Packaging method does alter boneless-skinless chicken breast fillet characteristics
Savannah L Douglas1, Gabriela M Bernardez-Morales1, Ricardo J Barrazueta-Cordero1, Tristan M Reyes2, Jason T Sawyer1*
1Department of Animal Sciences, Auburn University, Auburn, AL 36849, USA
2Winpak Ltd., 100 Saulteaux Crescent, Winnipeg, MB R3J 3T3, Canada
Received: 27 June 2025; Accepted: 08 July 2025; Published: 23 July 2025
*Corresponding author:Jason T Sawyer, Department of Animal Sciences, Auburn University, Auburn, AL 36849, USA.
Article Information
Citation: Savannah L Douglas, Gabriela M Bernardez-Morales, Ricardo J Barrazueta-Cordero, Tristan M Reyes, Jason T Sawyer. Packaging method does alter boneless-skinless chicken breast fillet characteristics. Journal of Food Science and Nutrition Research. 8 (2025): 65-73.
DOI: 10.26502/jfsnr.2642-110000178
View / Download Pdf Share at FacebookAbstract
Vacuum packaging of fresh boneless-skinless chicken breast is not widely used throughout the United States for marketing fresh chicken at the retail level. Evaluating technology such as vacuum packaging could improve the storage of fresh chicken. Boneless-skinless chicken breast was purchased from a commercial processor and packaged in one of six packaging treatments to evaluate the effects on surface color, odor evaluation, pH, microbial growth, and lipid oxidation throughout a 28-day refrigerated storage. Objective surface color of chicken breasts became lighter (p < 0.0001), less red (p = 0.0001) and less yellow (p < 0.0001) with increased storage time. Additionally, no change was observed in pH during storage duration (p = 0.2831). A spoilage threshold of 7-log cfu/g was established at the beginning of the study, and neither lactic acid bacteria (p = 0.2798), anerobic plate count (p < 0.0001) nor aerobic plate counts (p < 0.0001) reached the project threshold of 7-log cfu/g. Lipid oxidation was greatest (p < 0.0001) on day 21 of the storage period. In addition, subjective odor values increased over storage time (p < 0.0001) reaching an unacceptable threshold after 14 days. Results indicate that vacuum packaging is a method that can be used for extending storage life and quality attributes of fresh chicken retail products.
Keywords
<p>Chicken breast fillet, Instrumental color, Lipid oxidation, Shelf-life, Vacuum packaging</p>
Article Details
Introduction
Improving the storage-life of poultry products is often overlooked but necessary to meet consumer demands. In the United States, poultry cuts sold in retail settings are marketed in expanded polystyrene trays and wrapped in low barrier plastic packaging films. Over the last 60 years, global poultry meat production surged from 9 to 122 million tons, with poultry meat production accounting for nearly 40% of the global meat production in 2020 [1]. A rise in poultry production may be attributed to the increasing popularity of the protein source. Poultry is often favored due to its lower price, nutritional value, cooking convenience and a high value of polyunsaturated fatty acids in comparison to other proteins [2]. Unfortunately, there is minimal research evaluating storage duration when using vacuum packaging.
Poultry cuts possess characteristics that provide favorable conditions for microbial growth [3]. Susceptibility to microbial growth poses a consumer health hazard as poultry can harbor many microorganisms [4]. Microbial growth can lead to undesirable changes in meat quality, resulting in a product that is unappealing and unsuitable for consumption, often causing the consumer to dispose of fresh poultry products before consumption occurs [5,6]. Discarding poultry products prior to consumption not only impacts consumer satisfaction but contributes to increased food waste, creating more environmental volumes of meat packaging materials throughout the landfill systems in the United States [7]. According to the National Chicken Council, discarding one percent of the chicken produced each year could lead to over 460 million pounds of chicken wasted annually [7]. Developing interventions such as alternative packaging could greatly reduce food waste as 43% of all waste occurs at the household level [8]. Identifying newer technologies such as packaging could extend the storage duration of poultry products while minimizing waste and decreasing food costs [9].
Economic consequences of food spoilage and food waste are substantial for consumers and the poultry industry. Frequent disposal of poultry products caused by spoilage or a lack of sales at the retail counter results in increased costs to the manufacturing industry and additional waste management [10]. Reducing food waste by improving packaging and preservation methods could result in significant savings for both manufacturers and consumers improving the overall market efficiency [10]. A recent study suggests that consumers are willing to pay a premium for products in packages designed to reduce food waste [8]. Thus, enhancing the shelf-life poultry products through better packaging addresses consumer health, environmental concerns, and economic benefits.
