improve recovery by down regulating inflammatory cytokines like IFN-g, IL-6, and TNF-a. Furthermore, bone marrow MSC-derived exosomes demonstrated neuroprotective effects by decreasing inflammation in murine models with traumatic brain injury through influence macrophage polarization [69]. Additionally, these exosomes have also been shown to modulate neurovascular remodeling and increased recovery in the diabetic murine model with peripheral neuropathy [70]. In a study using a sciatic nerve injury murine model, umbilical cord MSC-derived EVs were researched on peripheral nerve regeneration. This study concluded that EVs can decrease levels of IL-6, IL-1b, and increase anti-inflammatory cytokines such as IL-10 at distal nerve stumps [71]. SC-derived exosomes have also shown promising data and played a crucial role in modulating the inflammatory phase of nerve regeneration through the presence of a-Crystallin B and galectin-1 [72]. Overall, research on exosomes in peripheral nerve repair has demonstrated that control of neuroinflammation can lead to increased recovery in peripheral nerve injury.
Exosomes and Vascular Regeneration in Peripheral Nerve Repair
The vascular network is imperative for nerve regeneration as it facilitates axonal growth during nerve repair [73]. Maintaining vascular integrity becomes integral following peripheral nerve injury and is another major target for therapy. MSC-derived exosomes have gained attention as paracrine promoters for angiogenesis and have been studied as a possible therapy option for peripheral nerve repair. Exosomes from pluripotent stem cell-derived mesenchymal stem cells influence angiogenesis by initiating the PI3K/AKT pathway in cells of endothelial origin [74]. Research has shown that miRNAs that are proangiogenic can be transported within endothelial cells through exosomes generated from MSCs and subsequently can improve vascularity post peripheral nerve injury [75]. Exosomes have also been shown to induce angiogenesis. A study involving intravenous infusion of MSC-derived exosomes exhibited improved neurite remodeling, angiogenesis, and overall recovery [76]. Similar results were shown in another study where MSC-derived exosomes promoted neurogenesis and angiogenesis in the murine models of peripheral nerve injury [77]. Moreover, bone marrow MSC generated exosomes have been shown to decrease ischemic brain injury by increasing revascularization of endothelial cells and decreased rates of neuron apoptosis through delivering miRNA-29b-3p. This might subsequently activate PTEN-mediated Akt pathway to carry out its protective and angiogenic promoting effects [78]. Endothelial progenitor cell generated exosomes have also reduced rates of neuron apoptosis and improved revascularization through the miR-126/PI3k pathway [79]. In summary, these studies portray exosomes derived from MSC origin as mediators of vascular endothelial cells which improve blood supply to peripheral nerves. Exosomes from MSC can provide new avenues of therapy for peripheral nerve injury repair through enhancing angiogenesis. However, further studies are needed to elucidate the full effects exosomes may have on angiogenesis in the setting of peripheral nerve injury.
Challenges using Exosomes
Recent research revealed exosomes being a top contender for promoting peripheral nerve regeneration after peripheral nerve injury. Exosome therapy is expected to be a feasible therapeutic option; however, challenges remain that need to be addressed. The methods of obtaining and purifying exosomes are a major setback that hampers the application of exosomes in a clinical setting. Several kits for isolating exosomes have been created to combat this setback which have been proven to be effective and reliable [80]. Additionally, the heterogeneity of the cargo exosomes poses a challenge due to the variety of functions that various proteins and RNA molecules possess. Thus, regulation of exosomes to the desired target is a challenging process. Administration routes have also been a point of concern for exosome therapy. The effectiveness of administration in a clinical setting needs to be further studied. Research has shown nerve regeneration increased when exosomes were integrated into tissue engineered nerve grafts [81]. Exosomes also have the potential to replicate and propagate transmissible diseases and its mass production should not be ignored to ensure the safety of patients. Much work has yet to be done to overcome the various limitations in the therapeutic application of exosomes in a clinical setting.
Conclusion
Exosomes have been widely studied as main modulators of tissue regeneration [82]. Exosomes exert their therapeutic effect by mediating intercellular communication. Their ability to transfer genetic material, proteins, and neurotrophic factors to axons allows restoration of homeostasis in the microenvironment of peripheral nerve injury. This allows axonal regrowth, hence promoting recovery after peripheral nerve injury. The regenerative effect of exosomes reinforces the paradigm that promoting nerve repair by MSCs is mediated by a paracrine pathway and provides new avenues for therapies [83]. The use of MSC exosomes can reduce the issues of stem cell transplant and in the future MSC exosome therapy represents a promising therapy for peripheral nerve injury repair. Although many studies show the use of MSC exosomes for peripheral nerve regeneration is effective, it would be best to reprogram or manipulate the secretome contents and functions by modifying the exosome-secreting cells or directly modifying the isolated and purified vesicles to develop the engineered exosomes for peripheral nerve regeneration. Obviously, further research is yet to be conducted to fully elucidate the potential of exosome therapy in a clinical setting.
