Emergency Contraception from Historical Myth to Modern Reality: A Historical Timeline and Updated Interpretation
Giulia Manzini1*, Michael Kremer2, Ian Hines3, Marko Kornmann4
1Department of General and Visceral Surgery, Hospital of Aarau, Aarau, Switzerland.
2Department of Surgery, Hospital of Lachen, Lachen, Switzerland.
3Department of Nutrition Science, College of Allied Health Sciences, East Carolina University, Greenville, NC 27834, USA.
4Department of General and Visceral Surgery, University Hospital of Ulm, Ulm, Germany.
*Corresponding Author: Giulia Manzini, Department of General and Visceral Surgery, Hospital of Aarau, Aarau, Switzerland.
Received: 19 August 2023; Accepted: 28 August 2023; Published: 25 October 2023
Article Information
Citation: Norman D Goldstuck. Emergency Contraception from Historical Myth to Modern Reality: A Historical Timeline and Updated Interpretation. Journal of Biotechnology and Biomedicine. 6 (2023): 524 - 536.
DOI: 10.26502/jbb.2642-91280114
View / Download Pdf Share at FacebookAbstract
Introduction: Emergency contraception is the use of a birth control method after coitus has taken place and there is a fear that it may lead to a pregnancy. Historical attempts were more likely to be harmful rather than effective. Oral estrogens, progestins, anti- progesterone, and partial agonist/antagonists of progesterone have all been used with varying degrees of efficacy. Currently ethinyl estradiol/levonorgestrel combinations, levonorgestrel alone, ulipristal acetate, and mifepristone are the usual oral methods depending on availability. Copper carrying and more recently levonorgestrel releasing intrauterine devices have also been used successfully. The intrauterine devices appear to be more effective than the oral methods and are also regular contraceptive methods and in addition have therapeutic properties.
Background: The evolution from longer duration oral treatments with side effects to the current single tablet of levonorgestrel, ulipristal acetate, or mifepristone with low side effects and reasonable efficacy is described. The role of the highly effective copper intrauterine device and now also the levonorgestrel intrauterine device for emergency contraception is examined.
Conclusion: Oral emergency contraception is a short term solution. Expanding emergency contraception to include the levonorgestrel releasing intrauterine device may provide long term contraception and health benefits as well as providing emergency contraception.
Keywords
<p>Emergency; Contraception; Estrogens; Progestins; Anti-progestins selective progesterone modulators; Copper intrauterine device; Levonorgestrel intrauterine device</p>
Article Details
Introduction
Pregnancy is a continuum from fertilization to parturition. Under normal circumstances the process takes approximately 40 weeks. Humans understand what initiates this process and have developed technologies to disrupt the process anywhere along the continuum. At what stage this may be done legally varies from country and locality around the world. Attempts to disrupt the continuum are easier and more likely to be legal the earlier it is attempted. Emergency contraception (EC) is the attempt to disrupt this continuum at the interface of where pre-conception and conception meet. It therefore finds itself straddling the area of contraceptive versus abortifacient. This review is however, is concerned only with the physiology and pharmacology of emergency contraception and not with any legal or moral standpoints regarding this issue. These will be used to later challenge some long held perceptions regarding EC. Following unprotected sexual intercourse a woman has two options: i) do nothing and hope for the best with a 0.1% to 25% of becoming pregnant depending on the time of the cycle of exposure, or ii) use a form of EC. EC is often provided when a high sensitivity pregnancy test (HSPT) is negative and is usually not regarded as abortifacient as there is no way of knowing at this stage whether conception has indeed occurred and whether implantation has taken place, even if it may be suspected that it has, based on extrapolating the facts known about the physiology of conception. The rate of unintended pregnancy, while dropping, remains high [1]. The use of emergency contraception in the form of a long-acting reversible contraceptive (LARC) or when a short acting form of EC is given in combination with a LARC, may help to reduce this [2]. This review will examine the pharmacokinetics and pharmacodynamics and clinical results of previous and existing methods of emergency contraception to be able to determine the possible mechanism of action and efficacy. It will avoid one problem of most reviews on this subject of conflating pregnancy rates from studies of the various methods as necessarily being synonymous with efficacy, given how difficult true efficacy is to determine.
Historical aspects of emergency contraception
Historical methods of emergency contraception are of interest because they form the background against which to evaluate our advances. Methods of attempting to prevent conception after coitus have been documented as far back as ancient Roman times. Folklore has continued to promote useless methods such as douching up until modern times. The physiology is against the probability of these methods from working. If coitus has taken place in the peri-ovular period then spermatozoa can easily penetrate the cervical mucus plug which has a high Insler score [3]. at this time. Spermatazoa usually penetrate the mucus plug and may even reach the Fallopian tubes within a few minutes [4]. In order for a contraceptive method to work at this stage then i) the impending ovulation must be delayed so that viable spermatozoa cannot reach the ovum, or ii) if one of the spermatozoa has succeeded in breaching the membrane of the ovum then this ovum should be unable to implant in the already decidualized endometrium. Once this is understood it is clear that it is too late for the use of a douche, irrespective of what it is, and a systemic product which does not target the prevention of ovulation or the formation of a decidualized endometrium or act on the endometrium itself is not likely to be of value. Many different types of douches and potions have been used over the years and have been of little value and occasionally some have been toxic [4]. Finally in the twentieth century chemical compounds which specifically targeted the reproductive tract became available and the prospect of effective and safe emergency contraception had arrived [5].
Oral emergency contraception
Estrogens
The ability of estrogens to interfere with pregnancy in animals has been known since the 1920’s. A number of synthetic and semi-synthetic estrogens were synthesized in the late 1930’s and were soon licensed for medical use. These included diethyl- stilbestrol (DES), Ethinyl-estradiol (EE2), conjugated equine estrogens (CEE), and estradiol esters eg., estradiol propionate. The interest in using them as EC initially was to prevent pregnancy after sexual assault and was based on the fact that veterinarians had used them after ‘inappropriate’ mating [4,5]. Studies began on EC use in the 1960’s on DES in North America6,7 and EE2 in the Netherlands [8,9]. These compounds were generally administered for five days and were often given with anti-emetics. Administration had to be commenced within 72 hours of unprotected intercourse (UIC). Additionally, because of the side effects, they were given largely to women who were deemed at greater pregnancy risk because of these side effects. In one study they were given only to women at ‘midcycle’ and who were assumed to be post-ovulatory [10]. The mechanism of action of emergency estrogens is thought to be the more rapid transport of fertilized ova through the Fallopian tube and retarded maturation of the endometrium with long lasting basal vacuoles thus inhibiting implantation [9] A study on EE2 and CEE [11] was the first study which attempted to assess the efficacy of EC by attempting to correlate the pregnancy rate with the expected number of pregnancies without treatment using the method of Barrett and Marshall [12]. The dose regimes of the various estrogen formulations is given in Table 1 [13]. High dose estrogens are no longer used because they pose a thrombotic risk.
|
Type of estrogen |
Dose |
|
Diethylstilbestrol (DES) |
25-50 mg bid × 5 days |
|
Ethinyl Estradiol (EE2) |
2.5 mg bid × 5days |
|
Conjugated equine estrogens (CEE) |
10 mg bid × 5 days |
|
Estrone (E2) |
5 mg bid × 5 days |
Table 1: Doses of estrogens used for emergency contraception.