Meat and food packaging aids in protection and preservation of products during storage and distribution. Packaging of food products provides a barrier exposure to atmospheric gases such as oxygen, carbon dioxide, water, or other contaminants. Currently the majority of packaging for fresh meat involves polyvinyl chloride films for short-term storage, and vacuum packaging or modified atmosphere packaging for extended storage [11]. Vacuum packaging is less expensive and simpler than modified atmosphere packaging which is the advantage of using this packaging type [12]. Vacuum packaging is a method of packaging that has been used since the 1970s to package meat in bulk but the use of vacuum packaging in a retail sector has only recently become a more popular method used [13]. This packaging type provides a preservative effect as it creates an oxygen deficient environment. Without the presence of oxygen, microbial spoilage is inhibited causing an extension to the storage duration and wholesome quality of poultry products [13]. It has been reported that the shelf-life of broiler carcasses could be extended by 50% with the use of vacuum packaging [14]. Although this is known, most research regarding vacuum packaged poultry dates back over 40 years. Additionally, focusing on the effect vacuum packaging has on chicken breast has not been thoroughly studied. Thus, the objective of this study was to evaluate the effects vacuum packaging has on chicken breast quality characteristics and microbiological growth rate.
2. Materials and Methods
2.1. Raw materials
Fresh boneless skinless chicken breast fillets were purchased and transported from a commercial poultry processor (Wayne Farms, Union Springs, AL, USA) within 12 h after harvest to the Lambert-Powell Meat Laboratory (Auburn University, Auburn, AL, USA) on dry ice. Chicken breast fillets were stored in refrigeration (Model LEH0630, Larkin, Stone Mountain, GA, USA) at 2°C ± 1.5°C in the absence of light for 24 h until packaging activities were completed. Prior to packaging, breast fillets were randomly assigned to one of six packaging treatments.
2.2. Packaging and display conditions
Chicken breasts were assigned to one of six film combinations (Table 1). Packaging treatments (A, B, C) were packaged using a Multivac system (KGD-87787 R175, Sepp Haggenmüller GmbH & Co., Wolfertschwenden, Germany), and thermoforming packaging treatments (D, E, F) were packaged using a Variovac Optimus system (OL0924, Variovac, Zarrentin, Germany). Packaged chicken breast fillets were placed onto lighted shelves within a two-door, refrigerated, retail display case (Model 178GDC49HCB, Clark Associates Inc. DBA Avantco Equipment, Lancaster, PA, USA) under continuous LED lighting with an intensity of 2297 lux for each shelf. Samples were distributed evenly across the shelves and stored for 28 days at 2°C ± 1.5 °C. On days 0, 7, 14, 21, and 28 of the display period samples were evaluated for fresh meat characteristics of instrumental surface color, lipid oxidation, pH, and odor evaluation.
|
TRT1 |
Film Type |
Composition |
Thickness |
OTR2 |
VTR3 |
||
|
A |
Forming |
polyester/EVOH/polyethylene coextrusion |
300 μ |
1.8 mL / m/ 24h |
3.0 g/ m/ 24 h |
||
|
Non-Forming |
PE/EVOH/LLDPE |
102 μ |
0.80 mL / m/ 24h |
0.40 g/ m/ 24 h |
|||
|
B |
Forming |
nylon/EVOH/polyethylene coextrusion |
100 μ |
1.5 mL / m/ 24h |
4.5 g/ m/ 24 h |
||
|
Non-Forming |
PE/EVOH/LLDPE |
102 μ |
0.80 mL / m/ 24h |
0.40 g/ m/ 24 h |
|||
|
C |
Forming |
nylon / EVOH / enhanced polyethylene coextrusion |
175 μ |
0.4 mL / m/ 24h |
3.3 g/ sq. m/ 24 h |
||
|
Non-Forming |
nylon/EVOH/polyolefin plastomer coextrusion |
75 μ |
1.0 mL / m/ 24h |
4.0 g/ sq. m/ 24 h |
|||
|
D |
Forming |
nylon / EVOH / enhanced polyethylene coextrusion |
175 μ |
0.4 mL / m/ 24h |
3.3 g/ sq. m/ 24 h |
||
|
Non-Forming |
nylon / polyolefin lastomer coextrusion |
65 μ |
53.0 mL / m/ 24h |
6.5 g/ sq. m/ 24 h |
|||
|
E |
Forming |
polyolefin/nylon/enhanced polyethylene coextrusion |
150 μ |
31.0 mL / m/ 24h |
3.7 g/ sq. m/ 24 h |
||
|
Non-Forming |
nylon / polyolefin lastomer coextrusion |
65 μ |
53.0 mL / m/ 24h |
6.5 g/ sq. m/ 24 h |
|||
|
F |
Forming |
polyolefin/polyethylene |
150 μ |
1287 mL / m/ 24h |
3.5 g/ sq. m/ 24 h |
||
|
Non-Forming |
polypropylene/polyolefin plastomer coextrusion |
69 μ |
1400 mL / m/ 24h |
3.5 g/ sq. m/ 24 h |
1Packaging treatment defined as (A, B, C, D, E, F). 2 OTR: Oxygen transmission rates. 3VPR: Vapor transmission rates.