Author Contributions
Concept and design: RS, DKA; Literature Search: RS, DKA; Critical review and interpretation of the findings: RS, DKA; Drafting the article: RS; Revising and editing the manuscript: RS, DRW, DKA; Final approval of the article: RS, DRW, DKA.
Funding
The research work of DKA is supported by the research grants R01 HL144125 and R01HL147662 from the National Heart, Lung, and Blood Institute, National Institutes of Health, USA. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable
Data Availability Statement
Not applicable since the information is gathered from published articles.
Acknowledgments
None
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carvalho CR, Wrobel S, Meyer C, et al. Gellan Gum-based luminal fillers for peripheral nerve regeneration: an in vivo study in the rat sciatic nerve repair model. Biomater Sci 6 (2018): 1059-1075.
- Carvalho CR, Oliveira JM, Reis RL. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front Bioeng Biotechnol 7 (2019).
- Nocera G, Jacob C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cellular and Molecular Life Sciences 77 (2020): 3977-3989.
- Jia Y, Yang W, Zhang K, et al. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater 83 (2019): 291-301.
- Philips C, Cornelissen M, Carriel V. Evaluation methods as quality control in the generation of decellularized peripheral nerve allografts. J Neural Eng 15 (2018): 021003.
- Lin T, Liu S, Chen S, et al. Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects. Acta Biomater 73 (2018): 326-338.
- Chiono V, Tonda-Turo C. Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog Neurobiol 131 (2015): 87-104.
- Yu T, Wen L, He J, et al. Fabrication and evaluation of an optimized acellular nerve allograft with multiple axial channels. Acta Biomater 115 (2020): 235-249.
- Hill AF. Extracellular Vesicles and Neurodegenerative Diseases. The Journal of Neuroscience 39 (2019): 9269-9273.
- Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles 3 (2014): 24722.
- Kawikova I, Askenase PW. Diagnostic and therapeutic potentials of exosomes in CNS diseases. Brain Res 1617 (2015): 63-71.
- Ariston Gabriel AN, Wang F, Jiao Q, et al. The involvement of exosomes in the diagnosis and treatment of pancreatic cancer. Mol Cancer 19 (2020): 132.
- Tang BL. Promoting axonal regeneration through exosomes: An update of recent findings on exosomal PTEN and mTOR modifiers. Brain Res Bull 143 (2018): 123-131.
- Wieringa PA, Gonçalves de Pinho AR, Micera S, et al. Biomimetic Architectures for Peripheral Nerve Repair: A Review of Biofabrication Strategies. Adv Healthc Mater 7 (2018): 1701164.
- Pankajakshan D, Agrawal DK. Mesenchymal Stem Cell Paracrine Factors in Vascular Repair and Regeneration. J Biomed Technol Res 1 (2014).
- Thankam FG, Agrawal DK. Infarct Zone: a Novel Platform for Exosome Trade in Cardiac Tissue Regeneration. J Cardiovasc Transl Res 13 (2020): 686-701.
- Akers JC, Gonda D, Kim R, et al. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 113 (2013): 1–11.
- Thankam FG, Huynh J, Fang W, et al. Exosomal-ribosomal proteins-driven heterogeneity of epicardial adipose tissue derived stem cells under ischemia for cardiac regeneration. J Tissue Eng Regen Med 16 (2022): 396-408.
- Sheller S, Papaconstantinou J, Urrabaz-Garza R, et al. Amnion-Epithelial-Cell-Derived Exosomes Demonstrate Physiologic State of Cell under Oxidative Stress. PLoS One 11 (2016): e0157614.
- Littig JPB, Moellmer R, Agrawal DK, et al. Future applications of exosomes delivering resolvins and cytokines in facilitating diabetic foot ulcer healing. World J Diabetes 14 (2023): 35-47.
- Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol 29 (2014): 116-125.
- Thankam FG, Agrawal DK. Hypoxia-driven secretion of extracellular matrix proteins in the exosomes reflects the asymptomatic pathology of rotator cuff tendinopathies. Can J Physiol Pharmacol 99 (2021): 224-230.
- Colombo M, Raposo G, Théry C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu Rev Cell Dev Biol 30 (2014): 255-289.
