Biomechanical Forces in the Tissue Engineering and Regeneration of Shoulder, Hip, Knee, and Ankle Joints
Merlin Rajesh Lal LP, Devendra K Agrawal*
Department of Translational Research, College of the Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, California USA
*Corresponding author: Devendra K Agrawal, Department of Translational Research, College of the Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, California USA.
Received: 25 September 2023; Accepted: 12 October 2023; Published: 19 October 2023
Article Information
Citation: Merlin Rajesh Lal LP, Devendra K Agrawal. Biomechanical Forces in the Tissue Engineering and Regeneration of Shoulder, Hip, Knee, and Ankle Joints. Journal of Biotechnology and Biomedicine 6 (2023): 491 - 500.
DOI: 10.26502/jbb.2642-91280111
View / Download Pdf Share at FacebookAbstract
Tear on the tendon, ligament and articular cartilage of the joints do not heal by itself and new modalities of treatment are required to address the need for full restoration of joint functions. Accompanied by degenerative diseases, the healing of these tissues does not occur naturally and hence requires surgical interventions, but with associated morbidity. Tissue engineering strategies are now focusing on the effective incorporation of biomechanical stimulation by the application of biomechanical forces relevant to the tissue of interest to regenerate and engineer functional tissues. Bioreactors are being continuously developed to accomplish this goal. Although bioreactors have been developed, the advancement in the field of biomaterial, basic science, and cell engineering warrant further refinement for their effective use. In this article we reviewed the application of biomechanical forces in the tissue engineering and regeneration of the joints such as rotator cuff of shoulder, ball and socket joint of the hip, articular cartilage of knee, and the ankle joints.
Keywords
<p>Ankle joint; Articular cartilage; Biomechanics of joints; Bioreactors; Hip joint; Knee joint; Shoulder joint; Tendon; Tissue engineering</p>
Article Details
Introduction
Ligament and tendon injuries account for 50% of musculoskeletal injuries with approximately 17 million ligamentous injuries that reported to have medical treatment and ~200,000 surgical procedures for cartilage defect are being performed in US annually [1,2]. Musculoskeletal injuries impart a huge economic burden significantly impacting the quality of life. Physiologically, the load generated by movement and inherent load of the body is counterbalanced by the force generated within the ligaments, tendons, muscles, and articular cartilage tissues. Due to overuse, tear or degeneration caused by diseases such as arthritis, the normal function of these tissues are impaired causing pain and dysfunction of the joint movements. The surgical restoration attempts are promising; however, often associated with morbidity resulting in repeated surgeries. Unfortunately, the joint instabilities, Achilles’ tendon tear, rotator cuff injuries and large cartilage defect are often irreparable warranting regenerative/tissue engineering strategies for regaining the biomechanical function [3-5]. Recently, tissue engineering strategies are focused on incorporating biomechanical stimuli in the development of functional tissues such as tendons, ligaments, and articular cartilage in vitro. Biomechanical stimulations on tissue samples and cells seeded on biomaterial constructs improved the mechanical properties [6]. Bioreactors are developed and used to apply biomechanical forces to the constructs, but to mimic the in vivo microenvironment has been a challenge. Hence, a deep understanding of the biomechanical forces in the tissues are required to develop treatment strategies, and to address the knowledge gap in tissue regeneration and pathological events associated with these load-bearing connective tissues.
Biomechanics of joints
Shoulder joint
The glenohumeral joint (GHJ) of the shoulder is stabilized by a group of tissues named rotator cuff [7]. The rotator cuff is composed of four muscles and their tendons: supraspinatus, infraspinatus, subscapularis, and teres minor, commonly referred as ‘SITS’. These muscles play a crucial role in stabilizing the shoulder joint (glenohumeral joint) and enabling its extensive range of motion [8]. The articulation surface is formed between the union of scapula and clavicle against the humerus. Rotator cuff muscles are responsible for holding up the tensile load and generating the tensile force to keep the GHJ intact. The glenoid cavity and its articulating surface of the humeral head holds up the compression force generated, and shear force generated during motion [9]. The glenohumeral joint reaction force generated by the articulating cartilage tissues balances the compression forces generated by the rotator cuff muscles and aid in the smooth gliding of the humeral head against the glenoid cavity resulting in shear load on the cartilage tissues. The transfer of the load across the joint during abduction and overhead elevation/lifting is shown in Figure 1. During abduction the acromion blocks the movement of the humerus at around 120o (Fig. 1B). The remaining range of motion (humoral elevation) is achieved by rotation of scapula in superior direction to another 60o (Fig. 1C), to make the 180o elevation. At this position the axis of the load is vertical, and the load is transferred to the body through the articular cartilage to the body. The tendons hold the joint complex intact throughout the movement by acting as force couple [10].