Table 1: Manufacturer specifications for packaging films used during storage of fresh boneless-skinless chicken: breast
2.3. Instrumental surface color
Surface color readings of each breast fillet were scanned using a HunterLab MiniScan EZ colorimeter (Model 45/0 LAV, Hunter Associates Laboratory Inc., Reston, WV, USA) through the packaging film with a 10° observer and 31.88 mm aperture. Prior to scanning, the colorimeter was calibrated using black and white tiles per the manufacturer’s guidelines. Surface color readings were determined from the mean of three readings on the surface of each fillet using illuminant D65 according to American Meat Science Association guidelines [15].
2.4. Odor evaluation and pH analysis
Packaged breast fillets were opened and within 5 seconds the odor was evaluated by trained laboratory personnel. Subjective odor evaluation scores of 1 to 5 were used based on the following: 1 = “fresh chicken, no off odor”; 2 = “slight off odor”; 3 = “small off odor”; 4 = “moderate off odor, unacceptable”; 5 = “extremely unacceptable, extreme off odor” using standards outlined by the American Meat Science Association guidelines [15]. Scores from the panel members were averaged for each breast fillet and the mean was used for statistical analysis. Postmortem pH of chicken breast was measured in duplicate by weighing 2g into a plastic centrifuge tube combined with 20 mL of deionized water, and homogenizing (Kinematica CH-6010, Brinkmann Instruments, Inc., Westbury, NY, USA) for 45 seconds. A pH meter equipped with a glass electrode was used to record pH values (Model-HI99163, Hanna Instruments, Woonsocket, RI, USA). Calibration of the pH meter was completed (pH 4.0 and pH 7.0) using 2-point standard buffers (Thermo Fisher Scientific, Chelmsford, MA, USA) prior to sampling.
2.5. Lipid oxidation
Packages of chicken breast were sampled for 2-thiobarbituric acid reactive substances (TBARS) using methods previously described [16]. Briefly, breast fillets were minced to provide a uniform sample of the entire fillet and 2g of minced meat was homogenized with 8mL of 50mM phosphate buffer containing 0.1% EDTA, 0.1% n-propyl gallate, and 2 mL trichloroacetic acid (Sigma-Aldrich, Saint Louis, MO, USA). Samples were homogenized for 45 seconds and filtered using Whatmann No. 1 filter paper. Duplicate 2-mL portions of the clear filtrate was transferred into 10-mL borosilicate tubes, mixed with 2 mL of 0.02 M 2-thiobarbituric acid reagent (Sigma-Aldrich, Saint Louis, MO, USA) and boiled for 20 min. After boiling, tubes were placed into an ice bath for 15 min to cool. Absorbance was measured at 533 nm using a spectrophotometer (Turner Model–SM110245, Barnstead International, Dubuque, IA, USA) and multiplied using a factor of 12.21 to obtain the TBARS value (mg malonaldehyde/kg of meat).
2.6. Microbiological analysis
Packaged samples were transported overnight to a contract laboratory for analysis (Food Safety Net Services, Tucker, GA, USA). Anaerobic plate count (ANA) was determined using the following method: Reagent and media preparation was conducted according to laboratory procedures. Samples were homogenized for 1 to 2 minutes with a stomacher (BagMixer 400W, Interscience, Saint Nom la Bretêche, France) on medium-high. Within 15 minutes of preparing the homogenate, 1mL of final dilution was placed on a sterile Petri dish. 12-15mL of molten SMA (standard methods agar) tempered between 44°C and 46°C was added to each plate within 15 minutes of homogenate preparation. Solutions were mixed and allowed to solidify on each plate. Petri plates were inverted and placed inside an anaerobic container with anaerobic generation sachets. Samples were incubated at 35 ± 2°C for 48 ± 2 hours before sample counting was completed by trained laboratory personnel.
Microbial analysis was conducted on packaged chicken breast using aerobic (APC) and lactic acid (LAB) plate counting using the following method. A 1:10 dilution was homogenized using a stomacher (BagMixer 400W, Interscience, Saint Nom la Bretêche, France) after stomaching, 1 mL was removed from each sample bag and placed in the center of the assigned Petrifilm (Neogen Corporation, Lasing, MI, USA). Using a 3M plastic petrifilm spreader, the homogenate was distributed evenly over the entire surface. Petrifilm were dried for 1 minute allowing the solidifying agent to gel. Samples were incubated aerobically at 35 ± 1°C for 48 ± 3 hours before plate counts were conducted.