Estrogen/progestin combinations
In 1972, Albert Yuzpe in Canada observed that a single dose of 0.05 mg (50μg) EE2 together with 0.5 mg (500μg) of dl-norgestrel (dl-NG) produced endometrial changes [14]. This was thought to be a possible mechanism of action, when using this combination as an EC. Current oral contraceptive however have less than 50 μg of EE2 (anywhere between 10-35μg) and use levonorgestrel (LNG), the active enantiomer of dl-NG. The era of the ‘emergency contraceptive pill’ (ECP) had begun.
A trial with an arbitrary dose of 0.1mg of EE2 and 1mg of dl-NG (given as two doses 12 hours apart) was conducted. This was two doses of two tablets of an existing oral contraceptive available at the time. The first dose had to be given within 72 hours of unprotected coitus. The first study was published in 1974 [15]. There was a pregnancy rate of 2.02%. Many other studies gave similar results. This method provides a dose of EE2 which is 125 times lower than that of the high dose estrogen regime. Since most practitioners were familiar with the oral contraceptive there was no reluctance to use this method as there had been with the high dose estrogens. This development would create an awareness in health professionals and later the public to the possibilities of emergency contraception.
A double-blind randomized controlled trial of high dose EE2 with the Yuzpe method showed no difference in pregnancy rates but far fewer side effects such as nausea, vomiting, and bleeding irregularities, so the use of high dose estrogens for emergency contraception was over [16]. In order to use this method with modern oral contraceptives the tablets must be juggled around to approximately fit these doses [17]. The maximum serum levels of LNG were found to be 15.6 9.2 ng/ml and 391 123 pg/ml for EE2 after a single dose of 500 μg LNG and 100 μg of EE2 [18]. A later analysis showed that the efficacy of the method was overestimated and was probably 51.4-66% effective [19] at best.
Androgen/progestin (Danazol)
Danazol (Danol©, Cipla, Mumbai, India) is a steroid with androgenic, progestogenic, anti-gonadotropic and anti- estrogenic activity. It was evaluated as an emergency contraceptive but was not as effective as the Yuzpe method [20,21]. Thus, its use was abandoned and it is of historical interest only.
Progesterone antagonists
In 1980 the French company Roussell-Uclaf synthesized Mifepristone (RU486) which was highly anti-progestogenic and anti-glucocorticoid. Anti-progestins bind to progesterone receptors and block its actions. This results in abortion in early pregnancies and can also block other luteal phase effects, including endometrial decidualization. It may also prevent ovulation if given before ovulation has taken place [22]. This compound became highly controversial and was later sold by the manufacturers to Exelgyn.
It has now become available in most countries as an abortifacient. Early emergency contraceptive studies of a 600 mg dose versus the Yuzpe method showed that it was as or more effective with fewer side effects [20,23]. Mid-level doses of 25-50 mg of mifepristone appear to be more effective and have fewer side effects than LNG [24]. A study comparing 600 mg, 50 mg, and 10 mg of mifepristone found no significant efficacy differences between the three while the 10 mg dosage was associated with less menstrual cycle disturbance [25]. There is evidence that doses as low as 5 mg are effective [26]. The mechanism of action of mifepristone depends on the dose and day of cycle when it is given. The most used 10 mg dose delays ovulation when it is given pre-ovulatory [27]. The calculated efficacy from 10 mg studies is 83% [28]. This is probably overstated due to the methodology of calculating efficacy in these studies. Mifepristone is not available for use for EC because of its role in medical abortions in most countries. It is available for EC in Russia, China, Cuba, and Vietnam. The antiprogestin gestrinone (10 mg) has also been used and appears to be as effective as 10 mg mifepristone [29].
Progestin
Racemic dl-norgestrel was discovered by Hughes at Wyeth in 1963 via modification of norethisterone, one of the earliest progestins. It was thus considered to be a ‘second-generation’ progestin. The active isomer was later shown to be d-norgestrel or d(-)-norgestrel. The ‘minus’ sign indicates that the ‘d’ structural form rotates polarized light to the left in a levorotatory manor, hence the name levonorgestrel (LNG), while the inactive enantiomer, dextronorgestrel (l(+)-norgestrel) does the opposite. Early papers on LNG still refer to it as d-NG. Only LNG is used nowadays.
By the end of the 1970’s LNG was available in combination with EE2 in combined oral contraceptive pills, on its own in progestin only pills, and was undergoing evaluation as a subcutaneous implant, an intrauterine device, and as a long- acting injectable contraceptive. The ovulatory delaying or suppressing effect of progesterone had been known since 1937 and the early oral contraceptives only added estrogen because the combination gave better cycle control [30]. The rationale for using a progestin only compound in place of the estrogen/progestin combination of the Yuzpe method was therefore evidence based. The side effects of EE2 would be avoided and possibly some aspects of estrogen/progestin interaction. Experimentation with varying doses of LNG began in the early 1970’s with the focus on using it as a modified ‘on demand’ birth control method rather than as a true emergency contraceptive.
Initial evaluation
Early formal studies were conducted in Hungary using 0.75 mg LNG as an emergency contraceptive [31] and also as a deliberate ‘morning-after’ contraceptive where women who had infrequent coitus and who were not suited to other forms of contraception would only take one or two doses of 0.75 mg LNG after coitus [32]. The efficacy and side effect profile appeared to be satisfactory for use as a postcoital emergency contraceptive but not as a deliberate ‘morning-after’ method with a frequency of once a week or more. A study in Germany using 0.4 mg and 0.75 mg LNG in the same ‘morning-after’ regimen came to the same conclusion that use as an infrequent emergency contraceptive would be preferable [33]. A multi-center EC study in Hungary had 23 pregnancies in 1,315 subjects (1.75%). This study used the regime consisting of 0.75 mg LNG twice daily for five days and was the forerunner of the very large studies later conducted by the World Health Organization (WHO). The results are similar to those later found by the WHO and, like the WHO results, are better than those achieved in ‘real world’ situations. The reasons for this are explained later [34].
Comparative studies
The first comparative study of LNG with the Yuzpe method was conducted in Hong Kong [35]. The Yuzpe method was compared with two doses of 0.75 mg LNG given 12 hours apart. The subjects had intercourse more than once in the treatment cycle so there was no attempt to calculate efficacy.
There was a 3.5% failure rate in the Yuzpe group and a 2.9% failure rate in the LNG group. The incidence of nausea, vomiting and fatigue was significantly higher in the Yuzpe group. This was followed up with a large WHO comparative study of the same two regimes commenced within 72 hours after unprotected coitus. The pregnancy rate was 1.1% for the LNG group and 3.2% for the Yuzpe group. An efficacy of 85% for the LNG group and 57% for the Yuzpe group was calculated. The efficacy declined with the time after starting treatment, especially after 24 hours. Nausea and vomiting were significantly higher in the Yuzpe group [36]. A further WHO trial compared mifepristone 10 mg, versus two doses of LNG 0.75 mg 12 hours apart and a single dose of LNG 1.5 mg (Plan-B©, Barr Pharmaceuticals Montvale, New Jersey, USA) within 72 hours of unprotected coitus. The pregnancy rates were 1.5% for both the mifepristone and single dose of 1.5 mg LNG and 1.8% for the two 0.75 mg doses of LNG. There were no significant side effects for all groups although the mifepristone tended to delay the next menses [37]. A later study showed a 1.3% failure rate for the two dose LNG regime and 2% for the mifepristone 10 mg regime.38 An earlier study had attempted to calculate the efficacy difference between LNG in two doses and mifepristone and found mifepristone to be 79.7% and LNG 59.1% effective using Dixon’s method [11,39].