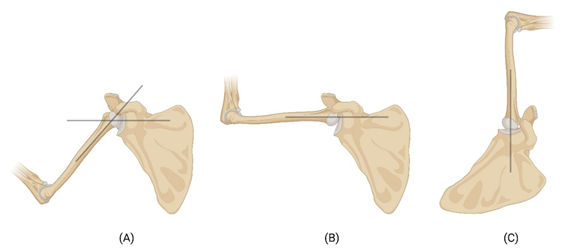
Figure 1: Schematic image showing the transfer of forces during the movement of shoulder joint. Black lines show the axis of load transfer. (A) Transfer of load during initial phase of abduction, (B) the acromion blocking the humerus at around 120o angle, and (C) the rotation of scapula to make the humoral elevation to 180o angle.
Hip joint
The acetabulofemoral joint or the ball-and-socket joint of the hip is stabilized by various ligaments, muscles, and tendons that perform as flexors, extensors, adductors, abductors, lateral rotators, and medial rotators [11]. The acetabulum has a smooth lining of articular cartilage called the lunate, and its crescent shaped incomplete ring bears most of the load that is transferred to the femur [12,13]. The articular cartilage of the hip joint experiences compressive forces that are directed vertically along the axis of the femur (Fig. 2A) and is stabilized by the ligaments and tendons of the joint. Shear forces occur parallel to the joint surfaces causing sliding movement between the articulating surfaces of the femur and acetabulum. The articular cartilage and the synovial fluid in the joint help to reduce shear forces and prevent excessive friction. A combination of shear and compression occurs during movements like rotation in the articular face, while the ligaments and tendons are under tensile load in the reciprocation of movements [14].
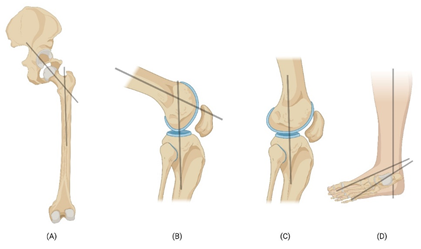
Figure 2: Schematic image showing the transfer of forces on the hip joint (A), Flexion of knee (B), Extension of knee (C), and ankle joint (D). Black lines show the axis of load transfer.
Knee joint
The knee joint is composed of two articulations namely the tibiofemoral joint and patellofemoral joint and is a hinge type synovial joint [15]. In humans it is the most stressed and largest articulating joint [6]. The network of ligaments and tendons of the knee contribute to the stability of the knee. The anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), medial collateral ligament (MCL), and lateral collateral ligament (LCL) give stability and integrity from excessive movement [16]. The medial collateral ligament has its origin at the medial epicondyle of the femur and inserts onto the medial aspect of the proximal tibia. On the other hand, the lateral collateral ligament originates from the lateral epicondyle of the femur and inserts onto the head of the fibula. The quadriceps tendon plays a crucial role in extending the knee joint and facilitating movements such as jumping and running. The hamstring tendon formed by the merging of the tendons of the hamstring muscles (semitendinosus, semimembranosus, and biceps femoris) imparts stability to knee joint. The transfer of load during flexion (Fig. 2B) and extension (Fig. 2C) occurs along the axis of tibia and fibula. The articular cartilage is constantly subjected to dynamic compression loading, hydrostatic force, and shear force. It holds the whole weight of the body during all physical activities and stabilized by the tensile force of the tendons and ligaments [6,16,17].
Ankle joint
The ankle joint complex includes the talocalcaneal (subtalar), tibiotalar (talocrural), and transverse-tarsal (talocalcaneonavicular) joints [18]. Achilles tendon is attached to the calcaneus and the interosseous talocalcaneal ligament, the lateral talocalcaneal ligament, anterior talocalcaneal ligament, calcaneofibular part of the lateral collateral ligament, tibiocalcaneal ligament of the deltoid provide stabilization to the talocalcaneal joint [19]. In normal walking the ankle joint holds up to five times the load of the body and it increases to thirteen times while running [20,21]. It was reported that approximately 83% of this load is transmitted through the tibiotalar joint. The representation of the axis of load transfer in ankle complex is shown in Figure 2D. The load bearing area of the ankle is 11-13 cm2 which results in lower stress level compared to the hip or knee joints [18].