2.7. Statistical analysis
Data were analyzed using the GLIMMIX procedures of SAS (version 9.2; SAS Inst., Cary, NC, USA). Least square means were computed for all dependent variables (instrumental color, odor evaluation, pH, lipid oxidation, and microbial growth) with treatment and day serving as independent variables. Significant (p ≤ 0.05) means were separated using pairwise t-tests (PDIFF option).
3. Results and Discussion
3.1. Instrumental surface color
Acceptance of poultry meat by consumers in the retail sector can be linked to surface color [17]. In the United States, fresh poultry cuts are portioned and marketed to consumers as skin-on, skinless, bone-in, or boneless low barrier packaging film and expanded polystyrene trays. Products lacking the appropriate color can result in lost sales [18]. Instrumental surface color was measured on boneless-skinless chicken breast fillets throughout the 28-day display period. The interactive influence of packaging treatment and storage day (treatment × day) was not significant for lightness (p = 0.5125), redness (p = 0.6500), or yellowness (p = 0.9975). However, the main effects for lightness (L*) values (Table 2) increased as storage time increased (p = 0.0001). Samples progressively became lighter which may be attributed to changes in state of myoglobin and moisture content. In contrast to the current study, previous literature reports a decrease in L* values as storage duration increases suggesting drip loss occurring [18]. Samples packaged in treatments E and F had a greater oxygen transmission rate resulting in darker samples compared to those packaged with a lower oxygen transmission rate. Increased oxygen transmission rate allows more oxygen exposure to the product causing a change in the myoglobin protein that results in a darker color. Moreover, this study shows a decrease in redness (a*) values over time. Higher redness values are indicative of a redder product. Due to the nature of the product, redness values are much lower than studies containing beef, lamb or pork due to the myoglobin content of the protein [19]. Current study results suggest that vacuum packaging will affect the surface color of poultry products, but the surface color changes are minimal. Additional research on surface color of poultry throughout storage duration is necessary to identify methods that alter consumer purchasing of fresh vacuum packaged chicken (Table 3).
|
Day of storage |
|||||||
|
0 |
7 |
14 |
21 |
28 |
SEM* |
p-Value |
|
|
Lightness (L*) 1 |
64.38b |
64.36b |
65.26ab |
65.82a |
66.07a |
0.326 |
0.0001 |
|
Redness (a*) 2 |
3.29bc |
3.83a |
3.59ab |
3.20c |
3.01c |
0.134 |
0.0001 |
|
Yellowness (b*) 3 |
12.91a |
12.17b |
12.22b |
11.87b |
12.04b |
0.197 |
0.0029 |
a–c Mean values within a row lacking common superscripts differ (p < 0.05). *SEM, standard error of the mean. 1 Lightness (L*)—values are a measure of darkness to lightness where 100 is white, and 0 is black; 2 Redness (a*)— a larger value indicates a redder color where +60 is red and -60 is green; and 3 Yellowness (b*)— a larger value indicates a more yellow color where +60 is yellow and -60 is blue.
Table 2: Main effect of storage day on instrumental surface color values (L*, a*, b*) of boneless-skinless chicken breast fillets. Traits lacking common letters differ (p ≤ 0.05)
|
Treatment1 |
||||||||
|
A |
B |
C |
D |
E |
F |
SEM* |
p-Value |
|
|
Lightness (L*)2 |
66.82a |
67.11a |
63.41d |
63.77cd |
65.46b |
64.51bc |
0.357 |
<0.0001 |
|
Redness (a*)3 |
2.66bc |
2.73bc |
4.85a |
4.54a |
2.51c |
3.01b |
0.146 |
<0.0001 |
|
Yellowness (b*)4 |
10.91b |
10.42b |
15.26a |
15.15a |
10.99b |
10.71b |
0.215 |
<0.0001 |
1Packaging treatment: (A) Forming: 300 μ polyester/EVOH/polyethylene coextrusion. OTR: 1.8mL/m/24hr Non-forming: 102 μ PE/EVOH/LLDPE. OTR: 0.80mL/m/24hr. (B) Forming: 100 μ nylon/EVOH/polyethylene coextrusion. OTR: 1.5mL/m/24hr. Non-forming: 102 μ PE/EVOH/LLDPE. OTR: 0.80mL\m\24hr. (C) Forming: 175 μ nylon / EVOH / enhanced polyethylene coextrusion. OTR: 0.4mL/m/24hr. Non-forming: 75 μ nylon/EVOH/polyolefin plastomer coextrusion. OTR: 1.0 ML/m/24hr. (D) Forming: 175 μ nylon / EVOH / enhanced polyethylene coextrusion. OTR: 0.4ML/m/24hr. Non-forming: 65 μ nylon / polyolefin plastomer coextrusion. OTR: 53.0mL/m/24hr. (E) Forming: 150 μ polyolefin/nylon/enhanced polyethylene coextrusion. OTR: 31.0mL/m/24hr. Non-forming: 65 μ nylon / polyolefin plastomer coextrusion. OTR: 53.0mL/m/24hr. (F) Forming: 150 μ5 polyolefin/polyethylene. OTR: 1287 mL/m/24hr. Non-forming: 69 μ polypropylene/polyolefin plastomer coextrusion. OTR: 1400mL/m/24hr. 2 Lightness (L*) —values are a measure of darkness to lightness where 100 is white, and 0 is black; 3 Redness (a*)— a larger value indicates a redder color where +60 is red and -60 is green; and 4 Yellowness (b*)— a larger value indicates a more yellow color where +60 is yellow and -60 is blue. a–c Mean values within a row lacking common superscripts differ (p < 0.05). *SEM, standard error of the mean.