Pharmacokinetics and mechanism of action
The maximum serum levels of LNG are around 15.2 ng/ml for the 750 μg dose which is similar to that of the Yuzpe regime18 and a maximum level of 19.1 (9.7) ng/ml was reported for the 1.5 mg dose [40]. LNG serum levels are very variable so the data for the different doses are not directly comparable. LNG 1.5 mg appears to act by delaying or preventing ovulation. It does this if given 48 hours before the luteinizing hormone LH surge. It is ineffective if LH levels are on the rise. There is no effect on the endometrium, progesterone levels or fertilization or implantation or ectopic pregnancy or a developing fetus [41]. The International Federation of Gynecology and Obstetrics (FIGO) considers the mechanism of action of LNG as “inhibition of ovulation and thickening of cervical mucus” and that language on “implantation” should not be included on product labelling [42]. A limited and constrained mechanism of action must limit the efficacy. More recently there has been some concern that LNG 1.5 mg is much less effective in women who weigh more than 75 kg or have a body mass index (BMI) of more than 30 kg/m2 [43]. LNG for EC has been available for about 20 years in most countries’ worldwide. It is available mostly without prescription and even ‘off the shelf’ in some jurisdictions because of its proven safety. Currently LNG 1.5 mg is is the benchmark or ‘gold-standard’ against which past and future oral emergency contraception can be judged.
Selective progesterone receptor modulators (SPRM)
The exploitation of pharmaceutical ligand molecules which bind selectively to different receptors has been used since the 1950’s. These molecules may have agonist activities at some receptors and antagonistic activities at the same or other receptors depending on dosage. Beta-blockers, opioids, and anti-histamines are good examples. Ulipristal acetate (UPA) is closely chemically related to mifepristone and has both agonistic and antagonistic effects on the progesterone receptor site and is therefore a selective progesterone receptor modulator (SPRM). Unlike mifepristone it stimulates rather than inhibits glucocorticoid activity but these effects have no influence on its use as an ECP [41].
Pharmacokinetics and pharmacodynamics
UPA, unlike the other molecules used as ECP was specifically developed as an emergency contraceptive pill by Laboratoire HRA Pharma. It has been licensed for EC in many countries as UPA 30 mg (Ella® or EllaOne®. HRA Pharma, London, UK). The pharmacokinetics and pharmacodynamics were studied before its widespread clinical usage [44]. Following a single dose administration of UPA in 20 women under fasting conditions, maximum plasma concentrations of UPA are around 180 ng/ml with a half-life of around 32 hours. It is highly bound to plasma proteins and mainly metabolized by cytochrome P450. It can be taken with or without food. UPA 30 mg has been studied in relation to the LH peak and at different ovarian follicle diameters and in relationship to ovulation [45]. UPA inhibits follicle rupture if given before the LH rise and even if the leading follicle was up to 18 mm. If given on the day of the LH peak UPA can delay ovulation, and thus exhibit efficacy for up to 48 hours after the LH surge. If LNG is given at this stage, it is not effective [45]. UPA may also have an inhibitory effect on follicle rupture. This gives it a larger window of potential efficacy than LNG. The fertile window is normally from five days before ovulation to the day of ovulation itself (Figure 1). UPA like LNG does not appear to have any endometrial effects with short term use but UPA does when used long term [46]. Lack of endometrial effect is a factor restricting the window of action of LNG and UPA.
Clinical evaluation
UPA appears to be the most effective ECP with efficacy rates calculated to be between 62-85% [47]. UPA has been compared to LNG, the benchmark ECP, and been found to be more effective [48,49]. The pregnancy rate with UPA was 42% lower than LNG in the first 72 hours and 65% lower in the first 24 hours. UPA was significantly more effective in the 72-120 hour subgroup. LNG is known to only be partially effective at best after 72 hours because of its failure to be effective after ovarian follicular size of 15 mm.50 Side effects of UPA included headaches, nausea and dizziness and the next menstrual period was delayed by 2.5 days on average. UPA is also less effective in overweight and obese women but is less affected by BMI than LNG.51 UPA 30 mg becomes less effective after BMI ≥30 (LNG BMI ≥25) and becomes virtually ineffective at BMI ≥35(LNG ≥30). The current epidemic of obesity in some countries limits this option. There has been some speculation on the use of higher doses in these women [51].
UPA 5 mg daily as a treatment for fibroids has resulted in some cases of liver toxicity. While this is not likely with a single dose of UPA 30 mg there is some concern over instances of repeated doses of UPA 30 mg. For this reason, there is concern where it may be available without prescription and used repeatedly or even where it is prescribed repeatedly [52].
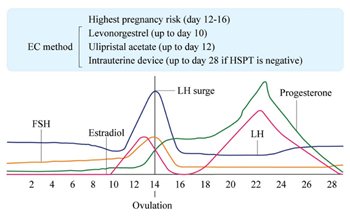
Figure 1: Relationship of menstrual cycle parameters to the potential usability of oral levonorgestrel (LNG), ulipristal acetate (UPA), or intrauterine device (IUD).
Non-steroidal anti-inflammatory agents
There is some evidence that meloxicam (a cyclo-oxygenase type-2 inhibitor) may be an ECP when given in a 30 mg dose for five days; the COX-2 inhibitor meloxicam when added to 1500μg of LNG significantly increased the proportion of cycles with no follicular rupture or ovulatory dysfunction. COX-2 inhibitors appear to be able to disturb the ovulatory process after the onset of the LH surge.53 There is no information on other newer COX-2 inhibitors like etoricoxib or celecoxib.
The efficacy of oral emergency contraception
There have not been any trials of the efficacy of oral emergency contraceptives (ECP) with a placebo as control, for ethical reasons. The determination of the effectiveness of oral emergency methods therefore rests on the probability that a particular coital act would result in a pregnancy. Initially the method of Dixon [11] which was based on values determined by Barrett and Marshall [12] was used. More recently, estimated risks of pregnancy by day of cycle have been based on the work of Wilcox and colleagues They conducted a detailed study relating the day of the menstrual cycle on which coitus occurred and how likely pregnancy would be to result on that day in women attempting to conceive [54]. This data has been used for the last 20 or more years to estimate the efficacy of ECP.