Injury and treatment
Repetitive eccentric forces, acute or chronic tendinopathies, rotator cuff tear are the cause of pain and dysfunction can lead to secondary arthritis of the joint if left untreated. The rotator cuff tear may be partial or full thickness tears. Full thickness defect may be graded as grade-1 (less than 3 mm), grade-2 (3-6 mm) and grade-3 (more than 6 mm) and the treatment varies in accordance with the grade of tear [22]. The general treatment options include rest, usage of non-steroidal anti-inflammatory drugs (NSAIDs), cytotherapy, steroids, continuous passive motion, restrictive bracing, surgically suturing the tendons and finally tissue grafting (Fig 3A). The graft may be autograft, allograft (biological derived or artificial) or xenograft [23]. The surgical procedures have a high failure rate while allograft and xenograft may end up with graft rejection and warrants life-long immunotherapy. The earlier reports state that there is inflammation and alteration in the collagen composition of the tendons with rotator cuff tear [24-26]. The restoration by rotator cuff repair surgery has a high failure rate (2% - 94%) and morbidity [27-30].
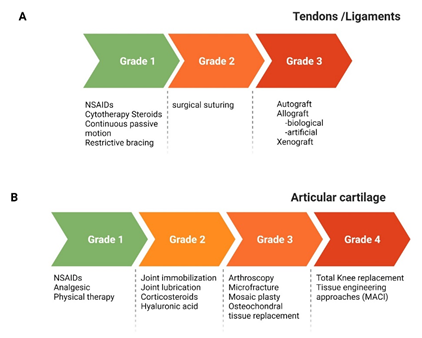
Figure 3: A schematic image representation of the grades of injury and current treatment/management approaches for load bearing tissues. MACI, matrix-induced autologous chondrocyte implantation; NSAIDs, non-steroidal anti-inflammatory drugs.
The articular cartilage full thickness defect larger than 6 mm do not heal by itself [31,32]. Depending on the grade of defect the use of NSAIDs to manage pain, joint immobilization, injection of hyaluronic acid to the joint to increase the joint lubrication are done. Arthroscopic procedures such as microfracture, mosaicplasty and osteochondral graft transplantation are being carried out (Fig 3B). But they come with the need of repeated surgery to remove/trim the formation of fibrous cartilage that grows in excess and the associated morbidity [33,34]. Autologous chondrocyte transplantation (ACI) is considered the gold standard treatment in the regeneration of articular cartilage [35]. This procedure has made immense development with the use of biomaterial of biological origin and established the matrix-induced autologous chondrocyte implantation (MACI). Tissue engineering is a viable option for non-treatable chronic disease conditions that combines the cells, biomaterials as scaffolds, biochemical factors, and biomechanical factors to generate functional tissues [5,36-38]. There is always a quest to identify the right cell source, a suitable biomaterial, and appropriate biochemical factors for the regeneration of tissues of the joints. These have been discussed in detail earlier for ligaments [23], articular cartilage [39,40], tendons and muscles.
Biomechanical forces and tissue engineering
The hierarchical organization, integrity and the ECM of the load bearing tissues such as tendons, ligaments and articular cartilage are responsive to mechanical loads [41,42]. In vivo models were used to study the response of these tissues to mechanical loading. The limited availability of animal models, variations between subjects, inability to achieve the precise mechanical loading and data collection were addressed by development of bioreactors [43]. In this regard the bioreactors are superior in having precise control over the use of biochemical factors and biomechanical stimulation of tissue engineered constructs [44-46]. Bioreactors may be used to study the (i) basic pathways, (ii) growing new tissues or (iii) priming the cells to mechanical stimulation before implantation [47]. A schematic representation of development of tissue engineered construct is shown in Figure 4. Few of the commonly employed bioreactors include the rotating wall bioreactor, a concentric cylinder bioreactor [48], perfusion bioreactor [49,50], and compression loading bioreactor [51-53] and tensile bioreactor system [54].
A list of bioreactors used for biomechanical stimulation is given in Table 1. Mechanical stimulation on cell seeded scaffolds increased the extracellular matrix (ECM) secretion and differentiation of stem cells [46,47,55-58]. For instance, the tensile forces applied to the porcine-derived acellular dermal matrix (PADM) increased the tenocyte markers [57].
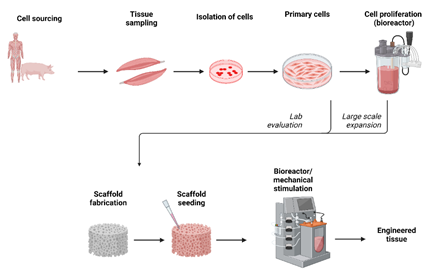
Figure 4: A schematic diagram showing the process of tissue engineering to develop functional tissue.