Table 3: Main effect of packaging treatment on instrumental surface color values (L*, a*, b*) of boneless-skinless chicken breast fillets. Traits lacking common letters differ (p ≤ 0.05)
3.2. Odor evaluation and pH analysis
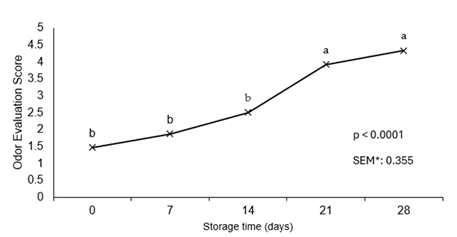
Consumers use their organoleptic senses of sight and odor to determine quality of a fresh meat product. With limitations throughout the literature on storage duration of fresh chicken, most fresh chicken in the U.S. is often packaged in low barrier materials which allows for higher oxygen transmission and short-term retail display. Thus, the measurement of odor is crucial in determining the quality perception of a packaged product. Results of the odor evaluation (Figure 1) show a significant pattern of decreasing odor acceptability of chicken breast as storage time increased. The maximum threshold of acceptability (score 4) was reached after day 14 of storage. At the conclusion of the study, samples reached an average score of 4.3 suggesting that the odor of chicken breast after 21 days (p < 0.0001) of vacuum packaged storage produced an unacceptable odor. It has been previously established throughout limited research that hydrogen sulfide odors increase with packaged chicken producing an off odor [18]. A shelf-life study of broiler carcasses reported a decline in subjective odor scores as microbial count increased [20]. Similarly, a study evaluating vacuum-packaged cooked chicken breast reported a decline in smell and taste scoring as storage time increased suggesting that odor is one limitation of vacuum packaging in poultry products [21].
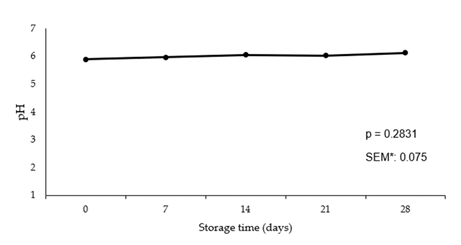
Acidity (pH) of the meat is commonly measured when looking at the quality of a product. The pH values have a substantial influence on the quality of meat due to the changes that occur to the muscle tissue when pH levels change [22]. In pork, it has been found that pH was significantly correlated with color, marbling, firmness, and color measurements [23]. Additionally, pork quality characteristics were more affected by the loin pH than the day of aging or retail display [23]. Literature reports that packaging type can affect pH values during storage due to the formation of lactic acid postmortem [24]. In the current study, results show no increase in pH levels during the 28-day storage duration (p = 0.2831) suggesting that there was no change in pH throughout the duration of the study.