Humans are the only species which seem to understand that pregnancies are produced by coitus. While most species engage in coitus for reproductive purposes only (the Benobo may be an exception), humans also use coitus for bonding purposes where reproduction is not wanted and not desired. The idea that measuring pregnancy rates in couples using coitus to create a pregnancy and applying them to those who are having coitus with the strict intention of not producing a pregnancy is not really logical. The act of coitus is complicated and cannot be singularly compared with for example, renal or liver function. Not all coital acts will therefore have the same possibility of producing a pregnancy, irrespective of all other factors but this cannot be quantified. Couples who are attempting to produce a pregnancy are pre-screened while assignations leading to requests for ECP may involve an unknowingly infertile partner. The male may have a low or non- existent sperm count or chronic epididymitis or prostatitis, while the female may have early endometriosis, adenomyosis, fibroids, or sub-clinical tubal infection. A full gynecological history and/or examination is rarely taken before ECP is prescribed and it obviously is not if the ECP is acquired over the counter or directly off the shelf. Some of the differences between coitus for planned pregnancy and non-reproductive intended coitus are given in Table 2. The conclusion from this is that coitus is not as efficient at producing a pregnancy when it is not specifically directed to pregnancy production but takes place none the less. Women who are not attempting to achieve a pregnancy will often not keep a good track of menstrual cycles. In one study pregnanediol concentrations show that 30% of women presenting for the ECP believed they were in the fertile phase of the cycle but were noy [55]. Some women are also not in the part of the cycle they think they are by calculation [56]. Women presenting for ECP often do not show the same numbers of sperm in the vagina as those who are trying to get pregnant at the same post-coital interval [57]. This information plus information on the relative efficacy of the intrauterine device for emergency contraception also appears to indicate the calculated efficacy of ECPs is overstated [2]. The circumstances surrounding ‘unprotected intercourse’ do not need any elaboration to those who have dispensed emergency contraception after taking a detailed history of said circumstances. The efficacy of oral emergency contraception is therefore probably overstated.
|
Planned |
||
|
Couple relationship |
Usually |
Not usually |
|
Female partner health |
Healthy |
Unknown |
|
Male partner health |
Healthy |
Unknown |
|
Coital site |
Usually optimal |
Possibly sub-optimal |
|
Mental state |
Relaxed |
Often stressed |
|
Intoxicants |
Not usually |
Often |
|
Ability to complete |
Usually |
May be impaired |
|
Contraception |
None |
Possible broken condom |
|
Withdrawal |
Never |
Sometimes attempted |
|
Menstrual cycle data |
Reliable |
Not reliable |
|
†Excluding failure of a contraceptive method other than condoms. |
||
Table 2: Coitus for planned pregnancy versus unprotected.
The intrauterine device as emergency contraception
More than a century after its introduction in Germany by Richter and Grafenberg [58,59] the intrauterine device (IUD) has finally come into its own. It has had a checkered history of being bedeviled by being associated with the problems of pain, bleeding, and infection. After World War II the use of thermoplastics was introduced by Lippes to solve these problems [60]. However, to be effective plastic IUDs need to have a minimum surface area of 600 mm2, [61] so the problems persisted to a large extent. In the late 1960’s Zipper, from Chile, the world’s largest copper producer, showed that copper had a powerful anti-fertility effect in rabbits and humans, [62] which was confirmed by Chang and Tatum [63]. This exciting find led to a series of clinical studies in Chile using increasing copper loads on a polyethylene ‘T’ frame from 30 mm2 to 200 mm2 to find a load which appeared to be effective.64 Freed of any constraints of the size and surface area of the plastic frame, the choice was made from anatomical data from Dickinson which Lippes had used earlier for the plastic devices.65 Modern day ultrasound measurements have been used to question the frame sizes that were chosen but they have largely persisted for the ‘T’ framed devices still used today.
The copper bearing IUD
Mechanism of action of copper as an emergency contraception
The mechanism of action of copper as an emergency contraceptive is probably the same as that of when it is used as a long-acting reversible contraceptive (LARC) except that it is placed after coitus so that some actions may not apply. The early copper IUDs had copper loads of 200 mm2 and released 45μg of copper per day [66]. Peak release of copper occurs in the first two cycles of use and since copper ionizes very rapidly in an ionic solution like endometrial fluid it is fair to assume this release begins immediately. Current copper IUDs tend to have greater surface area of copper and the release rate will be even greater. In the genital tract copper could act at any stage in the reproductive process. It could interfere with sperm directly, inhibit tubal sperm transport, be toxic to the fertilized egg or blastocyst, or disturb the intrauterine environment preventing implantation. There is reasonable evidence that all these mechanisms may occur [67]. Copper also reduces sperm penetration through cervical mucus [68] but this mechanism will not influence an emergency IUD.
Clinical evaluation of the emergency copper IUD
Lippes and colleagues published the first studies of the emergency copper IUD [69,70]. None of the 299 women became pregnant and a good percentage were assumed to be in the fertile window based on dates and ‘spinnbarkheit’ length. A large percentage were nulliparous. The use of IUDs in nulliparae was quite contentious at the time and had not yet gained more general acceptance as it has currently. The copper-7 200 (Gravigard® GD Searle High Wycombe, Bucks, UK), T copper 200 (TCu 220, Janssen-Cilag, High Wycombe, Bucks, UK) and the T copper 300 were the devices which were used. While most insertions took place within three days after UIC, some insertions took place up to seven days after UIC. These studies generated a lot of enthusiasm for the method as the high estrogen oral method was the only other method available at the time. The publication of the larger Yuzpe study [71] rapidly dampened enthusiasm for the emergency IUD. A secondary problem was that there was a high discontinuation rate for the emergency copper IUD in this study. This finding was to be replicated in other studies.
Two further studies in the United Kingdom included subjects up to 10 days after UIC [72,73] and one included a plastic IUD72 which were followed by another UK study which included some plastic IUDs [74]. These inclusions may have been unintentional due to protocol violation. The only randomized study of the copper IUD versus an oral emergency method was reported from Italy where EE2 for five days was compared. There were no pregnancies in either group [75]. Two further Italian studies followed in which insertions were conducted up to seven days after UIC [76,77]. At this stage in the mid-1980’s two interesting facts emerged; i) despite all completed studies using seven days after UIC as their cut-off point, all formal recommendations from the WHO, the Royal College of Obstetricians and Gynaecologists in the United Kingdom, and the American College of Obstetricians and Gynecologists, recommended five days as the cut-off point for insertion of an emergency IUD. The five day ruling was presumably based on the data of Croxatto and co-workers showing that implantation took place mainly on the 6th day after fertilization [78]. This was only changed years later to ‘five days after the expected day of ovulation’, following the realization of how inadequate this was for UIC in the early follicular phase. ii) The withdrawal of the Copper 7 IUD in the United States meant there was a short interval where no copper bearing IUDs were available there until the Copper T 380A (Paragard®, Cooper Cos Inc, Pleasanton, California, USA) was approved. Unlike in the US most other middle and higher income countries still had a variety of different types of copper IUDs at their disposal.
Shortly thereafter a study in Egypt reported four pregnancies in the copper IUD group and 22 in a control group who received no treatment [79]. This was the first study to report a pregnancy. While there are anecdotal reports of pregnancy with copper IUDs in western countries [80] there are at present no reports of a pregnancy in a single copper IUD for EC study in a western country following two extensive systematic reviews (8,550 insertions and 12 pregnancies, all from Egypt or China) [81,82]. Both reviews showed a pregnancy rate of about 0.1%. Only the Multiload IUDs (MLCu 250 and MLCu 375, Multilan SA, Fribourg, Switzerland) and GyneFix (Contrel, Ghent, Belgium) IUDs have an official indication for use as EC [41]. All other copper IUDS used as EC are being used ‘off label’.