The application of mechanical forces needed in the engineering of tissues of the joints should account for the biomechanical forces in the in vivo microenvironment to generate optimal regeneration. Young’s modulus of human Achilles tendon was reported as 816 ± 218 MPa and ultimate stress of 71 ± 17 MPa [59], while for the Achilles tendon of rat it is 405 ± 115 MPa (Young’s Modulus) and 51.6 ± 10.8 MPa (UTS), respectively [60]. These values get affected to a very lower values in disease conditions. This may be highly associated with the infiltration of inflammatory cells, loss of collagen in the tendons, fatty infiltration in the tendons and muscles of the joints [61, 62]. The shape of cells and the force sensed at the ECM induce cell differentiation. The cellular response to compression loading is different from tensile stress. Human mesenchymal stem cells (hMSCs) were seeded on to alginate hydrogels and mechanical loads were applied. Genes associated with fibroblast and osteogenesis were upregulated on cell seeded constructs that received tensile loading, whereas the constructs that received dynamic compression had upregulated gene expression related to chondrogenesis [63]. The stress-strain curve of tendons exhibits a nonlinearity strain (0-2%) and then a linear strain (2-6%) before it enters a microfracture leading to macrofracture (8%) and ultimate rupture. The physiological strain level equates to 2-6% strain, however prolonged cyclic loading causes molecular breakdown leading to tear of tendon. Strain valued greater than 8% caused macrofracture [64]. Hence to closely mimic the in vivo microenvironment, these strain % are used in bioreactors for generating tissue engineered constructs [65,66]. The creep test on flexor digitorum longus tendon of rat with 35% of ultimate tensile strength (UTS) broke the sample in 15 minutes, whereas 5% of UTS on rat tail tendon broke the sample at 15 hours [67,68]. Application cyclic tensile loading (10% strain) on collagen scaffolds seeded with porcine tenocytes were able to align the cells towards the direction of strain but the cells did not survive for longer period in culture [69]. It should be noted that the strain applied in this study was higher than the physiological percent strain and is equivalent to the macrofracture of tendons. Table 1 provides additional studies on mechanical stimulation and bioreactor used for stimulation [70-73].
|
Tissue |
Biomaterial construct |
Mechanical stimulation |
Bioreactor |
Reference |
|
Cartilage |
Polycaprolactone-β- tricalcium phosphate (PCL-TCP) blended scaffolds seeded with rat MSC |
0.22% cyclic compressive strain at 1 Hz for 4 h per day + 5 rpm biaxial rotation at an angle of rotation at 90° |
Custom made |
[70] |
|
Cartilage |
PCL/fibrin scaffolds scaffold seeded with KUM5 (Riken Cell Bank) |
15% cyclic compressive strain at a frequency of 1 Hz + 90 minutes static load |
Custom made |
[71] |
|
Cartilage |
3D-printed PCL scaffolds seeded cells (hADSCs) |
Media perfusion at flow rate of 1 ml/min |
Perfusion bioreactors (BOSE, TA Instruments) |
[72] |
|
Ligament/tendon |
porcine-derived acellular dermal matrix (PADM) seeded with rat MSC |
5% cyclic strain at a frequency of 0.2 Hz for 1 h each followed by 5 h no load for up to 3 days |
Flexcell®FX-5000TM system (Flexcell International, Burlington, NC, USA) |
[57] |
|
Tendon |
polycaprolactone (PCL) nanofiber seeded with HADMSC: HT: HUVEC (2:2:1) |
4% elongation at a frequency of 0.5 Hz for 2 h per day for 12 days. |
MechanoCulture T6 Mechanical Stimulation System (CellScale biomaterials testing, Canada) |
[65] |
|
Tendon |
poly(L-lactide-co-e-caprolactone)/collagen scaffold seeded with Tendon-derived stem cells |
4% elongation in length at 0.5 Hz, 2 h per day for a total of 14 days |
Information not available |
[66] |
|
Tendon |
Collagen scaffold seeded with porcine tenocytes |
10% elongation in length at 0.5, 1.0 and 2.0 Hz, for a total of 14 days |
Custom made |
[69] |
|
Tendon |
Oligo (polyethylene glycol) fumarate) gel seeded with hBMSC |
10 % cyclic tensile strain for 3 h at 1 Hz, for 21 days |
Custom made |
[73] |
Table 1: List of mechanical stimulation and bioreactor used for stimulation.