a-bMean values lacking a common superscript differ (p < 0.05). *SEM, standard error of the mean. Odor evaluation score anchors: 1 = “fresh chicken, no off odor”; 2 = “slight off odor”; 3 = “small off odor”; 4 = “moderate off odor, unacceptable”; 5 = “extremely
3.3. Lipid oxidation
It is well known that chicken meat contains greater quantities of unsaturated fatty acids [25], and these fatty acid concentrations can lead to oxidative deterioration during refrigerated storage. Research has been conducted on effective methods for reducing lipid oxidation [26]. Specifically, research focused on thermal processing and immediate hot packaging of turkey patties, reported that limiting oxygen exposure during the cooling phase effectively inhibited oxidative degradation of lipids. Oxygen is the most common reason for accelerating the deterioration of meat that is caused by lipid oxidation [26]. Historically, literature reports that vacuum packaging or modified atmosphere packaging can reduce lipid oxidation and extending storage periods of fresh meats by 7 to 21 days depending on gas composition and storage conditions [26,27]. Vacuum packaging is the best method for products such as turkey bacon as it hinders lipid oxidation which is represented in the current study with high barrier and thicker film treatments (treatment a and treatment b) [27]. In a previous study looking at the results of vitamin E concentration and packaging type on chicken thighs it was found that chicken thighs packaged in vacuum packaging had much lower TBARs values compared to those in an oxygen-permeable packaging [25]. In the current study, there was an interactive effect (p < 0.0001) of the packaging type ´ day of storage of lipid oxidation in chicken breast (Table 4). Treatment F consisted of a low barrier film combination allowing more oxygen to penetrate the packaging resulting in a higher oxidative value on day 21 (p < 0.0001) in comparison to the other treatments. Throughout the storage duration, TBAR values for each treatment of fresh chicken increased supporting theories that lipid oxidation will increase during storage, but greater exposure to oxygen can cause this deterioration to occur faster. However, it is important to note that the values presented in the current study were within the acceptable limit of <2.0 mg malonaldehyde/kilogram of meat sample suggesting that although the lipid oxidation values increased, they were still considered acceptable [28].
|
Day of storage |
|||||||
|
Treatment1 |
0 |
7 |
12 |
21 |
28 |
SEM* |
p-Value |
|
A |
0.32ij |
0.34ghij |
0.36ghi |
0.50d |
0.49d |
0.017 |
<0.0001 |
|
B |
0.30j |
0.33ij |
0.39fg |
0.57c |
0.42ef |
0.017 |
<0.0001 |
|
C |
0.35ghij |
0.35ghij |
0.36ghi |
0.44e |
0.43ef |
0.017 |
<0.0001 |
|
D |
0.33ij |
0.34hij |
0.36ghi |
0.62b |
0.42ef |
0.017 |
<0.0001 |
|
E |
0.32ij |
0.34ghij |
0.43ef |
0.51d |
0.38fgh |
0.017 |
<0.0001 |
|
F |
0.34ghij |
0.34hij |
0.49d |
0.71a |
0.49d |
0.01 |
<0.0001 |
1Packaging treatment: (A) Forming: 300 μ polyester/EVOH/polyethylene coextrusion.; OTR: 1.8mL/m/24hr Non-forming: 102 μ PE/EVOH/LLDPE. OTR: 0.80mL/m/24hr. (B) Forming: 100 μ nylon/EVOH/polyethylene coextrusion. OTR: 1.5mL/m/24hr. Non-forming: 102 μ PE/EVOH/LLDPE. OTR: 0.80mL\m\24hr. (C) Forming: 175 μ nylon / EVOH / enhanced polyethylene coextrusion. OTR: 0.4mL/m/24hr. Non-forming: 75 μ nylon/EVOH/polyolefin plastomer coextrusion. OTR: 1.0 ML/m/24hr. (D) Forming: 175 μ nylon / EVOH / enhanced polyethylene coextrusion. OTR: 0.4ML/m/24hr. Non-forming: 65 μ nylon / polyolefin plastomer coextrusion. OTR: 53.0mL/m/24hr. (E) Forming: 150 μ polyolefin/nylon/enhanced polyethylene coextrusion. OTR: 31.0mL/m/24hr. Non-forming: 65 μ nylon / polyolefin plastomer coextrusion. OTR: 53.0mL/m/24hr. (F) Forming: 150 μ5 polyolefin/polyethylene. OTR: 1287 mL/m/24hr. Non-forming: 69 μ polypropylene/polyolefin plastomer coextrusion. OTR: 1400mL/m/24hr.
a–j Mean values lacking common superscripts differ (p < 0.05). *SEM, standard error of the mean
Table 4: Impact of packaging treatment and day of storage for lipid oxidation on boneless skinless chicken breast fillets.