Newer clinical studies
The temporary unavailability of the copper IUD in the US and extreme interest in LNG oral emergency contraception led to a hiatus in emergency copper IUD evaluations in Western countries. The bulk of experience with various types of copper IUDs for EC now started coming from China [83]. Chinese studies are often comparative with oral EC methods but there have not been any controlled studies. IUD insertion for EC in Chinese studies is always five or fewer days after UIC. From the mid 1990’s the LNG releasing IUD started to become available and shortly thereafter sub dermal implants. These together with the copper IUD and sometimes including the injectables came to be known as long-acting reversible contraceptives (LARCs). LARCs were largely independent of user input and this led to the idea that they would be more successful as contraceptives. There is evidence that this is true.84 This increased the use of IUDs and interest in the copper IUD was rekindled. This in turn provided the impetus for a slew of new copper IUD for EC studies, mainly from Turok and colleagues [85,86].
Among the advances these studies have demonstrated is that the previous and current recommendations regarding emergency IUD use are not scientifically based. The original recommendation of five days after UIC was based on the estimated time to implantation of six days [78] which is in any case variable. The updated recommendations of five days after the presumed day of ovulation is also speculative as there is no way of determining this. Recent evidence shows clearly that an emergency copper IUD can be inserted anytime during the cycle provided a HSPT is negative as this also allows for multiple episodes of unprotected coitus in the same cycle [87-89]. This approach avoids being dependent on answers to questions regarding the time of the cycle and delay after UIC which may be either incorrect or untruthful. The limitations as previously accepted for IUDs are not scientifically based and the scientific limitations for the major EC methods are shown in Figure 1. Naturally physicians and providers are always free to exercise their own preference, for reasons other than scientific evidence.
The role of LNG-IUDs
Women who request emergency contraception are at risk for pregnancy at the time and presumably in the future. As well as being given an LNG oral emergency contraceptive, many are given a conventional contraceptive at the same time. A LARC method is often suggested and for those who do not want a copper IUD, one of the LNG containing IUDs [90] is an option, as is the sub dermal implant unless the woman was given UPA which might theoretically interfere with the initial action of the levonorgestrel-releasing intrauterine system (LNG-IUS) or implant and require a waiting period. A non- randomized study of the TCu 380A versus oral LNG 1.5 mg plus insertion of an LNG-IUS 52mg at the same time produced only a single pregnancy in the LNG oral plus LNG-IUS group. This was less than would have been expected for LNG alone [91]
The levonorgestrel IUD
Mechanism of action of intrauterine LNG as an emergency contraceptive
The levonorgestrel-releasing intrauterine systems (LNG-IUS) (LNG-IUS 52 mg Mirena©, Bayer Pharma, Berlin, Germany, Levosert©, Lilletta© Actavis, Reykjavik, Iceland), LNG-IUS 19.5 mg (Kyleena©, Bayer Pharma, Berlin, Germany, LNG-IUS13.5 mg, Jaydess©/Skyla©, Bayer Pharma, Berlin, Germany) are very effective for contraception and work by three main methods; they i) act on cervical mucus to reduce sperm penetration (decrease Insler score), ii) alter tubal mobility, and iii) produce a pseudo-decidualized atrophic endometrium, and less importantly, iv) a partial inhibition of the LH surge leading to anovulation (40%) with the LNG-IUS 52 mg device [92]. These effects are achieved over a number of cycles. Serum levels of LNG are around 200 picogram/ml (0.2 ng/ml) compared with 20 ng/ml for the oral LNG 1.5 mg pill which is achieved within hours [40]. The silastic membrane of the LNG-IUS 52mg device allows a constant release of about 20 μg/day of LNG so there is no immediate surge of LNG. This is known as zero order kinetics whereas pills for EC and the copper IUD will deliver using largely first order kinetics which are dose dependent if all other factors e.g., first pass metabolism, absorption etc. are excluded. An LNG-IUS will produce a tissue concentration of LNG of 800 ng/gm in the endometrium after 36 days when LNG is released at 30 μg/day (higher than the current LNG-IUS 52mg which releases 20μg/day) [93]. The mechanism of action of the LNG-IUS as an emergency contraceptive is therefore difficult to explain in terms of the foregoing and is therefore unknown at present.
Clinical evaluation of the emergency LNG IUD
Turok et al., performed a randomized non-inferiority trial of the TCu 380A versus the LNG-IUS 52mg [94]. The study was encouraged by the possible effectiveness of plain plastic devices [72,74]. There were 321 subjects in the TCu38A group and 317 in the LNG-IUS 52mg.There was one pregnancy in the LNG-IUS 52mg group and none in the TCu 380A group. These differences were not statistically significantly different.
The efficacy of emergency IUD insertion
Relatively few physicians recommend the emergency IUD [95]. It is an invasive and time-consuming procedure and, unless specifically requested, it is probably more often advised and inserted when the woman requesting EC is perceived to be at most risk. The majority of emergency IUD studies do not correlate the day of insertion with the cycle day on which it was inserted because in many cases there have been multiple episodes of UIC. There has only been one report of a randomized study [75] but many group comparative studies [2,81]. Although not explicitly stated it is reasonable to assume that, overall, the IUDs were placed more often when the clinician thought the woman was at a higher risk of pregnancy. Despite this bias (against the IUD) the relative risk for pregnancy with an IUD versus the main oral methods (these were not segregated in most studies) was anywhere from one third to one thousand times lower [2]. Assuming an efficacy of about 50-60% for oral methods the emergency IUD efficacy must asymptotically approach 99%,2 especially when considering insertions by inexperienced personnel may fail to correctly place an emergency copper IUD, which unlike an interval IUD insertion, only offers a limited window in which to re-attempt the insertion in an already stressed subject.
Emergency IUDs as LARC
The notion of providing LARC contraception at the same time as providing emergency contraception despite its appeal has not had much impact on providing IUDs for emergency contraception. There is a historically high discontinuation rate of most copper IUDs, especially in women of lower parity, for various reasons, despite counselling [96] except with the very small GyneFix® device which is also licensed as an emergency IUD [97-99] In some studies where recipients of an emergency IUD were followed up the discontinuation rate was very high [70,72,76,100,101] for a variety of reasons and for different devices (Table 3).
|
Study |
IUD type(s) |
Follow up (months) |
Discontinuation rate (%) |
Comments |
|
Lippes et al., 1979 [70] |
CuT200, CuT300, Cu 7 |
2 |
30 |
|
|
Black et al., 1980 [72] |
Cu7, Cu 7 mini, Lippes loop D |
2 |
20 |
|
|
Gottardi 1986 [76] |
Cu7, Cu7 mini, TCu 200, MLCu 250 |
21 |
43 |
Discontinuation was 25% in a control group. Four later pregnancies in emergency group, three in controls. |
|
Turok et al., 2014 [100] |
TCu 380A |
12 |
24 |
Nine pregnancies, eight after IUD removed, one while in use. |
|
Envall et al., 2016 [101] |
unspecified |
6 |
22.2 |
One pregnancy after emergency IUD removed. |
Table 3: Long term continuation rates of emergency copper intrauterine devices (IUDs).