Mechanical stimulation is sensed by Focal adhesion kinase (FAK), integrins and vinculin which in turn increases the intracellular calcium level. In a seminal study, the human tenocytes seeded on the unique uniaxial microgroove silicon membrane with cyclic stretching (4,8,12% strain at 1 Hz) at different time intervals (4,8,12 h) revealed maximum intracellular calcium at 12% strain than the 4% and 8% strain that increased with time [74]. Moreover, the stretch activated calcium channels have a positive effect in differentiation of human bone marrow mesenchymal stem cells to tenogenic lineage but at higher strain (8%) the viability of cells decreased beyond 72 hours [75]. Hydrostatic force and media perfusion (shear) have a positive effect in cartilage regeneration along with compression loading [76,77]. The native cartilage is subjected to repeated dynamic and static compression loading is degenerative to chondrocytes, but the ECM secretion is enhanced by the application of dynamic compression loading [78]. Canine chondrocytes were seeded on agarose hydrogels and dynamic compression loading (5% strain at 1 Hz) for 3 hours a day and 5 days a week. The constructs that received dynamic mechanical loading had significant increase in stiffness as measured by Young’s modulus, collagen-II and glycosaminoglycan (GAG) content [51]. The application of mechanical forces needed to engineer tissues of the joints should account for the biomechanical forces in the in vivo microenvironment to generate optimal regeneration. The results are encouraging but the bioreactor that could mimic the ideal microenvironment to produce load bearing tissue is yet to be designed. Hence the parameters such as loading time, frequency, interval of loading, and the strain percentage need to be carefully studied.
Outstanding questions and Future directions
There is a strong need to develop bioreactor for tissue engineering application due to significant challenge in mimicking the native microenvironment of the load bearing tissues like tendon, ligaments, and articular cartilage. Recent advancement in the field portrays the importance of biomechanical forces in regenerating tissues. But each tissue has a specific biomechanical regime, and the knowledge is largely incomplete. The biomechanical properties of tendons, ligaments or articular cartilage of each join have different values which add up to the complexity in designing the experimental studies further. To list the variables that need further consideration include: (i) force - tensile, compression, torsion, shear and hydrostatic force, (ii) nature of force - static, dynamic, intermittent, and alternating, (iii) frequency - has a wide physiological range, (iv) duration of culture, (v) duration of mechanical stimulation, and (vi) biochemical factors. One of the main limitations is the number of samples and its replicates that a bioreactor can hold, and the above variables can be accommodated in a single run. Multiple successful experiments need to be performed to study the differences in loading regimes and biochemical factors. Additional hurdles in performing vast experiments include capital and operational cost, and the requirement of large volume of cells. Lastly, in a dynamic environment the viability and attachment of cells for sufficiently long term to produce an appreciable functional tissue is a challenge. These may be further affected by mass transfer of oxygen, carbon dioxide, nutrients, and inappropriate mechanical stimulations/load. Hence, novel approaches need to be developed and create opportunities for advancing knowledge in developing tissues suitable for implantation.
Key Points:
- The joints are stabilized by load bearing tissues such as tendons, ligaments, and articular cartilage.
- Tear and degenerative diseases on the load bearing tissues do not heal by themselves.
- Conservative treatment procedures on higher grade of injuries fail and repeated surgeries are needed.
- Incorporation of biomechanical stimulation in tissue engineering strategies yields better functional tissues.
- Advances in bioreactor development and thorough understanding of the biomechanical forces of the in vivo microenvironment are necessary for development of functional tissues in vitro.
- Innovative approaches are needed to develop, understand, and mimic the response and survival of cells under mechanical load.
- Meticulous analysis of the role of biomechanical and biochemical factors needs further investigation to bridge the gap between the available knowledge and generation of new tissues in laboratory.
Author contribution:
MRL: literature search; design; critical review and interpretation of the published reports; preparation of figures and table; manuscript editing. DKA: conceptualization and design; manuscript preparation; manuscript editing; resources; funding.
Funding
The research work of DKA is supported by the R01 HL144125 and R01 HL147662 grants from the National Institutes of Health, USA. The content of this critical review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
Not applicable since the information is gathered from published articles.
Declarations
Competing interests
The authors declare no competing interests.
Competing interest
Both authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Consent for publication
Both authors have read the manuscript and consented for publication.
References
- Leong NL, Kator JL, Clemens TL, et al. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J Orthop Res 38 (2020): 7-12.
- Merkely G, Ackermann J, Lattermann C. Articular Cartilage Defects: Incidence, Diagnosis, and Natural History. Oper Tech Sports Med 26 (2018): 156-161.
- Wang L, Jiang J, Lin H, et al. Advances in Regenerative Sports Medicine Research. Front Bioeng Biotechnol 10 (2022): 908751.