3.4. Microbiological analysis
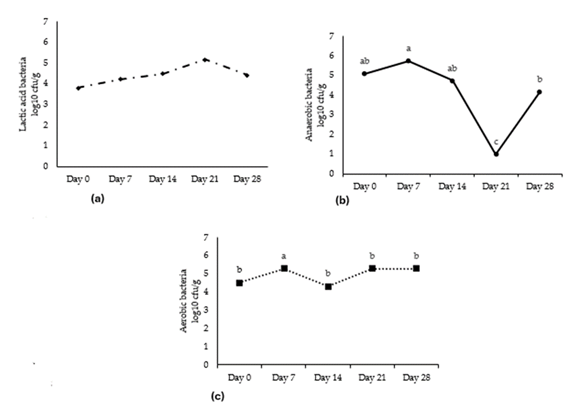
Quality and freshness of a meat product is often associated with the total number of microorganisms contributing to the spoilage characteristics of odor and appearance. It has been reported that fresh poultry meat can be considered unwholesome within 7 days of packaging [29]. In some instances, during the early stages of microbial growth, microorganisms may be present in small quantities. However, as storage time increases, the population of microorganisms will increase producing metabolites that cause off-flavors, off-odors, and textural changes in meat and food products [30]. Vacuum packaging minimizes the amount of oxygen that can reach the meat or food product and has been reported to prevent the growth of aerobic spoilage organisms [31]. Oxygen is the primary atmospheric gas being metabolized by aerobic organisms and it is well known throughout the literature that altering packaging atmospheres of oxygen, nitrogen, or even carbon dioxide can alter microbial growth. In most meat packaging, especially red meats, the absence of oxygen can impact the surface color of a product, but as the presence of increasing oxygen can decreases the storage life and increase spoilage organism growth [32]. In previous research, it has been suggested to incorporate small amounts of oxygen to reduce the anaerobic organism growth as well as the production of toxins since low levels of oxygen can cause raw products to turn green due to the metmyoglobin formation [32]. Literature reports that total plate count in tandoori chicken was within acceptable limits for up to 15 days in cold storage [32]. Vacuum packaged buffalo meat nuggets saw an increase in shelf life by 20 days as well as higher scores in flavor and acceptability [32]. Vienna sausages were vacuum packaged, and results show an increase in lactic acid bacteria as well as souring of the product [33]. Moreover, a study comparing retail display cases for steak and chicken reports no difference in microbial growth over storage time suggesting that storage type does not increase microbial growth [34]. There was no interaction for packaging treatment on microbial growth for anaerobic bacteria, (p = 0.9616), aerobic bacteria (p = 0.8864), or lactic acid bacteria (p = 0.2116) (Figure 2). Previous research of meat packaging using vacuum methods tends to agree with the results of the current study through growth of lactic acid bacteria tended to increase (p = 0.2798) but did not differ (Figure 3a). Anerobic bacteria growth (Figure 3b) increased slightly on day 7. Due to the limited oxygen in the vacuum packaged product, it is interesting to find a small significant difference in the growth of aerobic bacteria with an increase on day 7 (Figure 3c). However, it is important to note that microbial growth did not reach the established threshold of 7-log cfu/g throughout the duration of the study. Previous and current research concludes that the use of vacuum packaging in poultry products is a promising tool for extending storage life of fresh chicken and decreasing the growth of spoilage organisms.
Mean values lacking common superscripts differ (p ≤ 0.05). *SEM, standard error of the mean.
a-c Means lacking common superscripts differ (p < 0.05). *SEM, standard error of the mean.
Figure 3: (a) Growth of lactic acid bacteria of boneless-skinless chicken breast during 28-day storage period (b) Growth of anerobic bacteria of boneless-skinless chicken breast during 28-day storage period (c) Growth of aerobic bacteria of boneless-skinless chicken breast during 28-day storage period.
4. Conclusions
Vacuum packaging of fresh poultry products can aid in extending storage duration and preserving quality characteristics such as color if the correct packaging methods are used. Considerations should be focused on oxygen transmission rate to achieve maximum storage duration. Results from this study conclude that the use of vacuum packaging can limit the rate of microbial growth and lipid oxidation. Packaging materials that offer greater barrier to atmospheric conditions will slow the rate of microbial spoilage of fresh chicken. However, odor evaluation scores were considered unacceptable after day 14, and more research is needed to identify and minimize the off odors produced when vacuum packaging fresh poultry.
Acknowledgments
The authors would like to extend appreciation to WINPAK for providing the Variovac packaging machine and supplying packaging films to complete the study.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Food and Agriculture Organization of the United Nations. Production (2024).
- Brenes, A. and Roura, E. Essential oils in poultry nutrition: Main effects and modes of action. Animal Feed Science and Technology 158 (2010): 1-14.
- Cooreman-Algoed D, Boone L, Taelman SE, et al. Impact of consumer behavior on the environmental sustainability profile of food production and consumption chains – A case study on chicken meat. Resources, Conservation and Recycling 11 (2022): 178.
- Geornaras I, Jesus A, Zyl E, et al. Bacterial populations associated with the dirty area of the South African poultry abattoir. Journal of Food Protection 61 (1998): 700-703.
- Gram L, Ravn L, Rasch M, et al. Food spoilage- interactions between food spoilage bacteria. International Journal of Food Microbiology 78 (2002): 79-97.
- Fung DY. Microbial hazards in food: food-borne infections and intoxications. In: Toldra, F. (Ed.), Handbook of Meat Processing. Wiley-Blackwell Publishing, Ames, IA, USA (2010): 481-500.
- National Chicken Council, NCC Highlights Chicken Industry’s Efforts to Reduce Food Waste (2024).