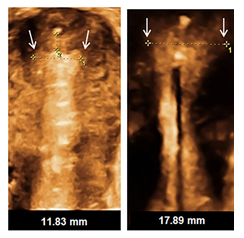
Figure 2: An endometrial cavity of width 11.83 mm containing a GyneFix device contrasted with a cavity width of 17.89 mm in which an levonorgestrel-releasing intrauterine system (LNG-IUS) 13.5 mg cannot fully open.
Determination of an emergency contraceptive agent as a contraceptive or an abortifacient
A compound which is given to disrupt an established pregnancy which may be determined i) clinically, ii) via imaging, or iii) biochemically is clearly an abortifacient e.g., mifepristone, misoprostol, or oxytocic’s when used for that purpose in the presence of a biochemical and/or image confirmation. In the absence of evidence of an established pregnancy the determination of emergency contraception as an abortifacient is purely speculative and based on the supposition that an ovum may have become fertilized and may have been either i) prevented from implanting or ii) having been implanted has been dislodged from the endometrium which had decidualized. Arguments to the contrary i.e., that emergency oral methods are abortifacient are unverifiable by physiological and pharmacological actions. All contraceptives work by one or more of the following mechanisms; i) preventing or incapacitating sperm from reaching the upper genital tract, ii) preventing folliculogenesis and/or ovulation, iii) preventing fertilization, iv) altering tubal mobility, or v) altering endometrial structure and/or function. All major methods of contraception including IUDs, combined oral contraceptives, implants, or injectables may fail to prevent pregnancy, however rarely. In that case all the above mechanisms failed. There must logically be occasions where all mechanisms fail, except one e.g., endometrial decidualization was not mature enough to support the fertilized ovum, in which case it may have implanted and/or failed to attach. Without a chemical test to confirm fertilization or imaging methods with sufficient resolution to observe this then all these suppositions are speculative, no matter how rational they seem to be as a thought experiment. All emergency contraception, especially the IUD if it is inserted more than seven days after unprotected sex, irrespective of the day of cycle, should always be preceded by a negative HSPT, to confirm the non- existence of a pregnancy as accurately as possible given current technology. Advocacy groups have done a remarkable job in getting ECPs off prescription and freely available over the counter in many countries, since they must be taken as soon as possible after UIC and their efficacy is compromised by time delay and increasing BMI. Delayed use beyond 72 hours and BMI over 25 start reducing efficacy markedly for LNG and significantly but less so for UPA, although a presumed minimum efficacy for LNG has been calculated [102]. This improved availability does not come without consequences and cost. There is both a lost opportunity to provide a more effective emergency contraceptive method which is a LARC, and so unintended pregnancy still remains a future threat, and there is also the possibility that repeated use of UPA may be harmful [52].
A confirmation of the LNG-IUD study [94] and establishing whether the LNG-IUS 13.5mg and LNG-IUS 19.5mg can operate as emergency contraception is a priority. This will be difficult to accomplish. The LNG release rates are lower and the physical sizes of these LNG-IUSs are smaller than the one tested. In contradistinction the early copper devices used for EC had lower copper loads than the newer ones used for EC so that it was logical that they would be as or more effective for EC. The LNG containing IUDs bring proven well established additional health benefits beyond their role as contraceptives and the copper IUD does as well albeit to a lesser extent [103,104]. The problem of discontinuation can be addressed, in part at least, by selecting the most appropriate device (Figure 2), a task which will become easier if a greater selection of sizes and choices for both copper and hormonal IUDs become available. Perhaps then the repeated calls for the use of more use of emergency IUDs will be satisfied [105]. It appears that the LNG-IUS can also be inserted up to 14 days after UIC [106] which makes it more versatile as an EC.
Conclusion
Currently all available evidence, including sensitive biochemical hormonal level estimations and high-resolution imaging techniques cannot demonstrate that emergency contraception is anything other than contraception despite protestations to the contrary [107]. We are living in the age of ‘hook-up’ culture with young people using dating platforms such as Tinder, Instagram, and Bumble to name a few. There is evidence that this has changed behavior somewhat making women more reluctant to demand condom use for the more attractive men and no reluctance on the part of men to not use protection [108]. This can only increase the need for EC (and treatment for sexually transmitted infections) in the future.
Data availability
No data are associated with this article.
References
- Finer LB, Zolna MR: Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med 374 (2016): 843-852.
- Cheung TS, Goldstuck ND, Gebhardt GS. The intrauterine device versus oral hormonal methods as emergency contraceptives: A systematic review of recent comparative studies. Sex Reprod Healthc 28 (2021): 100615.
- Insler V, Melmed H, Eichenbrenner L, et al.: The cervical score. Int J Gynecol Obstet 10 (1972): 223-228.
- Van Look F. Post coital contraception: a cover up story. Diczfalusy E, Bygdeman M, editors. Fertility regulation today and tomorrow. New York: Serono symposia Publication, Raven Press 36 (1987): 29-42.
- Haspels AA. Emergency contraception: a review. Contraception 50 (1994): 101-108.
- Morris JM, van Wagenen G. Compounds interfering with ovum implantation and development. III. The role of estrogens. Am J Obstet Gynecol 96 (1966): 804-815.
- Kuchera KK. Postcoital contraception with diethylstibestrol- updated. Contraception 10 (1974): 47-54.
- Haspels AA. The morning-after pill: a preliminary report. IPPF Med Bull 3 (1968): 6.
- Haspels AA. Interception. Posycoital estrogens in 3,016 women. Contraception 14 (1976): 375-381.
- Notelovitz M, Bard DS. Conjugated estrogen as a post-ovulatory interceptive. Contraception 17 (1978): 443-454.
- Dixon GW, Schlesselman JJ, Ory HW, et al. Ethinyl-estradiol and conjugated estrogens as postcoital contraceptives. JAMA 244 (1980): 1336-1339.
- Barrett JC, Marshall J. The risk of conception on different days of the menstrual cycle. Popul Stud 23 (1969): 455-461.
- Sitka CS. Emergency postcoital contraception. Glob. Libr Women's Med (2008).
- Yuzpe AA. Post coital contraception.Saxena BN, Mokkapati S, Toteja GS, editors. Post-coital, once a month and menses inducing agents in the control of female fertility. New Delhi: Indian Council of Medical Research (1972): 149-163.
- Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception-a pilot study. J Reprd Med 13 (1974): 53-58.
- Van Santen MR, Haspels AA. Comparative randomized double-blind study of high dosage ethinyl-estradiol versus ethinyl-estradiol and norgestrel combination in postcoital contraception. Acta Endocrinol 246 (1982): 2-4.
- Trussell J, Schwarz EB. Emergency contraception. Hatcher RA, Trussell J, Nelson A, et al., editors. Contraceptive technology. New York NY Ardent Media (2011): 113-145.
- Kives S, Hahn PM, White E, et al. Bioavailability of the Yuzpe and levonorgestrel regimens of emergency contraception: vaginal vs. oral administration. Contraception 71 (2005): 197-201.
- Trussell J, Ellertson C, Von Hertzen H, et al.: Estimating the effectiveness of emergency contraceptive pills. Contraception 67 (2003): 259-265.
- Webb AM, Russel J, Elstein M. Comparison of the Yuzpe regimen, danazol, and mifepristone (RU486) in oral postcoital contraception. BMJ Clin Res 305 (1992): 927-931.