- Peterson JR, Krabak BJ. Anterior Cruciate Ligament Injury. Phys Med Rehabil Clin N Am 25 (2014): 813-828.
- Langer R, Vacanti JP. Tissue Engineering. Science 260 (1993): 920-926.
- Logerstedt DS, Ebert JR, MacLeod TD, et al. Effects of and Response to Mechanical Loading on the Knee. Sports Medicine 52 (2022): 201-235.
- Huegel J, Williams AA, Soslowsky LJ. Rotator Cuff Biology and Biomechanics: a Review of Normal and Pathological Conditions. Curr Rheumatol Rep 17 (2015): 476.
- Akhtar A, Richards J, Monga P. The biomechanics of the rotator cuff in health and disease - A narrative review. J Clin Orthop Trauma 18 (2021): 150-156.
- Kozono N, Okada T, Takeuchi N, et al. Dynamic kinematics of the glenohumeral joint in shoulders with rotator cuff tears. J Orthop Surg Res 13 (2018): 9.
- Thompson WO, Debski RE, Boardman ND, et al. A Biomechanical Analysis of Rotator Cuff Deficiency in a Cadaveric Model. Am J Sports Med 24 (1996): 286-292.
- Neumann DA. Kinesiology of the Hip: A Focus on Muscular Actions. Journal of Orthopaedic & Sports Physical Therapy 40 (2010): 82-94.
- Dee R. Structure and function of hip joint innervation. Ann R Coll Surg Engl 45 (1969): 357-374.
- Mansfield PJ, Neumann DA. Structure and Function of the Hip. Essentials of Kinesiology for the Physical Therapist Assistant (2019): 233-277.
- Layton R, Messenger N, Stewart T. Characteristics of hip joint reaction forces during a range of activities. Med Eng Phys 108 (2022): 103894.
- Reinholz GG, Lu L, Saris DBF, et al. Animal models for cartilage reconstruction. Biomaterials 25 (2004): 1511-1521.
- Little CJ, Bawolin NK, Chen X. Mechanical Properties of Natural Cartilage and Tissue-Engineered Constructs. Tissue Eng Part B Rev 17 (2011): 213-227.
- Eschweiler J, Horn N, Rath B, et al. The Biomechanics of Cartilage-An Overview. Life 11 (2021): 302.
- Brockett CL, Chapman GJ. Biomechanics of the ankle. Orthop Trauma 30 (2016): 232-238.
- Procter P, Paul JP. Ankle joint biomechanics. J Biomech 15 (1982): 627-634.
- Michael JM, Golshani A, Gargac S, et al. Biomechanics of the ankle joint and clinical outcomes of total ankle replacement. J Mech Behav Biomed Mater 1 (2008): 276-294.
- Burdett RG. Forces predicted at the ankle during running. Med Sci Sports Exerc 14 (1982): 308-316.
- Fouda MB, Thankam FG, Dilisio MF, et al. Alterations in tendon microenvironment in response to mechanical load: potential molecular targets for treatment strategies. Am J Transl Res 9 (2017): 4341-4360.
- Lim WL, Liau LL, Ng MH, et al. Current Progress in Tendon and Ligament Tissue Engineering. Tissue Eng Regen Med 16 (2019): 549-571.
- Rai V, Mathews G, Agrawal DK. Translational and Clinical Significance of DAMPs, PAMPs, and PRRs in Trauma-induced Inflammation. Arch Clin Biomed Res 6 (2022): 673-685.
- Supra R, Agrawal DK. Innate Immune Response in Orthopedic Implant Failure. J Orthop Sports Med 5 (2023): 1-19.
- Thankam FG, Roesch ZK, Dilisio MF, et al. Association of Inflammatory Responses and ECM Disorganization with HMGB1 Upregulation and NLRP3 Inflammasome Activation in the Injured Rotator Cuff Tendon. Sci Rep 8 (2018): 8918.
- Abate M, Gravare-Silbernagel K, Siljeholm C, et al. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther 11 (2009): 235.
- Thankam FG, Dilisio MF, Dougherty KA, et al. Triggering receptor expressed on myeloid cells and 5’adenosine monophosphate-activated protein kinase in the inflammatory response: a potential therapeutic target. Expert Rev Clin Immunol 12 (2016): 1239-1249.
- Thankam FG, Boosani CS, Dilisio MF, et al. MicroRNAs Associated with Shoulder Tendon Matrisome Disorganization in Glenohumeral Arthritis. PLoS One 11 (2016): e0168077.