- Thankam FG, Chandra I, Diaz C, et al. Matrix regeneration proteins in the hypoxia-triggered exosomes of shoulder tenocytes and adipose-derived mesenchymal stem cells. Mol Cell Biochem 465 (2020): 75-87.
- Vlassov A, Magdaleno S, Setterquist R, et al. Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et Biophysica Acta (BBA) - General Subjects 1820 (2012): 940-948.
- Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol 428 (2016): 688-692.
- Connor DE, Paulus JA, Dabestani PJ, et al. Therapeutic potential of exosomes in rotator cuff tendon healing. J Bone Miner Metab 37 (2019): 759-767.
- Zappulli V, Friis KP, Fitzpatrick Z, et al. Extracellular vesicles and intercellular communication within the nervous system. Journal of Clinical Investigation 126 (2016): 1198-1207.
- Yang J, Wu S, Hou L, et al. Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol Ther Nucleic Acids 21 (2020): 512-522.
- Zhang G, Yang P. A novel cell-cell communication mechanism in the nervous system: exosomes. J Neurosci Res 96 (2018): 45-52.
- Fang WH, Agrawal DK, Thankam FG. “Smart Exosomes”: A Smart Approach for Tendon Regeneration. Tissue Eng Part B Rev 28 (2022): 613-625.
- Xia W, Zhu J, Wang X, et al. ANXA1 directs Schwann cells proliferation and migration to accelerate nerve regeneration through the FPR2/AMPK pathway. The FASEB Journal 34 (2020): 13993-14005.
- Xia B, Gao J, Li S, et al. Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle-mediated transfer of microRNA 23b-3p. Theranostics 10 (2020): 8974-8995.
- Chen B, Hu R, Min Q, et al. FGF5 Regulates Schwann Cell Migration and Adhesion. Front Cell Neurosci 4 (2020): 14.
- Li R, Li D, Wu C, et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics 10 (2020): 1649-1677.
- Lotfi L, Khakbiz M, Moosazadeh Moghaddam M, et al. A biomaterials approach to Schwann cell development in neural tissue engineering. J Biomed Mater Res A 107 (2019): 2425–2446.
- Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol 594 (2016): 3521-3531.
- Chen J, Ren S, Duscher D, et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J Cell Physiol 234 (2019): 23097-23110.
- Yin G, Yu B, Liu C, et al. Exosomes produced by adipose-derived stem cells inhibit schwann cells autophagy and promote the regeneration of the myelin sheath. Int J Biochem Cell Biol 132 (2021): 105921.
- Liu C, Yin G, Sun Y, et al. Effect of exosomes from adipose-derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neurosci Ther 26 (2020): 189-196.
- Haertinger M, Weiss T, Mann A, et al. Adipose Stem Cell-Derived Extracellular Vesicles Induce Proliferation of Schwann Cells via Internalization. Cells 9 (2020): 163.
- Rao F, Zhang D, Fang T, et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int 2019 (2019):1-12.
- Santos TE, Schaffran B, Broguière N, et al. Axon Growth of CNS Neurons in Three Dimensions Is Amoeboid and Independent of Adhesions. Cell Rep 32 (2020): 107907.
- Sarker M, Naghieh S, McInnes AD, et al. Strategic Design and Fabrication of Nerve Guidance Conduits for Peripheral Nerve Regeneration. Biotechnol J 13 (2018): 1700635.
- Rodemer W, Gallo G, Selzer ME. Mechanisms of Axon Elongation Following CNS Injury: What Is Happening at the Axon Tip? Front Cell Neurosci 14 (2020).
- Yamauchi J, Miyamoto Y, Chan JR, et al. ErbB2 directly activates the exchange factor Dock7 to promote Schwann cell migration. Journal of Cell Biology 181 (2008): 351-365.
- Min Q, Parkinson DB, Dun X. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 69 (2021): 235-254.
- Höke A, Brushart T. Introduction to special issue: Challenges and opportunities for regeneration in the peripheral nervous system. Exp Neurol 223(2010): 1-4.
- Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 61 (2013): 1795-806.
- López-Leal R, Díaz-Viraqué F, Catalan RJ, et al. Schwann cell reprogramming into repair cells increases exosome-loaded miRNA-21 promoting axonal growth. J Cell Sci (2020).
- López-Leal R, Díaz-Viraqué F, Catalan RJ, et al. Schwann cell reprogramming into repair cells increases exosome-loaded miRNA-21 promoting axonal growth. J Cell Sci (2020).