- Guillebaud J, Kubba A, Rowlands S, et al. Post-coital contraception with danazol, compared with an ethinyloestradiol- norgestrel combination or insertion of an intra-uterine device. J Obstet Gynaecol 3 (1983): S64-S68.
- Van Look PF, von Hertzen H. Clin use of antiprogestogens. Hum Reprod Update 1 (1995): 19-34.
- Glasier A, Thong KJ, Dewar M, et al.: Mifepristone (RU 486) compared with high-dose estrogen and progestogen for emergency postcoital contraception. N Engl J Med 327 (1992): 1041-1044.
- Shen J, Che Y, Showell E, et al.: Interventions for emergency contraception. Cochrane Database Syst Rev (2019).
- Task Force on Postovulatory Methods of Fertility Regulation: Comparison of three single doses of mifepristone as emergency contraception: a randomized trial. Lancet 353 (1999): 697-702.
- Carbonell JL, Garcia R, Breto A, et al.: Mifepristone 5 mg versus 10 mg for emergency contraception: double -blind randomized clinical trial. In. J Women’s Health 7 (2015): 95-102.
- Gemzell-Danielsson K, Mandl I, Marions L. Mechanisms of action of mifepristone when used for emergency contraception. Contraception 68 (2003): 471-476.
- Piaggio G, Heng Z, von Hertzen H, et al. Combined estimates of efficacy of mifepristone 10 mg in emergency contraception. Contraception 68 (2003): 439-446.
- Wu S, Dong J, Cong J, et al. Gestrinone compared with mifepristone for emergency contraception: a randomized controlled trial. Obstet Gynecol 115 (2010): 740-744.
- Dhont M. History of oral contraception. Eur J Contracept Reprod Health; 15 (2010): S12-S18.
- Seregely G, Vero T. Postcoital contraception with 0.75 mg d-norgestrel (Postinor). Ther Hung 29 (1981): 31-34.
- Farkas M, Apro G, Sas M. Clinico-pharmacological examination of Postinor (0.75 mg d-norgestrel). Ther. Hung 29 (1981): 22-30.
- Canzler E, Ahrendt HJ, Ahrendt S. Experiences with levonorgestrel in postcoital contraception. Zentralbl. Gynakol 106 (1984): 1182-1191.
- Seregely G. Results of a multicentre trial of Postinor. Ther Hung 30 (1982): 72-78.
- Ho PC, Kwan MSW. A prospective randomized comparison of levonorgestrel with Yuzpe regimen in postcoital contraception. Hum. Reprod 8 (1993): 389-392.
- Task Force on Postovulatory Methods of Fertility Regulation: Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet 352 (1998): 428-433.
- Von Hertzen H, Piaggio G, Peregoudov A, et al.: Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomized trial. Lancet 360 (2002): 1803-1810.
- Hamoda H, Ashok PW, Stalder C, et al.: a randomized trial of mifepristone (10 mg) and levonorgestrel for emergency contraception. Obstet Gynecol 104 (2004): 1307-1313.
- Wu SC, Wang CP, Wang YQ, et al. A randomized double-blind multicenter study comparing levonorgestrel and mifepristone for emergency contraception. J Reprod Med 8 (1999): S43-S46.
- Barr Pharmaceuticals Inc: Plan B One-Step (levonorgestrel) tablet, 1.5 mg, for oral use. Summary of product characteristics (2009).
- Gemzell-Danielsson K, Rabe T, Cheng L. Emergency Contraception. Gynecol. Endroc 29 (2013): 1-14.
- “Mechanism of action: How do levonorgestrel-only emergency contraceptive pills (LNG ECPs) prevent pregnancy?” (PDF). London: International Federation of Gynecology and Obstetrics. Archived (PDF) (2014).
- Festin MP, Peregoudov A, Seuc A, et al. Effect of BMI and body weight on pregnancy rates with LNG as emergency contraception: analysis of four WHO HRP studies. Contraception 95 (2017): 50-54.
- Watson Pharma, Inc: Ella 30 mg. Summary of product characteristics (2010).
- Gemzell-Danielsson K. Mechanism of action of emergency. Contraception 82 (2010): 404-409.
- Mutter GL, Bergeron C, Delegdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol 21 (2008): 591-598.
- Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48-120 hours after intercourse for emergency contraception. Obstet Gynecol 115 (2010): 257-263.
- Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol 108 (2006): 1087-1089.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta- analysis. Lancet 375 (2010): 555-562.
- Croxatto HB, Brache V, Pvez M, et al. Pituitary-ovarian function following the standard levonorgestrel emergency contraceptive dose or a single 0.75- mg dose given on the days preceding ovulation. Contraception 70 (2004): 442-450.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception 84 (2011): 363-367.
- Mozzanega B. Ulipristal acetate and liver-injuries: while Esmya is revoked, EllaOne is allowed in repeated self-administrations possibly exceeding UPA toxic-dosing with Esmya. J Hepatol 74 (2020): 750-751.
- Jesam C, Salvatirra AM, Schwarz JL, et al. suppression of follicular rupture with meloxicam, a cyclooxygenase-2 inhibitor: potential for emergency contraception. Hum Reprod 25 (2010): 368-373.
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 333 (1995): 1517-1521.
- Stirling A, Glasier A. Estimating the efficacy of emergency contraception-how good are the data? Contraception 66 (2002): 19-22.
- Espinos JJ, Rodriguez-Espinosa J, Senosiain R, et al. The role of matching menstrual data with hormonal measurements in evaluating effectiveness of postcoital contraception. Contraception 60 (1999): 243-247.
- Espinos-Gomez JJ, Senosiain R, Mata A, et al. What is the seminal exposition among women requiring emergency contraception? A prospective, observational study. Eur J Obstet Gynecol Reprod Biol 131 (2007): 57-60.
- Richter R. Ein Mittal zur Verhuetung der Konzeption. Deutsch Med. Wschr 35 (1909): 1525-1527.
- Grafenberg E. Silk as anticoncipient. Bendix K, editor. Geborten Regelung - Vortraege und Verhandlung en des Aerztekursus vom 28-30 Dezember 1928. Berlin: Selbsverlag (1929).
- Lippes J. Contraception with intrauterine plastic loops. Am J Obstet Gynecol 93 (1965): 1024-1030.
- Moyer DL, Mishell DR. Reactions of human endometrium to the intrauterine foreign body. II. Long term effects on the endometrial histology and cytology. Am J Obstet Gynecol 111 (1971): 66-80.
- Zipper J, Medel M, Prager R. Suppression of fertility by intrauterine copper and zinc in rabbits: A new approach to intrauterine contraception. Am J Obstet Gynecol 105 (1969): 529-534.
- Chang CC, Tatum HJ. A study of the antifertility effect of intrauterine copper. Contraception 1 (1970): 265-270.
- Zipper JA, Tatum HJ, Medel M, et al. Contraception through the use of intrauterine metals. I. Copper as an adjunct to the “T” device. The endouterine copper “T”. Am J Obstet Gynecol 109 (1971): 771-774.
- Dickinson RL. Human Sex Anatomy. Second ed. Huntington, NY: Robert E. Krieger Publishing Co (1971).