- Fennell K, Lu G, Mahmoudi M, et al. US Consumers’ Awareness, Purchase Intent, and Willingness to Pay for Packaging That Reduces Household Food. Waste Foods 12 (2023): 23.
- Nychas GJ, Skandamis PN, Tassou CC, et al. Meat spoilage during distribution. Meat Science 78 (2008): 77-89.
- Food and Agriculture Organization of the United Nations, The State of Food and Agriculture: Moving forward on food loss and waste reduction (2019).
- Kerry JP, O’Grady MN, Hogan SA. Past, current and potential utilization of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Science 74 (2006): 113-130.
- Wezemael LV, Ueland O, Verbeke W. European consumer response to packaging technologies for improved beef safety. Meat Science 89 (2011): 45-51.
- Narasimha D, Sachindra NM. Modified Atmosphere and Vacuum Packaging of Meat and Poultry Products. Food Reviews International (2002).
- Barnes EM, Impey CS, Griffiths NM. The spoilage flora and shelf-life of duck carcasses stored at 2 and -1°C in oxygen-permeable or oxygen impermeable film. British Poultry Science (1979).
- King DA, Hunt MC, Barbut S, et al. American Meat Science Association Guidelines for Meat Color Measurement. Meat and Muscle Biology 6 (2023): 1-81.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 52 (1978): 302-310.
- Fletcher DL. Poultry meat colour. World's Poultry Science Journal 58 (2002).
- Nollet LM, Boylston T, Chen F, et al. Handbook of Meat, Poultry and Seafood Quality, Wiley-Blackwell Publishing, Hoboken, NJ, USA, Chapter 35 (2012).
- Schweihofer J. The color of meat depends on myoglobin: Part 1, Michigan State University (2024).
- Russel SM. Rapid Prediction of the Potential Shelf-Life of Fresh Broiler Chicken Carcasses Under Commercial Conditions. Applied Poultry Science (1997).
- Rodriguez-Perez MR, Zurera-Cosano G, Garcia-Gimeno R, et al. Sensory and Microbiological Quality Evaluation of Vacuum-Packed Sliced Cooked Chicken Breast. Journal of Food Quality 26 (2003): 105-122.
- Yue K, Cao Q, Shaukat A, et al. Insights into the evaluation, influential factors and improvement strategies for poultry meat quality: A review. npj Science of Food 8 (2024): 62.
- Gurunathan K, Tahseen A, Manyam S. Effect of aerobic and modified atmosphere packaging on quality characteristics of chicken leg meat at refrigerated storage. Poultry Science 101 (2022): 102-170.
- Holmer SF, McKeith RO, Boler DD, et al. The effect of pH on shelf-life of pork during aging and simulated retail display. Meat Science 82 (2009): 86-93.
- Xiao S, Zhang WG, Lee EJ, et al. Effects of diet, packaging, and irradiation on protein oxidation, lipid oxidation, and color of raw broiler thigh meat during refrigerated storage. Poultry Science 90 (2011): 1348-1357.
- Ahn DU, Wolfe FH, Sim JS, et al. Packaging cooked turkey meat patties while hot reduces lipid oxidation. Journal of Food Science 75 (2006): 1075-1115.
- Phillips CA. Review: Modified atmosphere packaging and its effects on the microbiological quality and safety of produce. International Journal of Food Science and Technology 31 (1996): 463-479.
- Marsh K, Bugusu B. Food Packaging – Roles, Materials, and Environmental Issues. Journal of Food science 72 (2007): R39-R55.
- Witte VC, Krauze GF, Bailey ME. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. Journal of Food Science 35 (1970): 582-585.
- Balamatsia CC, Paleologos E, Kontominas MG, et al. Correlation between microbial flora, sensory changes and biogenic amines formation in fresh chicken meat stored aerobically or under modified atmosphere packaging at 4°C: Possible role of biogenic amines as spoilage indicators, Antonie Van Leeuwenhoek 89 (2006): 9-17.
- Rukchon C, Nopwinyuwong A, Trevanich S, et al. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 130 (2014): 547-554.
- Narasimha DR, Sachindra NM. Modified atmosphere and vacuum packaging of meat and poultry products. Food Reviews International 18 (2002): 263-293.
- Holy AV, Cleote TE, Holzapfel WH. Quantification and characterization of microbial populations associated with spoiled, vacuum-packaged Vienna sausages. Food Microbiology 8 (1991): 95-104.
- Vorst K, Shivalinngaiah N, Brenes ALM, et al. Residential Refrigerator Performance Based on Microbial Indicators of Ground Beef Preservation Assessed Using Predictive Microbiology Tools. Food Control (2018): 56-64.


 Impact Factor: * 3.8
Impact Factor: * 3.8 Acceptance Rate: 77.96%
Acceptance Rate: 77.96%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 