- Bucan V, Vaslaitis D, Peck CT, et al. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol Neurobiol 56 (2019): 1812-1824.
- Zhao J, Ding Y, He R, et al. Dose-effect relationship and molecular mechanism by which BMSC-derived exosomes promote peripheral nerve regeneration after crush injury. Stem Cell Res Ther 11 (2020): 360.
- Tassew NG, Charish J, Shabanzadeh AP, et al. Exosomes Mediate Mobilization of Autocrine Wnt10b to Promote Axonal Regeneration in the Injured CNS. Cell Rep 20 (2017): 99-111.
- Rao F, Zhang D, Fang T, et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int 2019 (2019): 1-12.
- Huang S, Ge X, Yu J, et al. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. The FASEB Journal 32 (2018): 512-528.
- Matsui H, Sopko NA, Hannan JL, et al. M1 Macrophages Are Predominantly Recruited to the Major Pelvic Ganglion of the Rat Following Cavernous Nerve Injury. J Sex Med 14 (2017): 187-195.
- Tomlinson JE, Zygelyte E, Grenier JK, et al. Temporal changes in macrophage phenotype after peripheral nerve injury. J Neuroinflammation 15 (2018): 185.
- lo Sicco C, Reverberi D, Balbi C, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl Med 6 (2017): 1018-1028.
- Sun G, Li G, Li D, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Materials Science and Engineering: C 89 (2018): 194-204.
- Cattin AL, Burden JJ, Van Emmenis L, et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 162 (2015): 1127-1139.
- Jia Y, Yang W, Zhang K, et al. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater 83 (2019): 291-301.
- Ma Z, Wang Y, Li H. Applications of extracellular vesicles in tissue regeneration. Biomicrofluidics 14 (2020): 011501.
- Rotshenker S. Wallerian degeneration. the innate-immune response to traumatic nerve injury. J Neuroinflammation 8 (2011): 109.
- Chen T, Li Y, Ni W, et al. Human Neural Stem Cell-Conditioned Medium Inhibits Inflammation in Macrophages Via Sirt-1 Signaling Pathway In Vitro and Promotes Sciatic Nerve Injury Recovery in Rats. Stem Cells Dev 29 (2020): 1084-1095.
- Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation 11 (2014): 68.
- Liu W, Wang Y, Gong F, et al. Exosomes Derived from Bone Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Suppressing the Activation of A1 Neurotoxic Reactive Astrocytes. J Neurotrauma 36 (2019): 469-484.
- Sun G, Li G, Li D, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Materials Science and Engineering: C 89 (2018): 194-204.
- Ni H, Yang S, Siaw-Debrah F, et al. Exosomes Derived From Bone Mesenchymal Stem Cells Ameliorate Early Inflammatory Responses Following Traumatic Brain Injury. Front Neurosci 13 (2019).
- Fan B, Li C, Szalad A, et al. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 63 (2020): 431-43.
- Ma Y, Dong L, Zhou D, et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J Cell Mol Med 23 (2019): 2822-2835.
- Wei Z, Fan B, Ding H, et al. Proteomics analysis of Schwann cell-derived exosomes: a novel therapeutic strategy for central nervous system injury. Mol Cell Biochem 457 (2019): 51-59.
- Wang H, Zhu H, Guo Q, et al. Overlapping Mechanisms of Peripheral Nerve Regeneration and Angiogenesis Following Sciatic Nerve Transection. Front Cell Neurosci 11 (2017).
- Liu X, Li Q, Niu X, et al. Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis. Int J Biol Sci 13 (2017): 232-244.
- Gong M, Yu B, Wang J, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 8 (2017): 45200-45212.
- Xin H, Li Y, Cui Y, et al. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity After Stroke in Rats. Journal of Cerebral Blood Flow & Metabolism 33 (2013): 1711-1715.
- Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg 122 (2015): 856-867.
- Supra R, Agrawal DK. Peripheral Nerve Regeneration: Opportunities and Challenges. J Spine Res Surg 5 (2023): 10-18.
- Wang J, Liu H, Chen S, et al. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Exp Neurol 330 (2020): 113325.
- Zhang Y, Liu Y, Liu H, et al. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9 (2019): 19.
- Rao F, Zhang D, Fang T, et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int 2019 (2019): 1-12.
- Newton WC, Kim JW, Luo JZQ, et al. Stem cell-derived exosomes: a novel vector for tissue repair and diabetic therapy. J Mol Endocrinol 59 (2017): R155-165.
- Marote A, Teixeira FG, Mendes-Pinheiro B, et al. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front Pharmacol 7 (2016).


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 