- Agrawal DK, Dougherty K, Dilisio M. Vitamin D and the immunomodulation of rotator cuff injury. J Inflamm Res 9 (2016): 123-131.
- Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am 75 (1993): 532-553
- Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am 76 (1994): 579-592
- Fritz J, Janssen P, Gaissmaier C, et al. Articular cartilage defects in the knee—Basics, therapies, and results. Injury 39 (2008): 50-57.
- Rai V, Radwan MM, Agrawal DK. IL-33, IL-37, and Vitamin D Interaction Mediate Immunomodulation of Inflammation in Degenerating Cartilage. Antibodies 10 (2021): 41.
- Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331 (1994): 889-895.
- Mollon B, Kandel R, Chahal J, et al. The clinical status of cartilage tissue regeneration in humans. Osteoarthritis Cartilage 21 (2013): 1824-1833.
- Thankam FG, Wilson VED, Agrawal DK. Animal models of inflammatory musculoskeletal diseases for tissue engineering and regenerative medicine: updates and translational application. Advances in Animal Experimentation and Modeling: Understanding Life Phenomena. Ed: RC Sobti 2022: 123-135.
- Connor DE, Paulus JA, Dabestani PJ, et al. Therapeutic potential of exosomes in rotator cuff tendon healing. J Bone Miner Metab 37 (2019): 759-767.
- Armiento AR, Stoddart MJ, Alini M, et al. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater 65 (2018): 1-20
- Kwon H, Paschos NK, Hu JC, et al. Articular cartilage tissue engineering: the role of signaling molecules. Cellular and Molecular Life Sciences 73 (2016): 1173-1194.
- Screen HRC. Hierarchical Approaches to Understanding Tendon Mechanics. Journal of Biomechanical Science and Engineering 4 (2009): 481-499.
- Woo SL-Y, Abramowitch SD, Kilger R, et al. Biomechanics of knee ligaments: injury, healing, and repair. J Biomech 39 (2006): 1-20.
- Dyment NA, Barrett JG, Awad HA, et al. A brief history of tendon and ligament bioreactors: Impact and future prospects. J Orthop Res 38 (2020): 2318-2330.
- Bian L, Zhai DY, Mauck RL, et al. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A 17 (2011): 1137-1146.
- Bian L, Zhai DY, Zhang EC, et al. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A 18 (2012): 715-724.
- Darling EM, Athanasiou K a. Articular Cartilage Bioreactors and Bioprocesses. Tissue Eng 9 (2003): 9-26.
- Youngstrom DW, Barrett JG. Engineering Tendon: Scaffolds, Bioreactors, and Models of Regeneration. Stem Cells Int 2016 (2016): 3919030.
- Saini S, Wick TM. Concentric Cylinder Bioreactor for Production of Tissue Engineered Cartilage: Effect of Seeding Density and Hydrodynamic Loading on Construct Development. Biotechnol Prog 19 (2003): 510-521.
- Zhao F, Ma T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: dynamic cell seeding and construct development. Biotechnol Bioeng 91 (2005): 482-493.
- Tigli RS, Cannizaro C, Gümüsderelioglu M, et al. Chondrogenesis in perfusion bioreactors using porous silk scaffolds and hESC-derived MSCs. J Biomed Mater Res A 96 (2011): 21-28.
- Bian L, Fong JV, Lima EG, et al. Dynamic Mechanical Loading Enhances Functional Properties of Tissue-Engineered Cartilage Using Mature Canine Chondrocytes. 16 (2010): 1781-1790.
- Mauck RL, Soltz M a, Wang CC, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122 (2000): 252-260.
- Spitters TWGM, Leijten JCH, Deus FD, et al. A dual flow bioreactor with controlled mechanical stimulation for cartilage tissue engineering. Tissue Eng Part C Methods 19 (2013): 774-783.
- Matsiko A, Levingstone TJ, Gleeson JP, et al. Incorporation of TGF-Beta 3 within Collagen-Hyaluronic Acid Scaffolds Improves their Chondrogenic Potential. Adv Healthc Mater 4 (2015): 1175-1179.
- Stevens MM, Marini RP, Schaefer D, et al. In vivo engineering of organs: The bone bioreactor. Proc Natl Acad Sci U S A 102 (2005): 11450-11455.
- Obregon R, Ramón-Azcon J, Ahadian S. Bioreactors in Tissue Engineering. Tissue Engineering for Artificial Organs (2016): 169-213.
- Delakowski AJ, Posselt JD, Wagner CT. Modular Bioreactor Design for Directed Tendon/Ligament Tissue Engineering. Bioengineering 9 (2022): 127.