- Hagenfeldt K. Intrauterine contraception with the copper T device. 1. Effect on trace elements in the endometrium, cervical mucus and plasma. Contraception 6 (1972): 37-54.
- Ortiz ME, Croxatto HB. Copper T intrauterine device and levonorgestrel intrauterine system: biological bases of their mechanism of action. Contraception 75 (2007): S16-S30.
- Elstein M, Ferrer K. The effect of a copper-releasing intrauterine device on sperm penetration in human cervical mucus in vitro. J Reprod. Fertil 32 (1973): 109-111.
- Lippes J, Malik T, Tatum HJ. The postcoital copper-T. Adv. Plan. Parent 11 (1976): 24-29.
- Lippes J, Tatum HJ, Maulik D, et al. Postcoital copper IUDs. Adv. Plan. Parent 14 (1979): 87-94.
- Yuzpe AA, Lance WJ. Ethinylestradiol and DL-norgestrel as a potential contraceptive. Fertil. Steril 28 (1977): 932-936.
- Black TRL, Goldstuck ND, Spence A. Post-coital intrauterine device insertion-a further evaluation. Contraception 22 (1980): 653-658.
- Goldstuck ND. Delayed postcoital IUD insertion. Contracept. Deliv. Syst 4 (1983): 293-296.
- Guillebaud J, Kubba A, Rowlands S, et al. Post-coital contraception with danazol, compared with an ethinyloestradiol-norgestrel combination or insertion of an intra-uterine device. J Obstet Gynaecol 3 (1983): S64-S68.
- Gottardi G, Marzi MM, Pozzi S. Postcoital estrogen or IUD? IPPF Reg Bull 8 (1979): 7-8.
- Gottardi G, Spreafico A, de Orchi L. The postcoital IUD as a an effective continuing contraceptive method. Contraception 34 (1986): 549-558.
- Luerti M, Tonta A, Ferla P, et al. Postcoital contraception by estrogen/progestagen combination or IUD insertion. Contraception 33 (1986): 61-68.
- Croxatto HD, Carillo D. Studies on the duration of egg transport in the human oviduct: Part I. Fertil. Steril 23 (1972): 447-458.
- Askalani AH, Al-Senity AM, Al-Agizy HM, et al. Egypt. Obstet Gynecol 13 (1987): 63-66.
- Kubba AA, Guillebaud Y. Failure of post-coital contraception after insertion of an intrauterine device. Case report Br. J Obstet Gynecol 91 (1984): 596-597.
- Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod 27 (2012): 1994-2000.
- Goldstuck ND, Cheung TS. The efficacy of intrauterine devices for emergency contraception and beyond: a systematic review update. Int J Women's Health 11 (2019): 471-479.
- Yang Y, Zhang XM, Wei DY, et al. Clinical study of mifepristone, A-nordrin and intrauterine device used for emergency contraception. J Reprod Med 6 (1997): 171-173.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 366 (2012): 1998-2007.
- Turok DK, Gurtcheff SE, Handley E, et al. A pilot study of the Copper T380A IUD and oral levonorgestrel for emergency contraception. Contraception 82 (2010): 520-525.
- Turok DK, Jacobson J, Dermish A, et al. Pregnancy rates 1 year after choosing the copper T380 IUD or oral levonorgestrel for emergency contraception: a prospective observational study. Contraception 86 (2012): 294.
- Turok DK, Godfrey EM, Wojdyla D, et al. Copper T380 intrauterine device highly effective at any time in the menstrual cycle. Hum. Reprod 28 (2013): 2672-2676.
- Thompson I, Sanders JN, Schwarz EB, et al. Copper intrauterine device placement 6-14 days after unprotected sex. Contraception 100 (2019): 219-221.
- Sanders JN, Howell L, Saltzman H, et al. Unprotected intercourse in the 2 weeks prior to requesting emergency intrauterine contraception. Am J Obstet Gynecol 215 (2016): 592.e1-592.e5.
- Goldstuck ND. Clarification of the Jaydess (Skyla) LNG-IUS 13.5mg and Kyleena LNG-IUS 19.5mg as intrauterine contraceptive systems. Exp Review Med. Dev 14 (2017): 593-599.
- Turok DK, Sanders JN, Thompson I, et al. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception 93 (2016): 526-532.
- Goldstuck ND, Le HP. Delivery of progestins via the subdermal versus the intrauterine route: comparison of the pharmacology and clinical outcomes. Expert Opin Drug Deliv 15 (2018): 717-727.
- Nilsson CG, Haukkamaa M, Vierola H, et al. Tissue concentrations of levonorgestrel in women using a levonorgestrel releasing IUD. Clin. Endocrinol 17 (1982): 529-536.
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med 384 (2021): 335-344.
- Harper CC, Speidel JJ, Drey EA, et al. Copper intrauterine device for emergency contraception: clinical practice among contraceptive providers. Obstet Gynecol 119 (2012): 220-226.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity and device type on complications and discontinuation of intrauterine devices. Obstet. Gynecol 123 (2014): 585-592.
- Wildemeersch D, Jandi S, Pett A, et al. Use of frameless intrauterine devices and systems in young nulliparous and adolescent women: results of a multicenter study. Int J Women's Health 6 (2014): 727-734.
- Goldstuck ND, Wildemeersch D. Practical advice for emergency contraception in young women. Obstet. Gynecol Int 2015 (2015): 1-6.
- Wildemeersch D, Pett A, Jandi S, et al. Precision intrauterine contraception may significantly increase continuation of use: a review of long-term clinical experience with frameless copper- releasing intrauterine contraception devices. Int J Women's Health 5 (2013): 215-225.
- Turok DK, Jacobsen JC, Dermish AI, et al. Emergency contraception with a copper IUD or oral levonorgestrel:an observational study of 1-year pregnancy rates. Contraception 89 (2014): 222-228.
- Envall N, Koefoed NG, Kopp-Kallner H. Use of effective contraception 6 months after emergency contraception with a copper intrauterine device or ulipristal acetate - a prospective observational cohort study. Acta Obstet Gynecol Scand 95 (2016): 887-893.
- Raymond E, Taylor D, Trussell J, et al. Minimum effectiveness of the levonorgestrel regimen of emergency contraception. Contraception 69 (2004): 79-81.
- Bahamondes L, Bahamondes MV, Shulman LP. Non- contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Hum. Reprod. Update 21 (2015): 640-651.
- Goldstuck ND. Modern menstruation: Is it abnormal and unhealthy? Med. Hypotheses 144 (2020): 109955.
- Belden P, Harper CC, Speidel JJ. The copper IUD for emergency contraception, a neglected option. Contraception 85 (2012): 338-339.
- Booras CM, Sanders JN, Schwarz EB, et al. Risk of pregnancy with levonorgestrel-releasing intrauterine system placement 6-14 days after unprotected sexual intercourse. Obstet Gynecol 137 (2021): 623-625.
- Kahlenborn C, Peck R, Severs WB. Mechanism of action of levonorgestrel emergency contraception. The Linacre Quart 82 (2015): 18-33.
- Eleftheriou A, Bullock S, Graham CA, et al. Does attractiveness influence condom use intentions in women who have sex with men? PLoS One 14 (2019): e0217152.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 