- Mohindra R, Mohindra R, Agrawal DK, et al. Bioactive extracellular matrix fragments in tendon repair. Cell Tissue Res 390 (2022): 131-140.
- Wren TA, Yerby SA, Beaupré GS, et al. Mechanical properties of the human achilles tendon. Clin Biomech (Bristol, Avon) 16 (2001): 245-251.
- Benage LG, Sweeney JD, Giers MB, et al. Dynamic Load Model Systems of Tendon Inflammation and Mechanobiology. Front Bioeng Biotechnol 10 (2022): 896336.
- Fang WH, Bonavida V, Agrawal DK, et al. Hyperlipidemia in tendon injury: chronicles of low-density lipoproteins. Cell Tissue Res 392 (2023): 431-442.
- Singh D, Rai V, Agrawal DK. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol Cardiovasc Med 7 (2023): 7-16.
- Haudenschild AK, Hsieh AH, Kapila S, et al. Pressure and distortion regulate human mesenchymal stem cell gene expression. Ann Biomed Eng 37 (2009): 492-502.
- Wang T, Chen P, Zheng M, et al. In vitro loading models for tendon mechanobiology. Journal of Orthopaedic Research 36 (2017): 566-575.
- Wu S, Wang Y, Streubel PN, et al. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation. Acta Biomater 62 (2017): 102-115.
- Xu Y, Dong S, Zhou Q, et al. The effect of mechanical stimulation on the maturation of TDSCs-poly(L-lactide-co-e-caprolactone)/collagen scaffold constructs for tendon tissue engineering. Biomaterials 35 (2014): 2760-2772.
- Fung DT, Wang VM, Laudier DM, et al. Subrupture tendon fatigue damage. Journal of Orthopaedic Research 27 (2009): 264-273.
- Parent G, Huppé N, Langelier E. Low Stress Tendon Fatigue is a Relatively Rapid Process in the Context of Overuse Injuries. Ann Biomed Eng 39 (2011): 1535-1545.
- Raimondi MT, Laganà M, Conci C, et al. Development and biological validation of a cyclic stretch culture system for the ex vivo engineering of tendons. Int J Artif Organs 41 (2018): 400-412.
- Ravichandran A, Wen F, Lim J, et al. Biomimetic fetal rotation bioreactor for engineering bone tissues—Effect of cyclic strains on upregulation of osteogenic gene expression. J Tissue Eng Regen Med 12 (2018): e2039-e2050.
- Panadero JA, Sencadas V, Silva SCM, et al. Mechanical fatigue performance of PCL-chondroprogenitor constructs after cell culture under bioreactor mechanical stimulus. J Biomed Mater Res B Appl Biomater 104 (2016): 330-338.
- Theodoridis K, Aggelidou E, Manthou M, et al. Assessment of cartilage regeneration on 3D collagen-polycaprolactone scaffolds: Evaluation of growth media in static and in perfusion bioreactor dynamic culture. Colloids Surf B Biointerfaces 183 (2019): 110403.
- Doroski DM, Levenston ME, Temenoff JS. Cyclic Tensile Culture Promotes Fibroblastic Differentiation of Marrow Stromal Cells Encapsulated in Poly(Ethylene Glycol)-Based Hydrogels. Tissue Eng Part A 16 (2010): 3457-3466.
- Chen W, Deng Y, Zhang J, et al. Uniaxial repetitive mechanical overloading induces influx of extracellular calcium and cytoskeleton disruption in human tenocytes. Cell Tissue Res 359 (2015): 577-587.
- Nam HY, Balaji Raghavendran HR, Pingguan-Murphy B, et al. Fate of tenogenic differentiation potential of human bone marrow stromal cells by uniaxial stretching affected by stretch-activated calcium channel agonist gadolinium. PLoS One 12 (2017): e0178117.
- Shahmoradi SR, Kabir Salmani M, Soleimanpour HR, et al. Induction of Chondrogenic Differentiation in Human Mesenchymal Stem Cells Cultured on Human Demineralized Bone Matrix Scaffold under Hydrostatic Pressure. Tissue Eng Regen Med 16 (2019): 69-80.
- Sharifi N, Gharravi AM. Shear bioreactors stimulating chondrocyte regeneration, a systematic review. Inflamm Regen 39 (2019): 16.
- Chai DH, Arner EC, Griggs DW, et al. αv and β1 integrins regulate dynamic compression-induced proteoglycan synthesis in 3D gel culture by distinct complementary pathways. Osteoarthritis Cartilage 18 (2010): 249-256.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 